Abstract
Humans have a diverse collection of neuropeptides that can influence a multitude of activities. There are now over 100 known neuropeptides and probably many more yet to be identified from the over 1000 predicted peptides encoded by the genome. While diverse, peptides generally share three common characteristics: (1) post-translational processing and release from vesicles, (2) activation of cell-surface receptors over a relatively large distance, and (3) modulation of target cells that are often in the brain and periphery. Within the brain, neuropeptides can modulate the activity of co-released neurotransmitters to either increase or decrease the strength of synaptic signaling. Within the periphery, neuropeptides can function similar to peptide hormones and modulate nearly all bodily functions. Given the clear involvement of the neuropeptide CGRP in migraine and the emerging evidence for other peptides, it seems likely that neuropeptides may help “awaken” the senses and contribute to the heightened sensory state of migraine.
Keywords: neuropeptide, CGRP, neuromodulation
What is a neuropeptide?
The objective of this overview is to provide an appreciation of how the diversity of neuropeptides in the body may contribute to the pathophysiology of migraine by modulating synaptic transmission in the CNS and acting in peripheral tissues to alter cellular activities.
Towards this objective, the first question is “just what are neuropeptides?” One definition is: “Neuropeptides are small proteinaceous substances produced and released by neurons through the regulated secretory route and acting on neural substrates”1. The key word in this definition is “neurons” because the only distinction between neuropeptides and other peptides, such as peptide hormones, is that a neuropeptide is synthesized and used by a neuron. Both neuropeptide and peptide hormones are synthesized, modified, and degraded by the same sets of enzymes. Furthermore, both can act nearby as autocrine and paracrine agents and at a distance as endocrine agents. Indeed, nearly all neuropeptides are also found as peptide hormones and vice/versa. So, while we use the term neuropeptide, it is important to keep in mind that neuropeptides are not just in the nervous system – they act both in and out of the CNS1.
The pleiotropic potential of neuropeptides was best stated by an early pioneer in the field, Candace Pert, who eloquently stated: “As our feelings change, this mixture of peptides travels throughout your body and your brain. And they’re literally changing the chemistry of every cell in your body”2. With this perspective, it is not surprising that neuropeptides are emerging as key players in migraine.
Diversity of neuropeptides
A little appreciated fact is that over the past several decades, the number of known neuropeptides in the human brain has grown to over 100 distinct molecules. An internet resource of data on all known neuropeptides expressed in the human brain has been compiled by the Human Genome Organization Gene Nomenclature Committee (http://www.neuropeptides.nl/tabel%20neuropeptides%20linked.htm)3, which has been further annotated in another database (http://isyslab.info/NeuroPep)4. Based on structural homologies, many of these peptides can be grouped into families (Table 1). This table of neuropeptide gene families includes only a partial, representative list of biologically active products within each family. For a complete listing of all the known neuropeptide genes and mature peptides, the reader is referred to the neuropeptides website listed above.
Table 1.
Neuropeptide families* and migraine.
| Families possibly involved in migraine+ | Other families |
|---|---|
| CGRP: CGRP (α, β), calcitonin, amylin, adrenomedullin (AM1,2) | Opioids: enkephalins, dynorphin, endorphins, nociceptin |
| Somatostatin/cortistatin | |
| Glucagon/secretin: PACAP, VIP, glucagon, secretin, GHRH, GIP | Natriuretic factors: ANF, BNF, CNP |
| GRP, neuromedins | |
| Vasopressin/oxytocin | Endothelins |
| CCK/gastrin | |
| F- and Y-amides: NPY, PPY, NPFF | Insulins: insulin, IGFs, relaxins |
| Motilin/ghrelin | |
| Tachykinins: Sub P, neurokinin A, neuropeptide K, neuropeptide gamma | Galanins |
| Gonadotropin releasing hormones | |
| Tensins: angiotensin, neurotensin, bradykinin | Neuropeptide B/W/S |
| Neurexophilins | |
| CRH-related: CRH, urocortins, urotensins | Cerebellins |
| Granins: chromogranins, secretogranins | |
| Adipose neuropeptides: leptin, adiponectin, resistins | |
| Family-less: orexins, MCH, TRH, PTHrP, CART, AGRP, prolactin, diazepam-binding inhibitor peptide, kisspeptins, etc | |
For full list of family members, see: http://www.neuropeptides.nl/tabel%20neuropeptides%20linked.htm
neuropeptides that may potentially be involved in migraine pathogenesis are indicated in bold.
A number of neuropeptides could potentially play roles in migraine. While in most cases, a role in migraine is very speculative, about a dozen candidates are highlighted in Table 1. Discussion of the speculation behind these candidates is beyond the scope of this introductory article and are indicated mainly to emphasize that there is a fairly wide frontier remaining to be explored. The two top peptides, CGRP and PACAP, and several candidates, oxytocin, orexin, and amylin, are discussed in the accompanying articles of this issue. It is intriguing that CGRP and PACAP share many features, which may underlie their roles in migraine5. In addition, special attention should be paid to NPY based on its autonomic and anti-nociceptive actions6, including recently described actions in the trigeminal nucleus7. Likewise, hypothalamic CRH is an interesting candidate based on its sexually dimorphic expression and activity (levels are higher and activation of stress/anxiety pathways is not inhibited by oxytocin in females)8, as well as its release from the paraventricular nucleus being controlled by CGRP9. The expression of many of these neuropeptides in the hypothalamus raises the possibility that they may contribute to descending pain modulation and autonomic and premonitory symptoms of migraine10, 11.
While the number of known neuropeptides is somewhat daunting, even more impressive is when all peptides, not just neuropeptides, are considered. There are > 1000 total peptides in Homo sapiens based on genome homologies of neuropeptides, peptide hormones, cytokines, growth factors, antimicrobial peptides, toxins & venom peptides, and antifreeze proteins (www.peptides.be/?p=home)12. While the functions of many of these peptides in human health and disease remains to be uncovered, the strong evolutionary conservation of peptides and their receptors13, argues that this cast of a thousand may play important undiscovered roles, including possibly in migraine.
Common features of neuropeptides
The common features of neuropeptides can be grouped into three categories: (1) post-translational processing from precursor proteins and release from dense core vesicles, (2) activation of cell-surface receptors over a relatively large distance, and (3) modulation of target cells in the periphery and the brain, which we suggest could alter sensory perception. These features are described below.
Neuropeptide processing and release
All neuropeptides are processed from precursor proteins and released from vesicles (Figure 1). The precursor proteins (called pro-peptides) are proteolytically cleaved and many, but not all, are also modified by C-terminal amidation, which is required for biological activity14. The biochemistry of peptide proteolysis and amidation was largely worked out in the labs of Eipper and Mains15 and has been recently reviewed16.
Figure 1. Neuropeptide activation requires multiple processing steps in the cell.
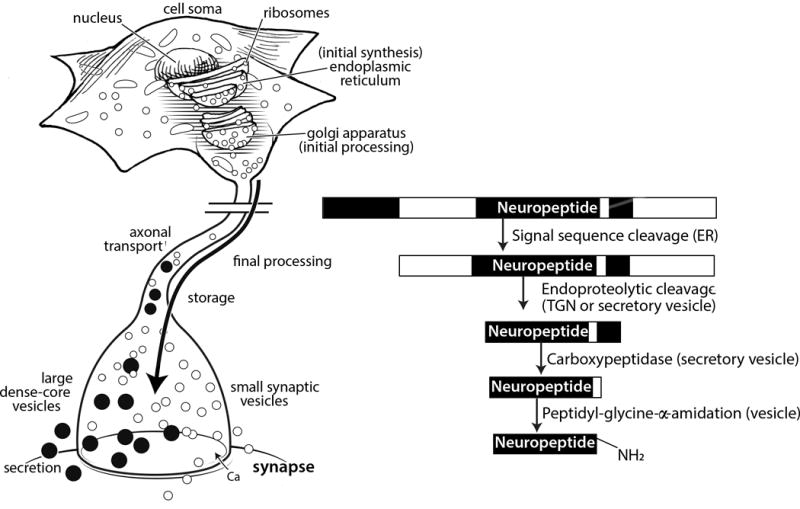
A representative neuropeptide is shown with sequential processing steps within the neuron beginning in the endoplasmic reticulum, followed by endoproteolytic cleavages in the trans-Golgi network (TGN) or secretory vesicles. Further processing in the vesicles at the C-terminus removes amino acids and adds a C-terminal amide group. The vesicles are transported down the axon and are stored at varicosities (not shown) and near the synapse. Neuropeptides are stored in dense core vesicles, which are larger and functionally distinct from the small, clear synaptic vesicles.
An important feature of the processing pathway is that it is a mechanism that can generate a diverse portfolio of peptides from a single gene. In this way, a single precursor protein can encode multiple neuropeptides based on processing, which can dictate generation of the final mature neuropeptides. For example, cell-specific cleavage of the proopiomelanocortin precursor protein can generate either adrenocorticotropic hormone or β-endorphin, which have very different biological activities15.
Peptide processing occurs as a progressive mechanism from the endoplasmic reticulum and Golgi apparatus into a subset of secretory vesicles called dense core vesicles (Figure 1). Synthesis and removal of the signal peptide sequence from the pre-propeptide occurs in the endoplasmic reticulum to generate the propeptide. As the peptides traverse the secretory route through the Golgi apparatus, proteolytic cleavage and other modifications occur, such as glycosylation on some peptides. Proteolytic cleavage by endopeptidases, often next to basic residues (lysine or arginine) continues in the secretory vesicles. In addition, cleavage of C-terminal residues and C-terminal amidation also occurs for some peptides in the vesicles. The secretory vesicles, which are called dense core vesicles because of accumulated peptides causing dense staining in electron micrographs, are transported from the cell body down the axon. Depending on the length of the axon, transport can take hours or even a day. The dense core vesicles are present throughout the neuron, but are especially abundant in dendrites, the cell body, and varicosities in the axon. At synapses, they are co-localized with clear synaptic vesicles that contain classical neurotransmitters, such as glutamate. Dense core and clear synaptic vesicles are often co-released, although by somewhat different machineries16–18. Like neurotransmitters, neuropeptides are released by calcium-dependent exocytosis in response to depolarization or other signals. However, in contrast to clear synaptic vesicles, dense core vesicles do not require specialized presynaptic machinery for release. Vesicular release of neuropeptides from dendrites and cell bodies has been observed in a variety of neurons19–23, including neuronal cell bodies in dorsal root ganglia24. Combined with features discussed below, these properties are consistent with the view that neuropeptides yield relatively slow and prolonged actions over a larger area than classical neurotransmitters.
Because of the vesicular storage of neuropeptides, when a neuron is activated it rapidly releases them in a relatively large bolus. This bulk release mechanism may be important in the context of CGRP-blocking monoclonal antibodies that are now in clinical trials for migraine. Specifically, would there be enough antibodies to immediately sequester the neuropeptide before it bound its receptor? While this is difficult to answer, a starting point is to ask is how many neuropeptides are in a secretory vesicle? Somewhat surprisingly, there have been very few measurements of peptide content in vesicles. Mains and Eipper provided a general estimate of 3–10 mM15, which is in rough agreement with the estimated concentration of 60 mM oxytocin and vasopressin in the posterior pituitary25. Assuming average dense core vesicle diameters of 100–200 nm, then each vesicle volume should be about 2 × 106 cubic nm, or 2 × 10−18 liter/vesicle. With these values, 10 mmol/l × 2 × 10−18 l = 2 × 10−20 mol/vesicle. Using Avogadro’s number (6 ×1023 molecules/mol), this yields 1.2 × 104 or ~10,000 peptides per dense core vesicle. Ludwig and Leng also calculated ~104 vesicle in hypothalamic neurons21. The number of vesicles released per cell and per stimulus will of course vary, but a rate of ~103 vesicles per sec was estimated from capacitance measurements of isolated somata of magnocellular hypothalamic neurons26. Each of these neurons has an estimated 106 dense core vesicles and releases ~107 molecules per sec21. Admittedly, these numbers may be on the higher end due to the abundance of peptides in hypothalamic neurons (e.g. chromaffin cells have ~20,000 dense core vesicles27). Thus, a conservative estimate is that at least hundreds of vesicles will be released from a neuron over a time scale of seconds. So, millions of neuropeptides are likely released in a short burst from a just a single cell. Based on this rough calculation, it seems likely that the blocking antibodies will be able to blunt, but are unlikely to completely block, neuropeptide signaling. In this regard, studies with knockout mice may not accurately reflect the physiology of mice treated with blocking antibodies. Furthermore, while speculative, perhaps the inability to completely block all CGRP in the body may contribute to the excellent safety profile that has been reported to date for the CGRP-blocking antibodies in clinical trials.
Activation of receptors at a distance
A perhaps under-appreciated feature of neuropeptides is that, in contrast to classical neurotransmitters, neuropeptides diffuse from their point of release and hence can act a relatively large distance28. This diffusion-driven distribution is referred to as volume transmission or dispersion (Figure 2). Volume transmission is a nonsynaptic dispersion in the extracellular fluid and cerebrospinal fluid. It should be noted that this mechanism can also apply to neurotransmitters in certain situations, for example dopamine and norepinephrine, which can be released from axonal varicosities.
Figure 2. Neuropeptides broadly diffuse and act beyond the synapse.
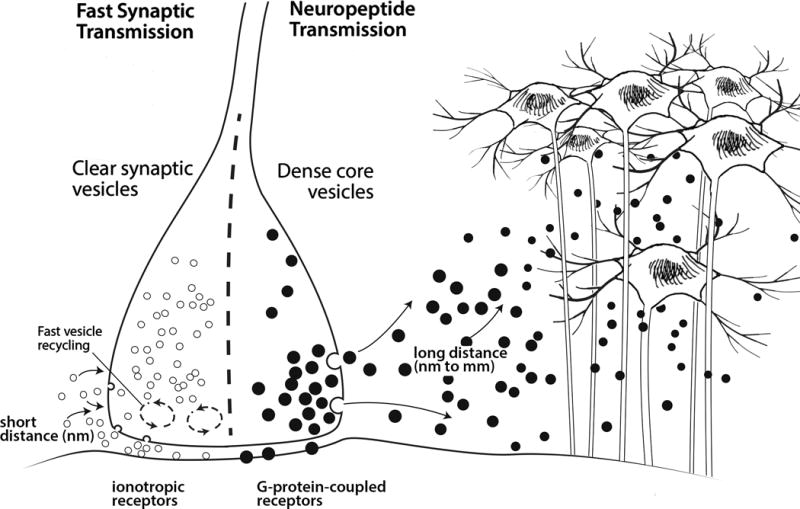
Distinguishing features of fast synaptic transmission versus neuropeptide transmission are shown. Classical small molecule transmitters, such as glutamate, are stored in clear synaptic vesicles, while peptides are stored in dense core vesicles. The synaptic vesicles are rapidly recycled and refilled with neurotransmitter close to the synaptic cleft. Once released, neuropeptides are not taken back up into the neuron, so dense core vesicles are not regenerated at the synapse. Instead, dense core vesicles are replenished by axonal transport of new vesicles from the cell body. In addition, dense core vesicles are released from non-synaptic sites as indicated. Once released, classical neurotransmitters bind to ion channel receptors (ionotropic receptors), while nearly all neuropeptides bind to G-protein coupled receptors. A major feature of neuropeptides is their ability to act by volume transmission due to diffusion over a relatively large distance from the point of release to act on targets far from the synapse.
Once released, the peptides are only slowly removed from the extracellular space. This slow removal is due to the lack of reuptake machinery for peptides. In contrast, classical neurotransmitters are rapidly removed from the synaptic cleft by reuptake pumps. The combination of volume transmission and lack of reuptake contributes to the relatively long lasting effects of neuropeptides.
However, while long-lived compared to neurotransmitters, neuropeptide actions are terminated. Inactivation occurs by extracellular proteases, which in some cases can actually create different bioactive peptides. The time scale of this inactivation for most peptides remains speculative28. Half-lives in the blood tend to be in the range of minutes, but this may not be the relevant measure. For example, plasma CGRP half-life is 7 min29, although it has much longer lasting actions in the skin on the order of hours30, and the half-life in extracellular fluid or cerebrospinal fluid is not known. Similarly, oxytocin and vasopressin have different half-lives of ~2 min in plasma versus ~20 min in cerebrospinal fluid21, 31. A challenge remains to understand how long peptides remain biologically active in different regions of the body.
All neuropeptides act as signal transducers via cell-surface receptors. Nearly all neuropeptides act at G-protein coupled receptors (Figure 2). This is an important distinction from ion channel-coupled receptors, since G-protein coupled signaling is consistent with neuropeptides inducing a slower and modulatory response compared to neurotransmitters. In addition, neuropeptide receptors have relatively high ligand affinities (nanomolar Kds), compared to neurotransmitter receptors. This allows a small amount of diffused peptide to still activate receptors. In summary, the combination of these features allows neuropeptides to be active at relatively large distances at relatively low concentrations.
Modulation in the brain and periphery
Neuropeptide modulation of target cells can occur by two distinct, but overlapping, mechanisms. They can act within the brain as neuromodulators and within the periphery as signaling molecules. Given these features, neuropeptides are well poised to alter sensory perception in migraine. An example is CGRP actions in the periphery and the central nervous system (Figure 3). CGRP can alter the microenvironment of the trigeminovasculature to potentially sensitize trigeminal nociceptors, as well as act in the brain to enhance glutamatergic signaling of CNS neurons32. The two mechanisms are not necessarily independent since altered peripheral signals could lead to altered neuromodulatory activity in the brain. For example, it has recently been reported that changes in vascular flow in parenchymal arterioles can alter pyramidal neuron activity in cortical slices33. And conversely, centrally released CGRP can also alter in vivo blood flow deep in the brain34. It is intriguing to speculate that these mechanisms might allow communication between peripheral and central CGRP actions in migraine.
Figure 3. Enhancement of neural activity by neuropeptides acting via peripheral and central mechanisms.
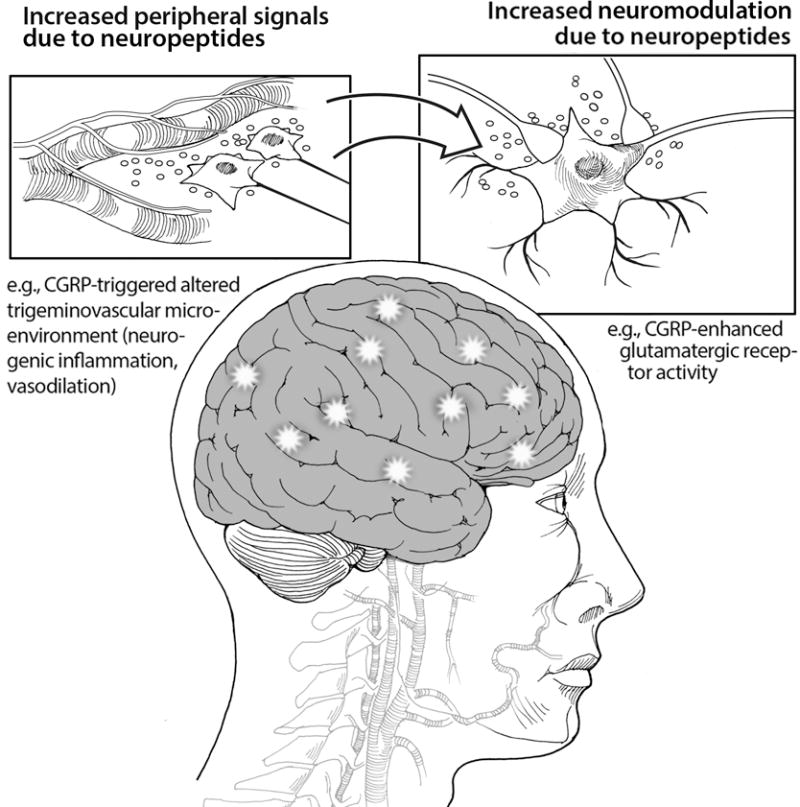
An example of peripheral actions of the neuropeptide CGRP to alter the microenvironment of the trigeminovasculature is shown. The release of inflammatory and pro-inflammatory molecules from mast cells, glia, and vascular cells can lead to sensitization of the trigeminal nerve. An example of central neuromodulation by CGRP to enhance glutamatergic signaling by cAMP-induced phosphorylation of glutamate receptors is shown. A conceptual link between peripheral and central CGRP actions is indicated by the arrow.
As neuromodulators in the CNS, neuropeptides can enhance or dampen synaptic activity. Furthermore, because of their ability to diffuse from the synaptic cleft and as a consequence of their release from nonsynaptic sites, such as cell bodies and axonal varicosities, neuropeptides can also act nonsynaptically by volume transmission discussed above. To illustrate how neuropeptides can modulate activity of co-released neurotransmitters to increase synaptic signaling, consider the actions of three simplified neurons on a post-synaptic neuron (Figure 4A). A neuron firing only classical small molecule neurotransmitters, such as glutamate, causes a rapid and brief excitatory postsynaptic potential (EPSP) (Figure 4B). In contrast, release of neuropeptides from neurons can either have no effect on their own or produce a slow onset and long-acting EPSP (Figure 4C). When combined together either as co-release from the same neuron or from different neurons, the neuropeptide can enhance the EPSP amplitude and duration, even if the neuropeptide on its own did not cause an EPSP (Figure 4D). Thus, a neuropeptide can modulate (increase or decrease) a postsynaptic response to a neurotransmitter. In this capacity, neuropeptides can influence many functions, including analgesia, reward, food intake, metabolism, reproduction, social behaviors, learning and memory.
Figure 4. Neuromodulation by neuropeptides in the central nervous system.
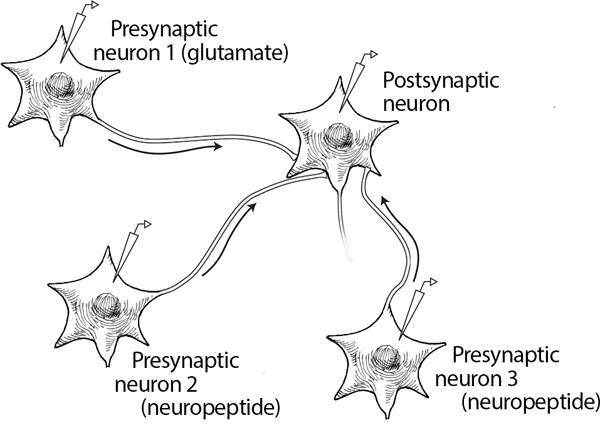
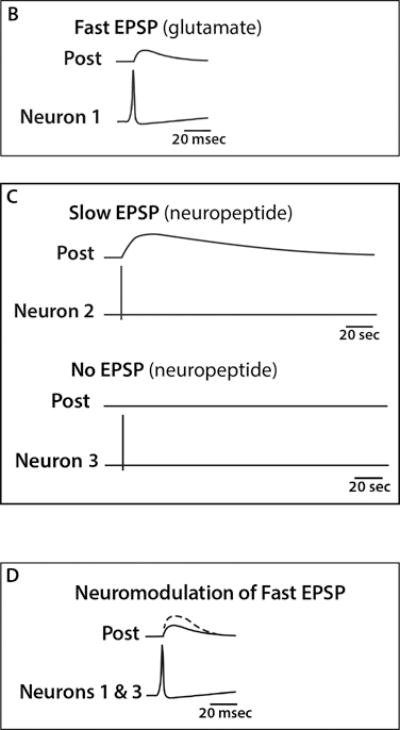
A) Schematic representation of three neurons (1–3) and a post-synaptic neuron with stimulating and recording electrodes. B) Representation of an action potential triggered in neuron 1, a glutamate releasing neuron, which produces a typical fast excitatory postsynaptic potential (EPSP). C) Representations of two consequences following an action potential triggered in neurons 2 and 3 that release neuropeptides, which produce either a slow onset and long-duration EPSP (top) or does not produce an EPSP (bottom). D) Neuromodulation by prior release or co-release of a neuropeptide and a neurotransmitter. In this scenario, the neuropeptide from neuron 3 has enhanced the EPSP caused by the neurotransmitter from neuron 1. The non-modulated EPSP from neuron 1 alone is shown as a solid line and the EPSP following stimulation of neurons 1 and 3 is shown as the dashed line. A similar enhancement of the EPSP might also be observed following stimulation of neurons 1 and 2 or if a neuropeptide was co-released from the same neuron with a transmitter (not shown).
Within the periphery, neuropeptides can function similar to peptide hormones and act on various target tissues, including peripheral nerves and ganglia, to modulate cellular signaling. These actions can occur locally or at a distance. The consequence is that nearly all bodily functions can be modulated by neuropeptides. An example of neuropeptide peripheral actions that is relevant to migraine might be at the cerebrovasculature. The vasculature is heavily innervated by sensory, parasympathetic, and sympathetic nerves35. These nerves release a panoply of neuropeptides, including CGRP, PACAP, NPY, along with neurotransmitters (e.g. acetylcholine, noradrenaline, nitric oxide) that can either increase or decrease vascular tone (Figure 5). Whether these actions contribute to migraine pathogenesis remains a hotly debated topic, although the more we learn, the more it seems that vascular targets are likely to be very important in migraine.
Figure 5. Perivascular convergence of neurotransmitters and neuropeptides in the periphery.
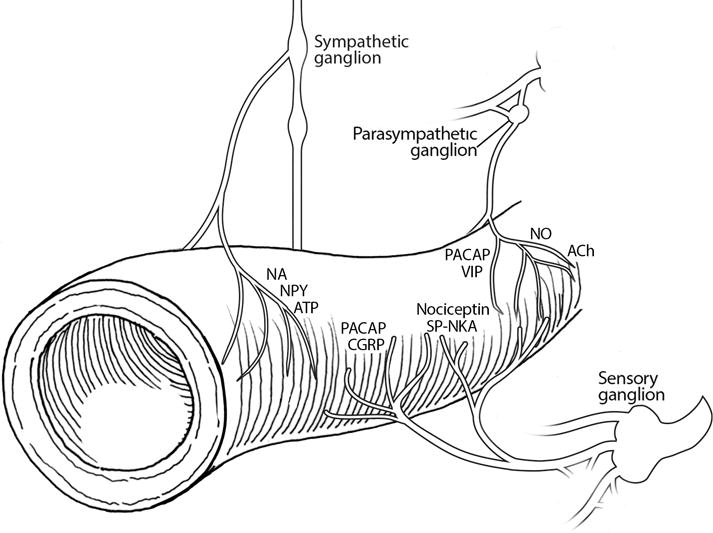
The vasculature is innervated by sensory, parasympathetic, and sympathetic autonomic nerves. Nerve fibers from sensory ganglia release CGRP, PACAP, nociceptin, and substance P-neurokinin A. Fibers from parasympathetic ganglia release PACAP, VIP, nitric oxide (NO) and the neurotransmitter acetylcholine (ACh). Fibers from sympathetic ganglia release neuropeptide Y (NPY), and the neurotransmitters noradrenaline (NA) and adenosine triphosphate (ATP).
Conclusion: do neuropeptides heighten sensory perception?
Members of the diverse families of neuropeptides share common features that could potentially contribute to migraine pathogenesis. Despite these shared properties, each neuropeptide has its own specific retinue of targets and activities that could add to the complexity of migraine. Clearly CGRP will not be the only neuropeptide involved in migraine and given the emerging evidence for other peptides, it seems likely that altered neuropeptide actions may be a general theme underlying the heightened sensory state of migraine. Such a mechanism could increase the perception of sensory input from the visual, auditory, gustatory, olfactory, and somatosensory systems (Figure 6). It is intriguing to speculate that if our senses are heightened by neuropeptides sensitizing the brain, then perhaps migraine may represent a state of too much of a good thing. It will be interesting to see how neuropeptides may help awaken our senses and why for some people that heightened sensitivity triggers the pain and discomfort associated with migraine.
Figure 6. Model of neuropeptide induction of a heightened sensory state in migraine.
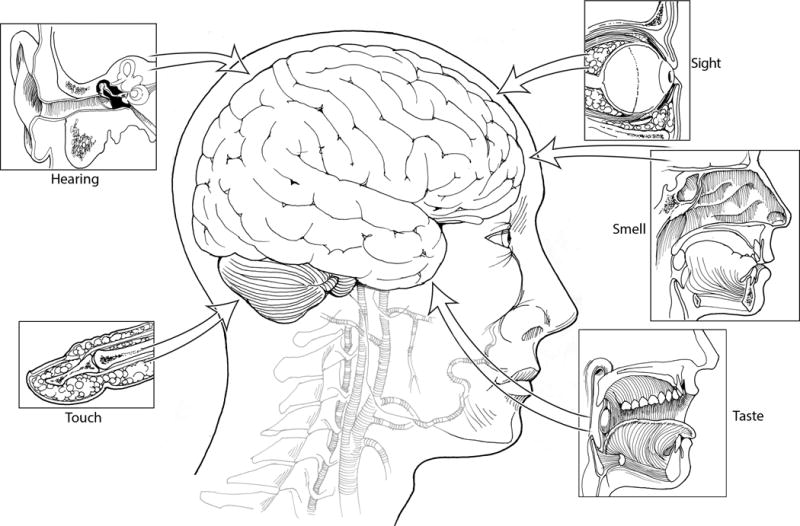
The senses of sight, smell, taste, hearing, and touch are indicated, with transmission of their sensory information to the CNS represented as arrows. Neuropeptides are predicted to enhance the perception of these sensory signals and in the case of migraine, the signals are suggested to reach a threshold that triggers a pain response.
Acknowledgments
Acknowledgments: The author acknowledges NIH (NS075599), Veterans Affairs Medical Center (1IO1RX002101), and Department of Defense USAMRAA (W81XWH-16-1-0071 and W81XWH-16-1-0211) for financial support and thanks members of the lab for interesting discussions.
Footnotes
Conflict of Interest: No conflict
References
- 1.Burbach JP. What are neuropeptides? Methods Mol Biol. 2011;789:1–36. doi: 10.1007/978-1-61779-310-3_1. [DOI] [PubMed] [Google Scholar]
- 2.Pert C. Molecules of emotion: The science behind mind-body medicine. New York: Scribner; 1997. [Google Scholar]
- 3.ScribnerHUGO gene nomenclature committee. Neuropeptide database. Human Genome Organization; 2017. [Google Scholar]
- 4.Wang Y, Wang M, Yin S, et al. NeuroPep: a comprehensive resource of neuropeptides. Database (Oxford) 2015;2015:bav038. doi: 10.1093/database/bav038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaiser EA, Russo AF. CGRP and migraine: could PACAP play a role too? Neuropeptides. 2013;47:451–461. doi: 10.1016/j.npep.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hokfelt T, Brumovsky P, Shi T, Pedrazzini T, Villar M. NPY and pain as seen from the histochemical side. Peptides. 2007;28:365–372. doi: 10.1016/j.peptides.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira MM, Akerman S, Tavares I, Goadsby PJ. Neuropeptide Y inhibits the trigeminovascular pathway through NPY Y1 receptor: implications for migraine. Pain. 2016;157:1666–1673. doi: 10.1097/j.pain.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li K, Nakajima M, Ibanez-Tallon I, Heintz N. A cortical circuit for sexually dimorphic oxytocin-dependent anxiety behaviors. Cell. 2016;167:60–72.e11. doi: 10.1016/j.cell.2016.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhillo WS, Small CJ, Jethwa PH, et al. Paraventricular nucleus administration of calcitonin gene-related peptide inhibits food intake and stimulates the hypothalamo-pituitary-adrenal axis. Endocrinology. 2003;144:1420–1425. doi: 10.1210/en.2002-220902. [DOI] [PubMed] [Google Scholar]
- 10.Robert C, Bourgeais L, Arreto CD, et al. Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci. 2013;33:8827–8840. doi: 10.1523/JNEUROSCI.0439-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154(Suppl 1):S44–53. doi: 10.1016/j.pain.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Data analysis and modeling group. Functional genomics and proteomics unit. Peptide DB: Bioactive peptide database. 2008 [Google Scholar]
- 13.Jekely G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc Natl Acad Sci U S A. 2013;110:8702–8707. doi: 10.1073/pnas.1221833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu Rev Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- 15.Mains RE, Eipper BA. Peptides. In: Siegel G, editor. Basic Neurochemistry, Molecular, Cellular and Medical Aspects. Philadelphia: Lippincott-Raven; 1999. [Google Scholar]
- 16.Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu Rev Pharmacol Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eiden LE. Signaling during exocytosis. In: Bradshaw RA, editor. Handbook of cell signaling. New York: Elsevier Science; 2003. pp. 375–392. [Google Scholar]
- 18.Bost A, Shaib AH, Schwarz Y, Niemeyer BA, Becherer U. Large dense-core vesicle exocytosis from mouse dorsal root ganglion neurons is regulated by neuropeptide Y. Neuroscience. 2017;346:1–13. doi: 10.1016/j.neuroscience.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Morris JF, Pow DV. Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytoses. Anat Rec. 1991;231:437–445. doi: 10.1002/ar.1092310406. [DOI] [PubMed] [Google Scholar]
- 20.Burke NV, Han W, Li D, Takimoto K, Watkins SC, Levitan ES. Neuronal peptide release is limited by secretory granule mobility. Neuron. 1997;19:1095–1102. doi: 10.1016/s0896-6273(00)80400-6. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 22.Trueta C, De-Miguel FF. Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front Physiol. 2012;3:319. doi: 10.3389/fphys.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De-Miguel FF, Nicholls JG. Release of chemical transmitters from cell bodies and dendrites of nerve cells. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang LY, Neher E. Ca(2+)-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17:135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- 25.Nordmann JJ, Morris JF. Method for quantitating the molecular content of a subcellular organelle: hormone and neurophysin content of newly formed and aged neurosecretory granules. Proc Natl Acad Sci U S A. 1984;81:180–184. doi: 10.1073/pnas.81.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soldo BL, Giovannucci DR, Stuenkel EL, Moises HC. Ca(2+) and frequency dependence of exocytosis in isolated somata of magnocellular supraoptic neurones of the rat hypothalamus. J Physiol. 2004;555:699–711. doi: 10.1113/jphysiol.2003.051136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plattner H, Artalejo AR, Neher E. Ultrastructural organization of bovine chromaffin cell cortex-analysis by cryofixation and morphometry of aspects pertinent to exocytosis. J Cell Biol. 1997;139:1709–1717. doi: 10.1083/jcb.139.7.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraenzlin ME, Ch’ng JL, Mulderry PK, Ghatei MA, Bloom SR. Infusion of a novel peptide, calcitonin gene-related peptide (CGRP) in man. Pharmacokinetics and effects on gastric acid secretion and on gastrointestinal hormones. Regul Pept. 1985;10:189–197. doi: 10.1016/0167-0115(85)90013-8. [DOI] [PubMed] [Google Scholar]
- 30.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 31.Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- 32.Russo AF. Calcitonin gene-related peptide (CGRP): A new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533–552. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim KJ, Ramiro Diaz J, Iddings JA, Filosa JA. Vasculo-neuronal coupling: Retrograde vascular communication to brain neurons. J Neurosci. 2016;36:12624–12639. doi: 10.1523/JNEUROSCI.1300-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai M, Slusarczyk AL, Chapin A, Barch M, Jasanoff A. Molecular imaging with engineered physiology. Nat Commun. 2016;7:13607. doi: 10.1038/ncomms13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edvinsson L, Uddman R. Neurobiology in primary headaches. Brain Res Brain Res Rev. 2005;48:438–456. doi: 10.1016/j.brainresrev.2004.09.007. [DOI] [PubMed] [Google Scholar]


