Abstract
Dynamic thalamic regulation of sensory signals allows the cortex to adjust better to rapidly changing behavioral, physiological and environmental demands. To fulfill this role, thalamic neurons must themselves be subjected to constantly changing modulatory inputs that originate in multiple neurochemical pathways involved in autonomic, affective and cognitive functions. This review defines a chemical framework for thinking about the complexity of factors that modulate the response properties of relay trigeminovascular thalamic neurons. Following the presentation of scientific evidence for monosynaptic connections between thalamic trigeminovascular neurons and axons containing glutamate, GABA, dopamine, noradrenaline, serotonin, histamine, orexin and melanin-concentrating hormone, this review synthesizes a large body of data to propose that the transmission of headache-related nociceptive signals from the thalamus to the cortex is modulated by potentially opposing forces and that the so-called ‘decision’ of which system (neuropeptide/neurotransmitter) will dominate the firing of a trigeminovascular thalamic neuron at any given time is determined by the constantly changing physiological (sleep, wakefulness, food intake, body temperature, heart rate, blood pressure), behavioral (addiction, isolation), cognitive (attention, learning, memory use) and affective (stress, anxiety, depression, anger) adjustment needed to keep homeostasis.
Keywords: Glutamate, Histamine, Serotonin, Orexin, Dopamine, Noradrenaline, Melanin-concentrating hormone, Stress, Anxiety, Sleep, Food intake
Historically, the thalamus was viewed as a simple relay station for sensory information from the periphery to the cortex. This view has been replaced by the concept that instead of ‘just’ transferring sensory signals from subcortical nuclei to the cortex, thalamic neurons play central role in the selection, amplification, and prioritization process that determines which type of information should be made available to the cortex at any given time1, 2. Being the so-called ‘gate-keeper’ of the cortex, thalamic neurons regulate the flow of rapidly-changing sensory signals, thus allowing the cortex to adjust to the constantly evolving behavioral and environmental demands1.
To regulate the amount of sensory signals that reach the cortex, thalamic neurons must themselves be subjected to a variety of modulatory inputs that originate in cortical, hypothalamic, brainstem, spinal and intrathalamic nuclei1, 3–6. In the context of somatosensory and nociceptive information, the more extensively studied networks that drive and/or modulate the activity of relay thalamic neurons are the excitatory glutamatergic input originating in corticothalamic, spinothalamic and medial lemniscus tract neurons, and the inhibitory GABAergic input involving the reticular thalamic nucleus (Kaneko and Mizuno, 1988; McCormick and von Krosigk, 1992; Jones, 2007). The excitatory glutamate input, acting through metabotropic mGluRs, is capable of producing sustained neuronal firing whereas the inhibitory GABA input, acting through the GABAb receptor is capable of switching off the sustained neuronal activity7.
Far less is known about the regulation of relay thalamic neurons by other neurotransmitters and neuropeptides3 from various brain regions. Candidates include those from the brainstem and hypothalamus. Brainstem inputs include serotonergic projections from raphe nuclei8, 9, noradrenergic projections from locus coeruleus and the A5 catecholamine group in the pons8–10, and dopaminergic projections from periaqueductal gray, and the lateral parabrachial nucleus11–15. Hypothalamic inputs include additional dopaminergic projections from A11/A1311–15, histaminergic projections from the tuberomammillary nucleus16, 17, orexinergic projections from the perifornical, dorsomedial and lateral hypothalamus18, 19, and melanin-concentrating hormone (MCH) projections from the lateral hypothalamus20–22.
The potential release of these neurotransmitters/neuropeptides on relay thalamic nuclei suggests that the modulation of individual neurons is rather complex, likely subjected to opposing forces driven by a variety of changing external and internal conditions that require constant behavioral, physiological, and affective adjustments. To understand how ‘a decision’ is made on whether or not a relay thalamic neuron fires, for how long, and at what frequency, it is necessary to determine which neuropeptides/neurotransmitters are in a position to govern the activity of individual thalamic neurons that share a common function. In the current review we describe an array of neuropeptides/neurotransmitters that may modulate individual, physiologically-identified thalamic trigeminovascular neurons believed to play a role in the generation of headache perception during migraine.
The discharge mode of relay thalamocortical neurons is either burst or tonic1, 23. The burst discharge is commonly associated with lower excitability, drowsiness, and in the context of headache, responses to acute pain, whereas the tonic discharge has been associated with higher excitability, wakefulness, and chronic pain state4, 24–26. In principle, each of the neurotransmitters/neuropeptides discussed below have close apposition with thalamic trigeminovascular neurons and as such, can potentially shift their firing mode from burst to tonic if it is excitatory, and from tonic to burst if it is inhibitory. As in other systems, the action of each neuropeptide/neurotransmitter on individual thalamic neuron depends on the type of release and reuptake, the type of receptor activated, and most likely the location of the neuron and its projection targets in the cortex. Since this information is not available for thalamic trigeminovascular neurons, which are the subject of this review, speculation on possible roles of the identified neuropeptides/neurotransmitters in setting thalamic transmission, as it may be related to migraine headache, is based on their known action in other systems.
A. Glutamatergic innervation
Axons immunoreactive to VGluT2, thus containing the excitatory neurotransmitter glutamate, are present at high density in all thalamic nuclei known to contain trigeminovascular neurons including VPM, Po, LP and LD (Fig. 1A). When examined in sections containing the trigeminovascular neuron(s), dense VGluT2 immunopositive vesicles are seen in close apposition to the cell body, proximal and distal dendrites (Fig. 1B).
Figure 1.
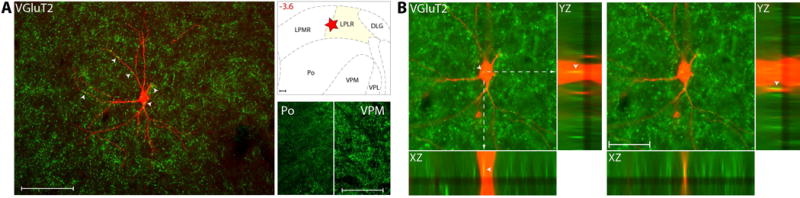
Glutamatergic innervation of thalamic trigeminovascular neurons. (A) Left: Immunopositive VGluT2 synaptic vesicles (green) surrounding a thalamic dura-sensitive neuron (red) labeled with TMR–dextran. Arrowheads indicate close apposition of VGluT2 positive axons and the cell body and dendrites of the labeled neuron. Upper right: Location of the dura-sensitive neuron (red star) shown at left. Number in red indicates distance from bregma (mm). Lower right: Fluorescent images showing VGluT2 axonal labeling in thalamic Po and VPM nuclei. Scale bars = 100 mm. (B) Close apposition between VGluT2 immunopositive vesicles and thalamic trigeminovascular neurons. The three views in the x-y, y-z and x-z planes provide evidence that VGluT2 immunopositive vesicles (green) may contact cell bodies, proximal and distal dendrites of trigeminovascular neurons in LP (red). Arrowheads indicate probable contact point on each view. Note that some green-labeled vesicles and red-labeled soma or dendrites are in the same focal plane (yellow). Scale bar = 50 μm. Adapted from Noseda et al., 201427
Vesicular glutamate transporters (VGluTs) are responsible for glutamate trafficking and for the subsequent regulated release of glutamate at the synapse. Glutamate excites relay thalamocortical neurons through NMDA receptors, if the sensory stimulus is prolong, and through non-NMDA receptors if the sensory stimulus is brief28, 29. Of the three isoforms of VGluT, we opted to study VGluT2 because it is expressed most densely in relay thalamic nuclei30–34 and in ascending trigeminal sensory neurons that project to VPM and Po35, 36. Since VGluT1 axons originate in corticothalamic neurons, we interpreted the presence of VGluT2 on thalamic trigeminovascular neurons as constituting the main drive for activation of these neurons by glutamatergic input they receive from ascending trigeminothalamic (possibly dura-sensitive) neurons in SpV.
B. Dopaminergic innervation
Axons immunoreactive to Tyrosine Hydroxylase (TH), thus containing the catecholamine neurotransmitter dopamine, are present at moderate density in all thalamic nuclei known to contain trigeminovascular neurons (Fig. 2A). When examined in sections containing the trigeminovascular neuron(s), moderate density of TH immunopositive axons and varicosities are seen in close apposition to proximal and distal dendrites (Fig. 2B). We interpreted some of the TH-positive axons as dopaminergic based on a recent retrograde tracing study where we showed that the dopaminergic cells group A11/A13 project to the same Po and LP areas in which trigeminovascular neurons are found37.
Figure 2.
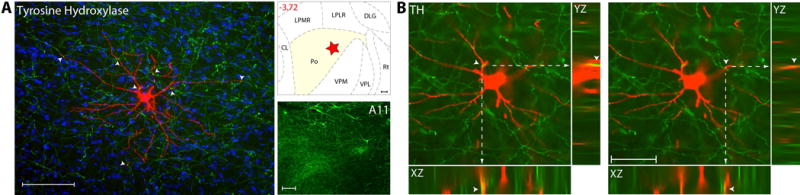
Dopaminergic innervation of thalamic trigeminovascular neurons. (A) Left: Immunopositive Tyrosine Hydroxylase axons (green) surrounding a thalamic dura-sensitive neuron (red) labeled with TMR–dextran. Nuclear counterstaining was performed with DAPI (blue). Arrowheads indicate close apposition of TH positive axons and the cell body and dendrites of the labeled neuron. Because TH is present in noradrenergic and dopaminergic cells, the interpretation of its labeling must take into consideration these two neurotransmitters. Upper right: Location of the dura-sensitive neuron (red star) shown at left. Number in red indicates distance from bregma (mm). Lower right: Fluorescent image showing TH labeling of cell bodies in the hypothalamic A11 nucleus. Scale bars = 100 mm. (B) Close apposition between TH immunopositive axons and thalamic trigeminovascular neurons. The three views in the x-y, y-z and x-z planes provide evidence that TH immunopositive fibers (green) may contact proximal and distal dendrites of trigeminovascular neurons in Po (red). Arrowheads indicate probable contact point on each view. Note that some green-labeled axons and red-labeled dendrites are in the same focal plane (yellow). Scale bar = 50 μm. Adapted from Noseda et al., 201427
In the context of migraine, dopamine has been considered for its role in promoting hypothalamic-mediated symptoms/prodromes such as yawning and nausea38, and more recently, modulation of dorsal horn trigeminovascular neurons39. Further supporting this hypothalamic connection is the finding that the A11 dopaminergic cell group in the medial hypothalamus innervates trigeminovascular neurons in both, the medullary dorsal horn40, 41 and the thalamic relay nuclei37. The rich innervation of thalamic trigeminovascular neurons by dopaminergic fibers suggests that modulation of transmission of nociceptive trigeminovascular signals by dopamine may also occur at the thalamus. When conceptualizing dopamine role in migraine, a consideration should be given to the activation of thalamic D1 and D2 receptors which facilitate membrane depolarization and increase spike discharge in somatosensory VPL/VPM thalamic neurons42, and to the selective uptake of cocaine by dopaminergic nerve terminals in the thalamus as these findings define the possibility that thalamic dopamine pathways may be critically involved in drug-addiction, impulse control, affect, attention and decision making43–49. Translating these into clinical implications, thalamic dopamine may thus be considered as a possible contributor to behaviors that lead to medication-overuse headache and exacerbation of headache by negative emotions, effort to control anger and irritability, cognitive tasks that require attention and the need to make mundane decisions.
C. Serotoninergic innervation
Serotoninergic innervation was determined using Serotonin Transporter – SERT (Fig. 3A), a stable marker of serotoninergic fibers in the brain50. Axons immunoreactive to SERT, thus containing the monoamine neurotransmitter serotonin, were present at high density in all thalamic nuclei known to contain trigeminovascular neurons (Fig. 3B). When examined in sections containing the trigeminovascular neuron(s), dense SERT immunopositive axons and varicosities were seen in close apposition to the cell body, proximal and distal dendrites.
Figure 3.
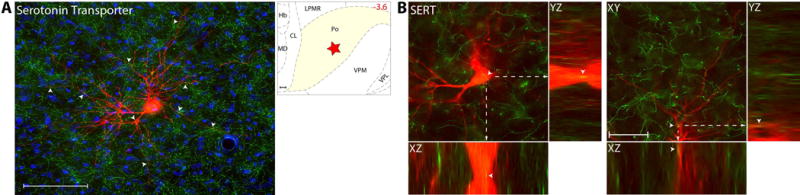
Serotoninergic innervation of thalamic trigeminovascular neurons. (A) Left: Immunopositive Serotonin Transporter axons (green) surrounding a thalamic dura-sensitive neuron (red) labeled with TMR–dextran. Nuclear counterstaining was performed with DAPI (blue). Arrowheads indicate close apposition of SERT positive axons and the cell body and dendrites of the labeled neuron. Upper right: Location of the dura-sensitive neuron (red star) shown at left. Number in red indicates distance from bregma (mm). Scale bars = 100 mm. Since SERT does not stain cell somas, it was not possible to use this marker to identify the serotoninergic neurons in the raphe nuclei that project to the thalamic nuclei containing trigeminovascular neurons. (B) Close apposition between Serotoninergic axons and thalamic trigeminovascular neurons. Images from the original z-stack (obtained every 1 μm) were used to create orthogonal views in the y-z and x-z planes. The three views provide evidence that SERT immunopositive fibers (green) may contact cell bodies, proximal and distal dendrites of trigeminovascular neurons in Po (red). Note that some green-labeled axons and red-labeled soma or dendrites are in the same focal plane (yellow). Scale bar = 50 μm. Adapted from Noseda et al., 201427
Relevant to this study is that serotonin has long been implicated in migraine pathophysiology51, 52, that this implication has lead to the development of 5HT1B/1D receptor agonists (i.e., triptans) for acute treatment of migraine, that serotonergic innervation of VPM and Po originating mainly in the rostral raphe8, 53–56, and that depending on the amount of serotonin release in the thalamus, it could be facilitatory (at low concentration) or inhibitory (at high concentration) to relay neurons in VPM and Po57. In principle, a high concentration of serotonin is inhibitory whereas a low concentration is excitatory. Accordingly, the very dense innervation of thalamic trigeminovasular neurons observed in the figure above can provide an anatomical substrate for a predominantly inhibitory effect of serotonin on transmission of trigeminovascular information between the thalamus and the cortex, as well as the inhibition of trigeminovascular thalamic neurons by local administration of 5HT1 agonists58. Given the latter, we were surprized by the total absence of 5HT1D receptors in the thalamus. This finding suggests that the inhibition of thalamic trigeminovascular neurons response to dural stimulation occur at an earlier synapse along the trigeminovasculat pathway59, rather than in the thalamus. On a more global view, serotonin, through its involvement in stress60, anxiety61, depression62, sleep63, apetite64, and learning61 may help facilitate the reciprocal relationship between these affective and physiological states and migraine.
D. Noradrenergic innervation
Axons immunoreactive to Dopamine β-Hydroxylase (DBH), thus containing the catecholamine neurotransmitter noradrenaline, are present at moderate-to-high density in all thalamic nuclei known to contain trigeminovascular neurons (Fig. 4A). When examined in sections containing the trigeminovascular neuron(s), moderate-to-high density of DBH immunopositive axons and varicosities are seen in close apposition to the cell body, proximal and distal dendrites (Fig. 4B). These DBH axons originate in the locus coeruleus, the main producer of noradrenalin in the brain.
Figure 4.
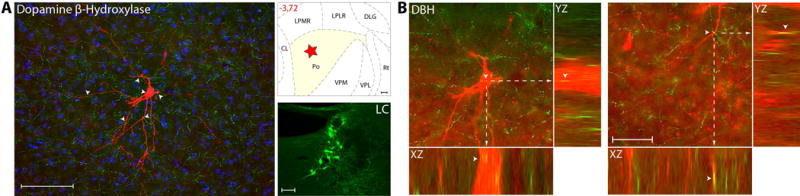
Noradrenergic innervation of thalamic trigeminovascular neurons. (A) Left: Immunopositive Dopamine β-Hydroxylase axons (green) surrounding a thalamic dura-sensitive neuron (red) labeled with TMR–dextran. Nuclear counterstaining was performed with DAPI (blue). Arrowheads indicate close apposition of DBH positive axons and the cell body and dendrites of the labeled neuron. Upper right: Location of the dura-sensitive neuron (red star) shown at left. Number in red indicates distance from bregma (mm). Lower right: Fluorescent image showing DBH labeling of cell bodies in the LC of the brainstem. Scale bars = 100 mm. (B) Close apposition between DBH immunopositive axons and thalamic trigeminovascular neurons. The three views in the x-y, y-z and x-z planes provide evidence that DBH immunopositive fibers (green) may contact cell bodies, proximal and distal dendrites of trigeminovascular neurons in Po (red). Arrowheads indicate probable contact point on each view. Note that some green-labeled axons and red-labeled soma or dendrites are in the same focal plane (yellow). Scale bar = 50 μm. Adapted from Noseda et al., 201427
Because of the wide distribution of noradrenergic fibers in the brain it is difficult to assign to this neurotransmitter a specific role in certain function. Rather, it is thought to improve signal-to-noise ratio in the firing of neurons that respond to sensory stimuli10, 65–67 when conditions involve anticipation, reward, and changing cognitive and emotional circumstances68. To be in a position to modulate thalamic neurons, noradrenergic fibers project heavily to all thalamic sensory nuclei69, 70 and act on both α and β adrenoceptors, which together modulate firing rate, set a pacemaker current, determine membrane resting potential, and synaptic strength71–74. In the context of migraine, noradrenaline, which usually prolongs the activation of thalamic neurons75–78, may be involved in setting abnormal excitability level in trigeminovascular neurons, centrally, and the magnitude of arterial hypertension, peripherally. This view is supported by the finding that β1 adrenoceptor blockers, which are among the very few drugs approved as migraine prophylactics79, inhibit the activity of thalamic trigeminovascular neurons80. The observed relationship between noradrenergic fibers and thalamic trigeminovascular neurons provide a direct anatomical substrate for the central action of β1 adrenoceptor blockers in migraine. Given that activation of β1 adrenoceptor enhances the hyperpolarization-activated cation current (Ih) responsible for setting the so-called pacemaker activity level and the resting membrane potential in those relay thalamic neurons that exhibit such current71, 73, 74, it is reasonable to speculate that thalamic trigeminovascular neurons exhibit the hyperpolarization-activated cation current – a current that may render them likely to exhibit a prolonged firing mode.
E. Histaminergic innervation
Axons immunoreactive to histamine neurotransmitter were present at moderate density in LP and LD, and at low density in VPM and Po (Fig. 5A). When examined in sections containing the trigeminovascular neuron(s), moderate density of histaminergic immunopositive axons and varicosities are seen in close apposition to the cell body, proximal and distal dendrites (Fig. 5B). This histaminergic innervation originates in the dorsal and ventral tuberomammillary nuclei of the hypothalamus, the sole producers of histamine in the brain.
Figure 5.
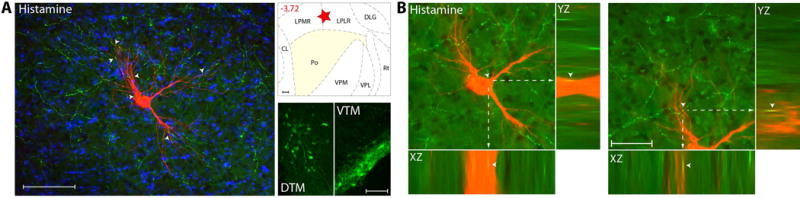
Histaminergic innervation of thalamic trigeminovascular neurons. (A) Left: Immunopositive Histamine axons (green) surrounding a thalamic dura-sensitive neuron (red) labeled with TMR–dextran. Nuclear counterstaining was performed with DAPI (blue). Arrowheads indicate close apposition of Histamine positive axons and the cell body and dendrites of the labeled neuron. Upper right: Location of the dura-sensitive neuron (red star) shown at left. Number in red indicates distance from bregma (mm). Lower right: Fluorescent image showing Histamine labeling of cell bodies in the hypothalamic DTM and VTM nuclei. Scale bars = 100 mm. (B) Close apposition between Histamine immunopositive axons and thalamic trigeminovascular neurons. The three views in the x-y, y-z and x-z planes provide evidence that Histamine immunopositive fibers (green) may contact cell bodies, proximal and distal dendrites of trigeminovascular neurons in LP (red). Arrowheads indicate probable contact point on each view. Note that some green-labeled axons and red-labeled soma or dendrites are in the same focal plane (yellow). Scale bar = 50 μm. Adapted from Noseda et al., 201427
In the context of migraine, histamine has been considered for its role in causing H1 receptor mediated arterial dilatation and the consequential induction of delayed headache81. The findings that histaminergic nerve terminals converge on thalamic trigeminovascular neurons suggest that histamine role in migraine may also include modulation of thalamic trigeminovascular neurons through excitatory H1 receptors whose action enhances slow depolarization current capable of switching neuronal discharge mode from burst to tonic82. In the CNS, histamine originates exclusively from neurons of the tuberomammillary hypothalamic nucleus16, 17. Given that these neurons are active during the wake-state and quiescent during the sleep state83–85 and that histamine switches the firing mode of relay thalamic neurons from burst to tonic3, 82, it is tempting to speculate that the modulation of thalamic trigeminovascular neurons by the histaminergic pathway may play a role in the partial, or even complete, headache relief provided by sleep.
F. Melanin-concentrating hormone innervation
Axons immunoreactive to MCH are present at low density in all thalamic nuclei known to contain trigeminovascular neurons (Fig. 6A). When examined in sections containing the trigeminovascular neuron(s), low density of MCH immunopositive axons and varicosities are seen in close apposition to the proximal and distal dendrites, but not the cell body (Fig. 6B). These MCH axons originate mainly in the lateral hypothalamus.
Figure 6.
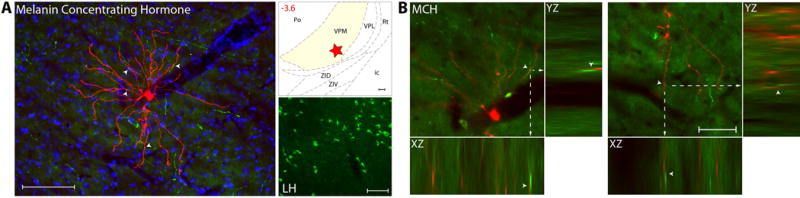
MCH innervation of thalamic trigeminovascular neurons. (A) Left: Immunopositive Melanin Concentrating Hormone axons (green) surrounding a thalamic dura-sensitive neuron (red) labeled with TMR–dextran. Nuclear counterstaining was performed with DAPI (blue). Arrowheads indicate close apposition of MCH positive axons and the cell body and dendrites of the labeled neuron. Upper right: Location of the dura-sensitive neuron (red star) shown at left. Number in red indicates distance from bregma (mm). Lower right: Fluorescent image showing MCH labeling of cell bodies in the lateral hypothalamus. Scale bars = 100 mm. (B) Close apposition between MCH immunopositive axons and thalamic trigeminovascular neurons. The three views in the x-y, y-z and x-z planes provide evidence that MCH immunopositive fibers (green) may contact distal dendrites of trigeminovascular neurons in VPM (red). Arrowheads indicate probable contact point on each view. Note that some green-labeled axons and red-labeled dendrites are in the same focal plane (yellow). Scale bar = 50 μm. Adapted from Noseda et al., 201427
The MCH system, which originates in the hypothalamus and contain GABA86 is thought to play a modulatory/inhibitory role in the regulation of energy expenditure, arousal, locomotion, sexual behavior and a variety of autonomic functions87–91. Being excited by increased glucose level after a meal, MCH neurons are thought to promote sleep and energy expenditure (i.e., cessation of food intake) by releasing GABA at multiple cortical, subcortical, brainstem and spinal areas they project to. To date, this system has not been considered in the pathophysiology of migraine or other headaches. The findings that hypothalamic MCH neurons issue axons that terminate on thalamic trigeminovascular neurons define a novel anatomo-functional substrate for hypothesizing about possible interactions between food intake, drowsiness and migraine. It is tempting to propose that the mechanism by which eating may make patients ‘feel better’ during migraine involves increased level of glucose, activation of hypothalamic MCH neurons92, and the consequential inhibition of relay thalamic trigeminovascular neurons. Conversely, this anatomo-functional substrate may also explain a part of the reasons for why migraine is promoted by skipping a meal. Skipping a meal inhibits MCH neurons (as glucose level goes down) that, when inactive, may release far less GABA around thalamic trigeminovascular neurons. Reduced GABA input might then enhance neuronal excitability, rendering them more likely to respond to subthreshold input they receive from ascending dura-sensitive neurons in the spinal trigeminal nucleus.
G. Orexinergic innervation
Axons immunoreactive to orexin A were present at low density in LP, LD and most medial part of Po, and at very low density in VPM and most lateral part of Po (Fig. 7A). When examined in sections containing the trigeminovascular neuron(s), low density of orexinergic immunopositive axons and varicosities were seen in close apposition to the proximal and distal dendrites, but not the cell body (Fig. 7B). These orexinergic axons originate mainly in the perifornical hypothalamic area.
Figure 7.
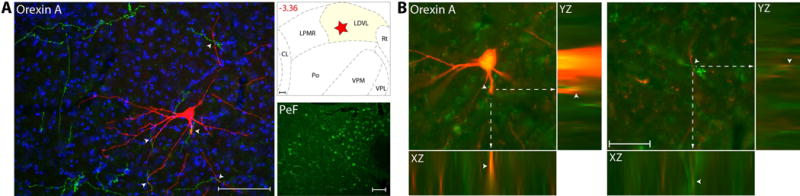
Orexinergic innervation of thalamic trigeminovascular neurons. (A) Left: Immunopositive Orexin A axons (green) surrounding a thalamic dura-sensitive neuron (red) labeled with TMR–dextran. Nuclear counterstaining was performed with DAPI (blue). Arrowheads indicate close apposition of OrA positive axons and the cell body and dendrites of the labeled neuron. Upper right: Location of the dura-sensitive neuron (red star) shown at left. Number in red indicates distance from bregma (mm). Lower right: Fluorescent image showing OrA labeling of cell bodies in the hypothalamic perifornical area (PeF). Scale bars = 100 mm. (B) Close apposition between Orexin A immunopositive axons and thalamic trigeminovascular neurons. The three views in the x-y, y-z and x-z planes provide evidence that Orexin A immunopositive fibers (green) may contact proximal and distal dendrites of trigeminovascular neurons in LD (red). Arrowheads indicate probable contact point on each view. Note that some green-labeled axons and red-labeled dendrites are in the same focal plane (yellow). Scale bar = 50 μm. Adapted from Noseda et al., 201427
The orexin system originates in the LH and projects to the cortex, thalamus, brainstem, spinal cord and other hypothalamic nuclei19, 93–96. It consists of 2 neuropeptides (orexin A, orexin B) that are synthesized by the same gene97 and act on 2 classes of receptors, the selective orexin receptor 1 (orexin A) and the non-selective orexin receptor 2 (orexin A and B). The wide distribution of orexin fibers in the brain support a role in regulating food intake, arousal, wakefulness and sympathetically-mediated increase in body temperature, heart rate and blood pressure98. Opposite to the function of the MCH system, orexin neurons are excited by falling glucose levels, and their activation promotes food intake and wakefulness99–101. Of potential relevance to the pathophysiology of migraine are orexinergic axons in nociceptive laminae of the medullary dorsal horn and in close apposition to thalamic trigeminovascular neurons. Although no information is available regarding the direction in which orexin modulates thalamic trigeminovascular neurons, in vitro slice recording of thalamic neurons suggests that both orexin B and, for a lesser extent, orexin A are capable of depolarizing these neurons sufficently to switch their firing from the sleep-associated burst mode to the wakefulness-associated tonic mode102. In the context of migraine, it is thus reasonable to hypothesize that the mechanism by which eating may reduce headache intensity involves not only local release of GABA from activated MCH neurons but also inhibition of facilitatory orexin input to thalamic trigeminovascular neurons induced by increased glucose level (orexin neurons are inhibited by glucose). And conversely, fasting-induced fall in glucose activates the orexinergic neurons which in turn facilitate excitability through local release of orexin B and orexin A.
The relative innervation density of each of the neuropeptides and neurotransmitters described above is shown in Figure 8. As a rule, when the density is higher, more opportunities are created for a chemical pathway to modulate the activity of the targeted neuron. Accordingly, it is concluded that the activity of thalamic trigeminovascular neurons is modulated mainly by glutamate, GABA, dopamine and serotonin, to a lesser extent by noradrenaline and histamine, and least by MCH and orexin.
Figure 8.
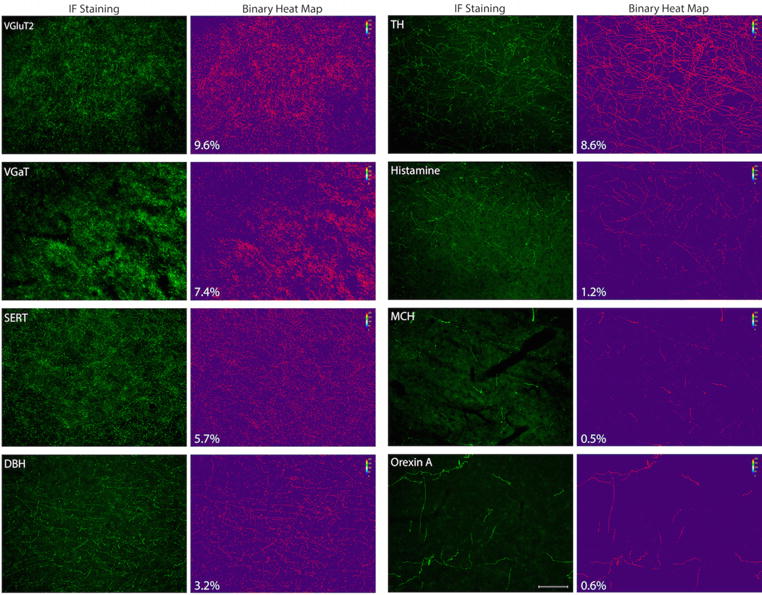
Density maps of thalamic innervation by neurotransmitters and neuropeptides. Photomicrographs showing immunofluorescence staining of each biomarker in thalamic areas where juxtacellularly labeled trigeminovascular neurons were recorded (for anatomical reference, see figures 1–7). Adjacent to each fluorescent image, binary heat maps showing all pixels (in red) containing positive immunostaining are shown as a quantitative measurement of innervation density. Numbers in white reflect the percent of pixels with staining. Scale bar = 100 μm.
Summary
The thalamus is intricately connected with multiple cortical, subcortical and brainstem regions. It is viewed as an important subcortical hub with respect to functional brain networks103 involved in processes that are altered in certain disease states104, 105. In the migraine brain, changes in modulation of thalamic neurons by various inputs may have significant effects on thalamic functional connectivity during both the interictal and the ictal state. The diverse neurochemical pathways that converge on thalamic trigeminovascular neurons (Fig. 9A–B) and the probability that many of them modulate neuronal activity in the same direction under certain conditions (e.g., sleep deprivation) and in opposite directions under other conditions (e.g., when satiated or scared) define a sophisticated neuroanatomical network that may help us conceptualize how sensory, physiological, cognitive and affective conditions trigger, worsen or improve migraine headache.
Figure 9.
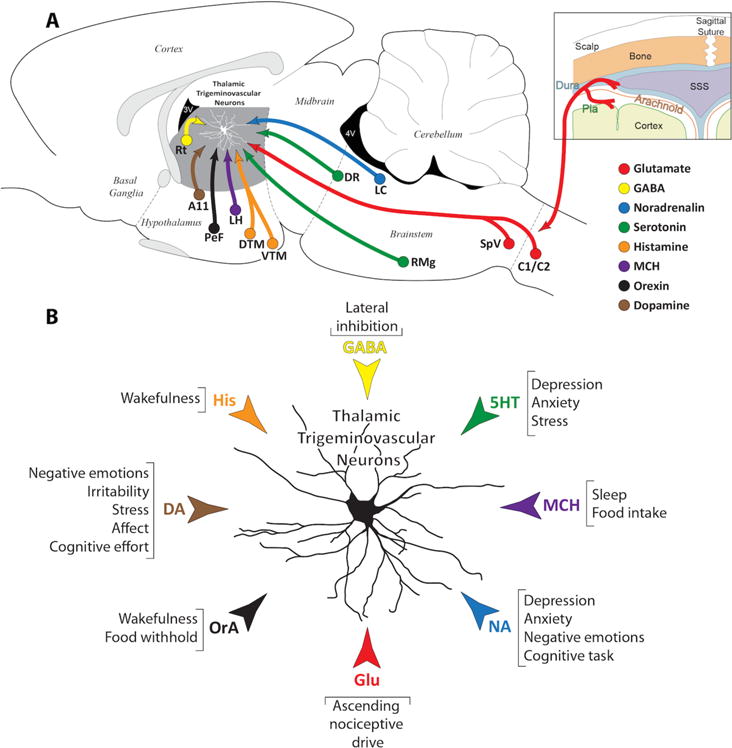
(A) Schematic illustration of the neurotransmitter and neuropeptidergic systems innervating thalamic trigeminovascular neurons in VPM, Po and LP. The peripheral (meningeal nociceptors) and central (trigemino-thalamic) components of the trigeminovascular pathway are shown in red. The neurotransmitter and neuropeptidergic systems are color coded as follow: (a) Glutamate from SpVC/C1-2 in red; (b) GABA from Rt in yellow; (c) Noradrenalin from LC in blue; (d) Serotonin from RMg and DR in green; (e) Histamine from DTM and VTM in orange; (f) Melanin Concentrating Hormone from LH in purple; (g) Orexin from PeF in black; (h) Dopamine from A11 in brown. (B) The diverse neurochemical pathways that converge on thalamic trigeminovascular neurons and the probability that many of them modulate neuronal activity in the same direction under certain conditions (e.g., sleep deprivation, wakefulness, food withhold, stress, anxiety) and in opposite directions under other conditions (e.g., food intake, sleep) define a sophisticated neuroanatomical network that may help us conceptualize how sensory, physiological, cognitive and affective conditions trigger, worsen or improve migraine headache. Adapted from Noseda et al., 201427
Acknowledgments
This research was supported by NIH Grants R01-NS069847, R37-NS079687 (R.B.) and R21-NS0902554 (R.N.).
References
- 1.Sherman SM. Thalamic relays and cortical functioning. Prog Brain Res. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- 2.Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: Distinguishing “drivers” from “modulators”. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- 4.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science New York, NY. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 5.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 6.Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- 7.McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci U S A. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westlund KN, Sorkin LS, Ferrington DG, Carlton SM, Willcockson HH, Willis WD. Serotoninergic and noradrenergic projections to the ventral posterolateral nucleus of the monkey thalamus. The Journal of comparative neurology. 1990;295:197–207. doi: 10.1002/cne.902950204. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos GC, Parnavelas JG. Distribution and synaptic organization of serotoninergic and noradrenergic axons in the lateral geniculate nucleus of the rat. The Journal of comparative neurology. 1990;294:345–355. doi: 10.1002/cne.902940304. [DOI] [PubMed] [Google Scholar]
- 10.Morrison JH, Foote SL. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. The Journal of comparative neurology. 1986;243:117–138. doi: 10.1002/cne.902430110. [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulos GC, Parnavelas JG. Distribution and synaptic organization of dopaminergic axons in the lateral geniculate nucleus of the rat. The Journal of comparative neurology. 1990;294:356–361. doi: 10.1002/cne.902940305. [DOI] [PubMed] [Google Scholar]
- 12.Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Cabezas MA, Rico B, Sanchez-Gonzalez MA, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. NeuroImage. 2007;34:965–984. doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Cabezas MA, Martinez-Sanchez P, Sanchez-Gonzalez MA, Garzon M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cereb Cortex. 2009;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M. Histaminergic transmission in the mammalian brain. Physiological reviews. 1991;71:1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- 18.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 19.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 20.Bittencourt JC, Frigo L, Rissman RA, Casatti CA, Nahon JL, Bauer JA. The distribution of melanin-concentrating hormone in the monkey brain (Cebus apella) Brain Res. 1998;804:140–143. doi: 10.1016/s0006-8993(98)00662-3. [DOI] [PubMed] [Google Scholar]
- 21.Adamantidis A, de Lecea L. A role for Melanin-Concentrating Hormone in learning and memory. Peptides. 2009;30:2066–2070. doi: 10.1016/j.peptides.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hervieu GJ, Cluderay JE, Harrison D, et al. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12:1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 23.Steriade M, Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984;320:1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- 24.McCarley RW, Benoit O, Barrionuevo G. Lateral geniculate nucleus unitary discharge in sleep and waking: state- and rate-specific aspects. J Neurophysiol. 1983;50:798–818. doi: 10.1152/jn.1983.50.4.798. [DOI] [PubMed] [Google Scholar]
- 25.Steriade M, Domich L, Oakson G. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci. 1986;6:68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 27.Noseda R, Kainz V, Borsook D, Burstein R. Neurochemical pathways that converge on thalamic trigeminovascular neurons: potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS One. 2014;9:e103929. doi: 10.1371/journal.pone.0103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salt TE, Eaton SA. Function of non-NMDA receptors and NMDA receptors in synaptic responses to natural somatosensory stimulation in the ventrobasal thalamus. Exp Brain Res. 1989;77:646–652. doi: 10.1007/BF00249618. [DOI] [PubMed] [Google Scholar]
- 29.Deschenes M, Hu B. Membrane resistance increase induced in thalamic neurons by stimulation of brainstem cholinergic afferents. Brain Res. 1990;513:339–342. doi: 10.1016/0006-8993(90)90478-t. [DOI] [PubMed] [Google Scholar]
- 30.Barroso-Chinea P, Castle M, Aymerich MS, Lanciego JL. Expression of vesicular glutamate transporters 1 and 2 in the cells of origin of the rat thalamostriatal pathway. Journal of chemical neuroanatomy. 2008;35:101–107. doi: 10.1016/j.jchemneu.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzog E, Bellenchi GC, Gras C, et al. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fremeau RT, Jr, Kam K, Qureshi T, et al. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science New York, NY. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- 34.Fremeau RT, Jr, Troyer MD, Pahner I, et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 35.Graziano A, Liu XB, Murray KD, Jones EG. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. The Journal of comparative neurology. 2008;507:1258–1276. doi: 10.1002/cne.21592. [DOI] [PubMed] [Google Scholar]
- 36.Ge SN, Ma YF, Hioki H, et al. Coexpression of VGLUT1 and VGLUT2 in trigeminothalamic projection neurons in the principal sensory trigeminal nucleus of the rat. The Journal of comparative neurology. 2010;518:3149–3168. doi: 10.1002/cne.22389. [DOI] [PubMed] [Google Scholar]
- 37.Kagan R, Kainz V, Burstein R, Noseda R. Hypothalamic and basal ganglia projections to the posterior thalamus: Possible role in modulation of migraine headache and photophobia. Neuroscience. 2013;248C:359–368. doi: 10.1016/j.neuroscience.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sicuteri F. Dopamine, the second putative protagonist in headache. Headache. 1977;17:129–131. doi: 10.1111/j.1526-4610.1977.hed1703129.x. [DOI] [PubMed] [Google Scholar]
- 39.Bergerot A, Storer RJ, Goadsby PJ. Dopamine inhibits trigeminovascular transmission in the rat. Ann Neurol. 2007;61:251–262. doi: 10.1002/ana.21077. [DOI] [PubMed] [Google Scholar]
- 40.Takada M, Li ZK, Hattori T. Single thalamic dopaminergic neurons project to both the neocortex and spinal cord. Brain Res. 1988;455:346–352. doi: 10.1016/0006-8993(88)90093-5. [DOI] [PubMed] [Google Scholar]
- 41.Skagerberg G, Lindvall O. Organization of diencephalic dopamine neurones projecting to the spinal cord in the rat. Brain Res. 1985;342:340–351. doi: 10.1016/0006-8993(85)91134-5. [DOI] [PubMed] [Google Scholar]
- 42.Govindaiah G, Wang Y, Cox CL. Dopamine enhances the excitability of somatosensory thalamocortical neurons. Neuroscience. 2010;170:981–991. doi: 10.1016/j.neuroscience.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 43.Rondou P, Haegeman G, Van Craenenbroeck K. The dopamine D4 receptor: biochemical and signalling properties. Cellular and molecular life sciences : CMLS. 2010;67:1971–1986. doi: 10.1007/s00018-010-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi H, Higuchi M, Suhara T. The role of extrastriatal dopamine D2 receptors in schizophrenia. Biol Psychiatry. 2006;59:919–928. doi: 10.1016/j.biopsych.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural brain research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 48.Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Current opinion in pharmacology. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological reviews. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen K, Brask D, Knudsen GM, Aznar S. Immunodetection of the serotonin transporter protein is a more valid marker for serotonergic fibers than serotonin. Synapse. 2006;59:270–276. doi: 10.1002/syn.20240. [DOI] [PubMed] [Google Scholar]
- 51.Hegerl U, Juckel G. Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry. 1993;33:173–187. doi: 10.1016/0006-3223(93)90137-3. [DOI] [PubMed] [Google Scholar]
- 52.Sicuteri F. Headache as possible expression of deficiency of brain 5-hydroxytryptamine (central denervation supersensitivity) Headache. 1972;12:69–72. doi: 10.1111/j.1526-4610.1972.hed1202069.x. [DOI] [PubMed] [Google Scholar]
- 53.Westlund KN, Sorkin LS, Ferrington DG, Carlton SM, Willcockson HH, Willis WD. Serotoninergic and noradrenergic projections to the ventral posterolateral nucleus of the monkey thalamus. Journal of Comparative Neurology. 1990;295:197–207. doi: 10.1002/cne.902950204. [DOI] [PubMed] [Google Scholar]
- 54.Nothias F, Onteniente B, Roudier F, Peschanksi M. Immunocytochemical study of serotoninergic and noradrenergic innervation of the ventrobasal complex of the rat thalamus. Neurosci Lett. 1988;95:59–63. doi: 10.1016/0304-3940(88)90632-5. [DOI] [PubMed] [Google Scholar]
- 55.Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: ascending projections. The Journal of comparative neurology. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- 56.Consolazione A, Priestley JV, Cuello AC. Serotonin-containing projections to the thalamus in the rat revealed by a horseradish peroxidase and peroxidase antiperoxidase double-staining technique. Brain Res. 1984;322:233–243. doi: 10.1016/0006-8993(84)90113-6. [DOI] [PubMed] [Google Scholar]
- 57.Eaton SA, Salt TE. Modulatory effects of serotonin on excitatory amino acid responses and sensory synaptic transmission in the ventrobasal thalamus. Neuroscience. 1989;33:285–292. doi: 10.1016/0306-4522(89)90208-x. [DOI] [PubMed] [Google Scholar]
- 58.Shields KG, Goadsby PJ. Serotonin receptors modulate trigeminovascular responses in ventroposteromedial nucleus of thalamus: a migraine target? Neurobiology of disease. 2006;23:491–501. doi: 10.1016/j.nbd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004;101:4274–4279. doi: 10.1073/pnas.0306147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez JF, Akil H, Watson SJ. Neural circuits mediating stress. Biol Psychiatry. 1999;46:1461–1471. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- 61.Homberg JR. Serotonin and decision making processes. Neuroscience and biobehavioral reviews. 2012;36:218–236. doi: 10.1016/j.neubiorev.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Meltzer H. Serotonergic dysfunction in depression. The British journal of psychiatry Supplement. 1989:25–31. [PubMed] [Google Scholar]
- 63.Green AR. Neuropharmacology of 5-hydroxytryptamine. Br J Pharmacol. 2006;147(Suppl 1):S145–152. doi: 10.1038/sj.bjp.0706427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeo GS, Heisler LK. Unraveling the brain regulation of appetite: lessons from genetics. Nat Neurosci. 2012;15:1343–1349. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- 65.Ego-Stengel V, Bringuier V, Shulz DE. Noradrenergic modulation of functional selectivity in the cat visual cortex: an in vivo extracellular and intracellular study. Neuroscience. 2002;111:275–289. doi: 10.1016/s0306-4522(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 66.Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci. 2004;24:10773–10785. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep medicine reviews. 2012;16:187–197. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain research. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 69.Lindvall O, Bjorklund A, Nobin A, Stenevi U. The adrenergic innervation of the rat thalamus as revealed by the glyoxylic acid fluorescence method. The Journal of comparative neurology. 1974;154:317–347. doi: 10.1002/cne.901540307. [DOI] [PubMed] [Google Scholar]
- 70.De Lima AD, Singer W. The brainstem projection to the lateral geniculate nucleus in the cat: identification of cholinergic and monoaminergic elements. The Journal of comparative neurology. 1987;259:92–121. doi: 10.1002/cne.902590107. [DOI] [PubMed] [Google Scholar]
- 71.Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- 72.Luthi A, McCormick DA. Periodicity of thalamic synchronized oscillations: the role of Ca2+-mediated upregulation of Ih. Neuron. 1998;20:553–563. doi: 10.1016/s0896-6273(00)80994-0. [DOI] [PubMed] [Google Scholar]
- 73.Luthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron. 1998;21:9–12. doi: 10.1016/s0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- 74.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annual review of physiology. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 75.Rogawski MA, Aghajanian GK. Norepinephrine and serotonin: opposite effects on the activity of lateral geniculate neurons evoked by optic pathway stimulation. Experimental neurology. 1980;69:678–694. doi: 10.1016/0014-4886(80)90060-6. [DOI] [PubMed] [Google Scholar]
- 76.Rogawski MA, Aghajanian GK. Modulation of lateral geniculate neurone excitability by noradrenaline microiontophoresis or locus coeruleus stimulation. Nature. 1980;287:731–734. doi: 10.1038/287731a0. [DOI] [PubMed] [Google Scholar]
- 77.Kayama Y, Negi T, Sugitani M, Iwama K. Effects of locus coeruleus stimulation on neuronal activities of dorsal lateral geniculate nucleus and perigeniculate reticular nucleus of the rat. Neuroscience. 1982;7:655–666. doi: 10.1016/0306-4522(82)90071-9. [DOI] [PubMed] [Google Scholar]
- 78.Kayama Y. Ascending, descending and local control of neuronal activity in the rat lateral geniculate nucleus. Vision Res. 1985;25:339–347. doi: 10.1016/0042-6989(85)90058-6. [DOI] [PubMed] [Google Scholar]
- 79.Welch KM. Drug therapy of migraine. N Engl J Med. 1993;329:1476–1483. doi: 10.1056/NEJM199311113292008. [DOI] [PubMed] [Google Scholar]
- 80.Shields KG, Goadsby PJ. Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: a role in migraine? Brain. 2005;128:86–97. doi: 10.1093/brain/awh298. [DOI] [PubMed] [Google Scholar]
- 81.Lassen LH, Christiansen I, Iversen HK, Jansen-Olesen I, Olesen J. The effect of nitric oxide synthase inhibition on histamine induced headache and arterial dilatation in migraineurs. Cephalalgia. 2003;23:877–886. doi: 10.1046/j.1468-2982.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 82.McCormick DA, Williamson A. Modulation of neuronal firing mode in cat and guinea pig LGNd by histamine: possible cellular mechanisms of histaminergic control of arousal. J Neurosci. 1991;11:3188–3199. doi: 10.1523/JNEUROSCI.11-10-03188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 84.Monti JM. Involvement of histamine in the control of the waking state. Life sciences. 1993;53:1331–1338. doi: 10.1016/0024-3205(93)90592-q. [DOI] [PubMed] [Google Scholar]
- 85.Lin JS, Sakai K, Jouvet M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology. 1988;27:111–122. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- 86.Elias CF, Lee CE, Kelly JF, et al. Characterization of CART neurons in the rat and human hypothalamus. The Journal of comparative neurology. 2001;432:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- 87.Marsh DJ, Weingarth DT, Novi DE, et al. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci U S A. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Astrand A, Bohlooly YM, Larsdotter S, et al. Mice lacking melanin-concentrating hormone receptor 1 demonstrate increased heart rate associated with altered autonomic activity. American journal of physiology Regulatory, integrative and comparative physiology. 2004;287:R749–758. doi: 10.1152/ajpregu.00134.2004. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez MI, Vaziri S, Wilson CA. Behavioral effects of alpha-MSH and MCH after central administration in the female rat. Peptides. 1996;17:171–177. doi: 10.1016/0196-9781(95)02092-6. [DOI] [PubMed] [Google Scholar]
- 91.Segal-Lieberman G, Bradley RL, Kokkotou E, et al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci U S A. 2003;100:10085–10090. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elias CF, Saper CB, Maratos-Flier E, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. The Journal of comparative neurology. 1998;402:442–459. [PubMed] [Google Scholar]
- 95.Date Y, Ueta Y, Yamashita H, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. The Journal of comparative neurology. 1998;402:460–474. [PubMed] [Google Scholar]
- 97.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 98.Girault EM, Yi CX, Fliers E, Kalsbeek A. Orexins, feeding, and energy balance. Prog Brain Res. 2012;198:47–64. doi: 10.1016/B978-0-444-59489-1.00005-7. [DOI] [PubMed] [Google Scholar]
- 99.Leinninger GM. Lateral thinking about leptin: a review of leptin action via the lateral hypothalamus. Physiol Behav. 2011;104:572–581. doi: 10.1016/j.physbeh.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 101.Kelz MB, Sun Y, Chen J, et al. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Govindaiah G, Cox CL. Modulation of thalamic neuron excitability by orexins. Neuropharmacology. 2006;51:414–425. doi: 10.1016/j.neuropharm.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 103.Tomasi D, Volkow ND. Association between functional connectivity hubs and brain networks. Cereb Cortex. 2011;21:2003–2013. doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Z, Jia X, Liang P, et al. Changes in thalamus connectivity in mild cognitive impairment: evidence from resting state fMRI. European journal of radiology. 2012;81:277–285. doi: 10.1016/j.ejrad.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 105.Nathan DE, Wang BQ, Wolfowitz RD, et al. Examining intrinsic thalamic resting state networks using graph theory analysis: implications for mTBI detection. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2012;2012:5445–5448. doi: 10.1109/EMBC.2012.6347226. [DOI] [PubMed] [Google Scholar]


