Abstract
Fucoidan has been reported to exhibit various beneficial activities ranging from to antivirus and anticancer properties. However, little information is available about the effects of fucoidan on cerebral ischemia-reperfusion injury (IRI). Our study aimed to explore the effects of fucoidan on cerebral IRI, as well as the underlying mechanisms. Sprague-Dawley (SD) rats were randomly subjected to four groups: Sham, IRI+saline (IRI+S), IRI+80 mg/kg fucoidan (IRI+F80), and IRI+160 mg/kg fucoidan (IRI+F160). Fucoidan (80 mg/kg or 160 mg/kg) was intraperitoneally injected from 7 days before the rats were induced to cerebral IRI model with middle cerebral artery occlusion (MCAO) method. At 24 h after reperfusion, neurological deficits and the total infarct volume were determined. The levels of inflammation-associated cytokines (interleukin (IL)-1β, IL-6, myeloperoxidase (MPO), and tumor necrosis factor (TNF)-α), oxidative stress-related proteins (malondialdehyde (MDA) and superoxide dismutase (SOD)) in the ischemic brain were measured by enzyme-linked immunosorbent assay (ELISA). Besides, the levels of apoptosis-related proteins (p-53, Bax, and B-cell lymphoma (Bcl)-2) and mitogen-activated protein kinase (MAPK) pathway (phosphorylation-extracellular signal-regulated kinase (p-ERK), p-c-Jun N-terminal kinase (JNK), and p-p38) were measured. Results showed that administration of fucoidan significantly reduced the neurological deficits and infarct volume compared to the IRI+S group in a dose-dependent manner. Also, fucoidan statistically decreased the levels of inflammation-associated cytokines, and oxidative stress-related proteins, inhibited apoptosis, and suppressed the MAPK pathway. So, Fucoidan plays a protective role in cerebral IRI might be by inhibition of MAPK pathway.
Keywords: Fucoidan, Cerebral ischemia-reperfusion injury, MAPK pathway
INTRODUCTION
Ischemic stroke, a devastating neurological disease, is the third leading cause of death and permanent disability in adults all over the world (Lakhan et al., 2009). Reperfusion to the ischemic brain is currently the best way of salvaging ischemic brain and limiting infarct development (Li et al., 2012). However, ischemia-reperfusion injury (IRI) is generally believed to be another significant clinical problem in treatment of cerebral damage. In addition, the exactly pathogenesis of cerebral IRI is not yet completely understood. Accumulating evidence has demonstrated that inflammation (Jean et al., 1998; Lakhan et al., 2009), reactive oxygen species (ROS) (Sugawara and Chan, 2003; Muralikrishna Adibhatla and Hatcher, 2006), and apoptosis (Duan et al., 2004; Eefting et al., 2004; Nakka et al., 2008) are involved in IRI. Moreover, many signaling pathways have been reported to be associated with the pathogenesis of cerebral IRI, including mitogen-activated protein kinase (MAPK) (Kovalska et al., 2012). Therefore, numerous investigations have been paid attention to inhibit the inflammation, ROS, apoptosis, and/or MAPK pathway as targets of neuroprotection.
Fucoidan, a sulfated polysaccharide, is mainly found in various species of brown algae and brown seaweed and is now used as an ingredient in some dietary supplement products. In recent years, it has gained a great deal of attention due to its wide range of pharmacological properties, such as anti-tumor (Koyanagi et al., 2003; Maruyama et al., 2003), antioxidant and anti-coagulant (Wang et al., 2010), anti-viral (Prokofjeva et al., 2013; Thuy et al., 2015) and anti-inflammatory (Cumashi et al., 2007). Fucoidan has been used to treat many health conditions, including myocardial and renal IRI (Bojakowski et al., 2001; Li et al., 2011). However, little information is available regarding the effect of fucoidan on cerebral IRI.
Therefore, in the present study, we aimed to investigate the effect of fucoidan on cerebral IRI, as well as the underlying mechanisms. The rats were pre-treated with 80 mg/kg or 160 mg/kg fucoidan for 7 days before the model of cerebral IRI was induced. After administration of fucoidan, we evaluated infarct volume, neurological deficit scores, and the levels of inflammation-associated cytokines, oxidative stress-related proteins, apoptosis-related proteins and MAPK pathway proteins. Our results might provide new strategies to improve the treatment of cerebral IRI.
MATERIALS AND METHODS
Preparation of fucoidan
Crude fucoidan was purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). Fucoidan was dissolved in physiological saline to 20 mg/mL for animal treatment, and stored at −20°C until for later analysis.
Animals and experimental designs
Adult male Sprague-Dawley (SD) rats weighing between 250 and 320 g were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences (Shanghai, China). All the animals were allowed to acclimate to the laboratory conditions for at least 1 week before the test. The animals were housed individually under standard conditions (12:12 h light-dark cycle, 20–22°C, and 50–65% humidity), with food and water ad libitum. The rats were randomly divided into four groups (n = 10 in each group): sham-operated group (Sham); IRI+saline group (IRI+S); IRI+80 mg/kg fucoidan group (IRI+F80); and IRI+160 mg/kg fucoidan group (IRI+F160). Fucoidan (80 mg/kg or 160 mg/kg) was intraperitoneally injected from 7 days before MCAO until sacrifice. In sham-operated rats, the arteries were separated without occlusion. The rats in the IRI+S group received physiological saline as control. All experimental protocols were carried out in accordance with the Principles of Laboratory Animal Care and approved by the Ethics Committee of Shaanxi Traditional Chinese Medicine Hospital (Xi’an, Shaanxi, China).
Induction of focal cerebral ischemia and reperfusion in rats
Focal brain ischemia-reperfusion was induced by intraluminal MCAO as described previously with minor modifications (Chang et al., 2007). Briefly, the rats were anesthetized and then a midline neck incision was performed under a stereo dissecting microscope (Nikon, Tokyo, Japan). After carefully separated the left common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA), the proximal left CCA and the ECA were ligated. A 5-0 monofilament suture (Harvard Apparatus, Holliston, MA, USA) was introduced through a small arteriotomy of the CCA into the distal ICA and was advanced 9–10 mm distal to the origin of middle cerebral artery (MCA) until the MCA was occluded. The suture was withdrawn from the carotid artery to ensure the reperfusion. Then, the wound was closed. The rectal temperature was maintained at 36.5–37.5°C with a homeothermic temperature system (Fine Science Tools Inc., Foster City, CA, USA) during the surgical procedure and up to 24 h after reperfusion. Twenty-four hours after reperfusion, the rats were euthanized by cervical dislocation under anesthesia with 5% isoflurane.
Evaluation of neurological deficits and determination of infarct volume
Neurological deficits were evaluated at 24 h after reperfusion by a neurological scoring system: 0 represents normal spontaneous movement; 1 represents failure to extend the contra lateral forelimb; 2 represents circling to affected side; 3 means partial paralysis on affected side; and 4 presents no spontaneous motor activity. The rats were killed at 24 h after MCAO under anesthesia. The brains were immediately collected, frozen, sectioned into coronal slices, incubated with 2% 2,3,5-triphenyltetrazolium chloride monohydrate (TTC) at 37°C for 15 min, and then maintained in 4% paraformaldehyde overnight. The brain slices were photographed and the total infarct volume was calculated.
Enzyme-linked immunosorbent assay (ELISA)
The secreted levels of interleukin (IL)-1β, IL-6, myeloperoxidase (MPO), tumor necrosis factor (TNF)-α, malondialdehyde (MDA), and superoxide dismutase (SOD) in the ischemic brain were measured by a commercially available ELISA kits (R&D System, Minneapolis, MN, USA) according to the manufacturer’s instructions. The absorbance at 450 nm was read on a microplate reader (Molecular Devices Corp., Sunnyvale, CA, USA).
Western blot analysis
Protein was extracted from the brain issues, and the concentrations of protein were measured by BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). The protein samples were separated by sodium dodecyl sulfonate (SDS) polyacrylamide gel electrophoresis, and blotted to polyvinylidene difluoride (PVDF) membranes. Blots were were blocked in 5% non-fat dried milk and incubated with antibodies against Bax, B-cell lymphoma (Bcl)-2, phosphorylationextracellular signal-regulated kinase (p-ERK), ERK, p- c-Jun N-terminal kinase (JNK), JNK, p-p38, p38, p-p53, and p53. All antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Then the bolts were incubated with goat anti-rabbi IgG for 2 h at room temperature, and detected by enhanced chemiluminescence and densitometric analysis. Densitometric values were normalized to the GAPDH control.
Statistical analysis
All data were shown as the mean ± SD. Statistical Package for the Social Sciences (SPSS) (version 18.0, SPSS Inc., Chicago, IL, USA) was performed to compare the significance of the differences among different groups. Student’s t test or a one-way analysis of variance (ANOVA) was carried out to calculate p-values. Differences were considered significant when p<0.05.
RESULTS
Effects of fucoidan on infarct volume
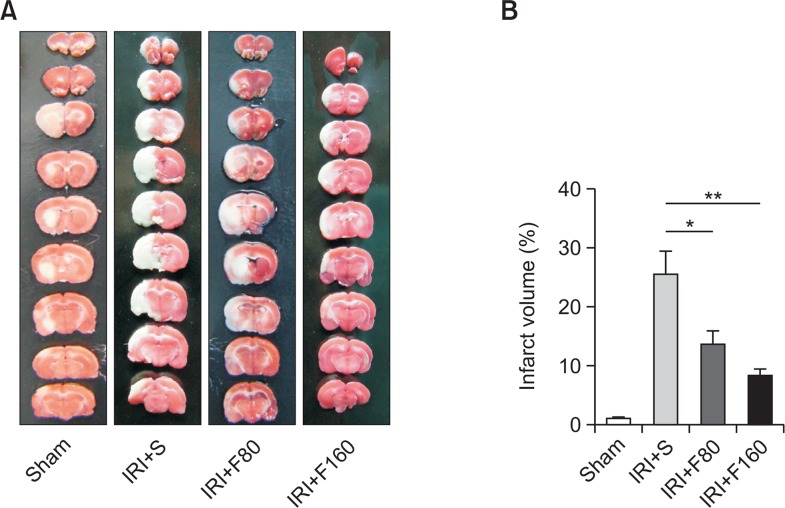
To evaluate the functional role of fucoidan in IRI in rats, we first administrated different doses of fucoidan (80 mg/kg or 160 mg/kg) to the rats and then induced the IRI model by MCAO method. The infarct volume was determined after administration of fucoidan. The representative photographs of brain tissues were shown in Fig. 1A, whereas the quantitative evaluation result were shown in Fig. 1B. Form these pictures, we found that administration of 80 mg/kg fucoidan significantly reduced the infarct volume compared to the IRI+S group (p<0.01), and while administration of 160 mg/kg fucoidan further significantly decreased the infarct volume compared to the IRI+S group (p<0.001), indicating that fucoidan might protect against IRI in a dose-dependent manner.
Fig. 1.
Effects of fucoidan on infarct volume. (A) Representative photographs of brain tissues, (B) The quantitative evaluation results. IRI: ischemia-reperfusion injury, S: saline, F80: 80 mg/kg fucoidan, F160: 160 mg/kg fucoidan. *p<0.01 compared to the IRI+S group. **p<0.001 compared to the IRI+S group.
Effects of fucoidan on neurological deficit score
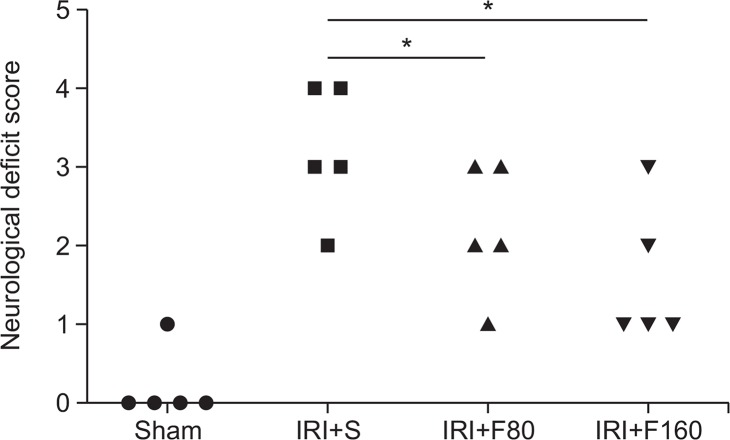
After successful induction of IRI by MCAO method, we evaluated the effect of fucoidan on neurological deficit score. The results showed that in the sham group, rats appeared with non-neurological impairment symptoms, in contrast, rats in the IRI+S group showed significantly increased the neurological deficit scores compared to the Sham group (neurological score: 3.2 ± 0.84 vs 0.2 ± 0.04, p<0.05). However, administration of fucoidan (80 mg/kg or 160 mg/kg) significantly decreased the neurological deficit scores (neurological score: 2.2 ± 0.83 or 1.6 ± 0.89) compared to the IRI+S group (both p<0.05, Fig. 2). These data indicated that treatment with fucoidan can significantly ameliorate these neurological impairment symptoms after cerebral ischemic injury in rats.
Fig. 2.
Effects of fucoidan on neurological deficit score. IRI: ischemia-reperfusion injury, S: saline, F80: 80 mg/kg fucoidan, F160: 160 mg/kg fucoidan. *p<0.05 compared to the IRI+S group.
Effects of fucoidan on inflammation-associated cytokines
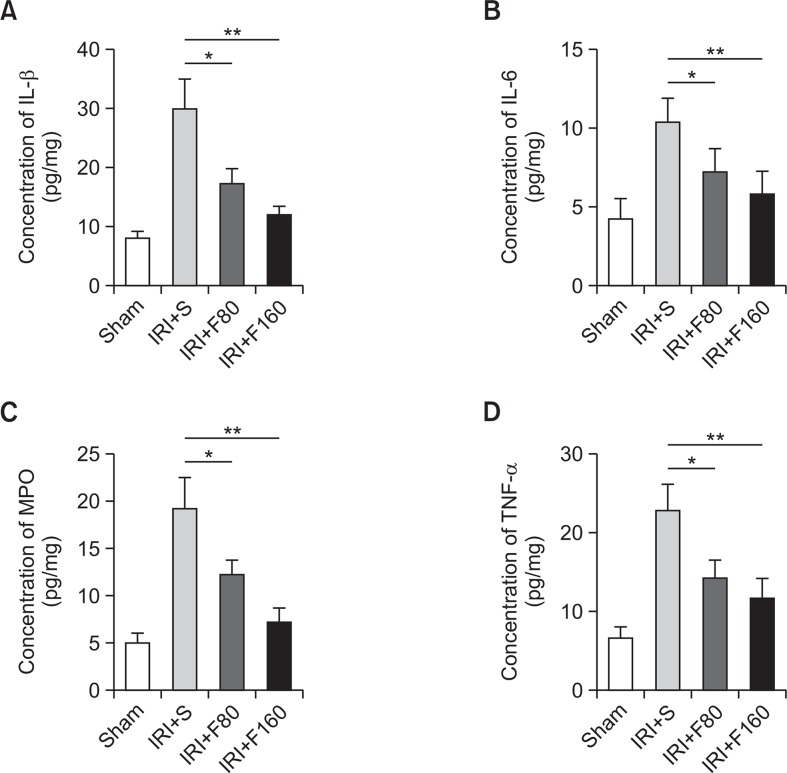
There is ample evidence that inflammatory-associated cytokines such as IL-1β, IL-6, and TNF-α, play a chief role in inflammatory cerebral IRI injury (Yi et al., 2007). MPO is a marker that indicates tissue infiltration of inflammatory cells (Calcagni and Elenkov, 2006). In this study, ELISA was performed to measure the content of IL-1β, IL-6, MPO, and TNF-α. As shown in Fig. 3, the results showed that the levels of IL-1β, IL-6, MPO, and TNF-α were all significantly decreased by treatment with fucoidan compared with the IRI+S group (p<0.05 for F80 and p<0.01 for F160). The results demonstrated that fucoidan can significantly relieve the inflammatory response after cerebral ischemic injury in rats.
Fig. 3.
Effects of fucoidan on inflammation-associated cytokines. (A) The levels of IL-1β, (B) The levels of IL-6, (C) The levels of MPO, (D) The levels of TNF-α. IRI: ischemia-reperfusion injury, S: saline, F80: 80 mg/kg fucoidan, F160: 160 mg/kg fucoidan, IL: interleukin, MPO: myeloperoxidase, TNF: tumor necrosis factor. *p<0.05 compared to the IRI+S group, **p<0.01 compared to the IRI+S group.
Effects of fucoidan on oxidative stress-related proteins
It has been well demonstrated that oxidative stress is involved in the pathogenesis of cerebral IRI (Wong and Crack, 2008). Therefore, we evaluated the effect of fucoidan on oxidative stress-related protein SOD, and MDA. The levels of SOD and MDA were measured by ELISA. As shown in Fig. 4, we observed that treatment with fucoidan significantly decreased the levels of SOD and MDA compared with the IRI+S group (p<0.05 for F80 and p<0.01 for F160). Our data indicated that fucoidan can significantly alleviate the oxidative stress response after cerebral ischemic injury in rats.
Fig. 4.
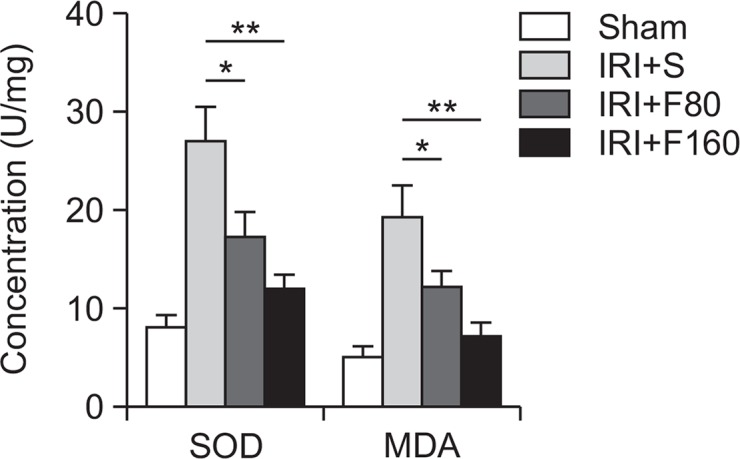
Effects of fucoidan on oxidative stress-related proteins. IRI: ischemia-reperfusion injury, S: saline, F80: 80 mg/kg fucoidan, F160: 160 mg/kg fucoidan, MDA: malondialdehyde, SOD: superoxide dismutase. *p<0.05 compared to the IRI+S group, **p<0.01 compared to the IRI+S group.
Effects of fucoidan on apoptosis-related proteins and MAPK pathway
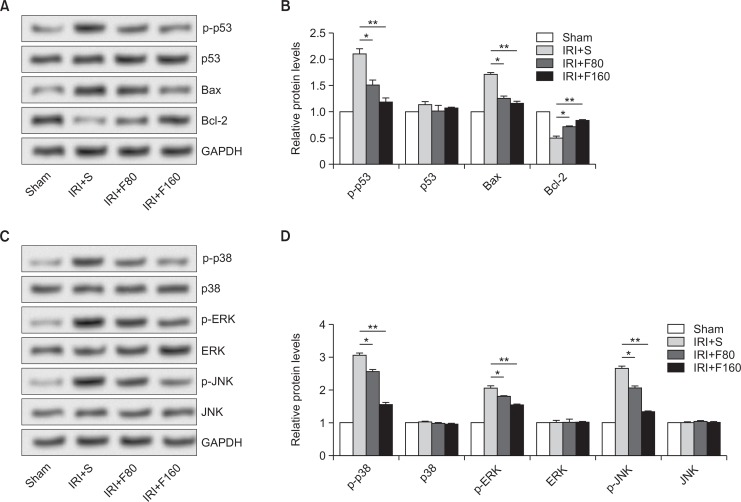
Neuronal apoptosis plays a significant role in neuronal loss after IRI, which is mediated by apoptosis-specific genes, oncogens, or anti-ongogens, such as Bcl-2, Bax, and p53 (Zhou et al., 2003). Hence, the effect of fucoidan on apoptosis-related protein was investigated. As shown in Fig. 5A and 5B, the representative photographs of Western blot showed that both the levels of p-p53 and Bax were significantly higher while the levels of Bcl-2 were markedly lower after IRI. However, administration of fucoidan statistically reduced the levels of p-p53 and Bax but obviously elevated the levels of Bcl-2 (p<0.05 for F80 and p<0.01 for F160). In addition, high concentration of fucoidan exerted different effects on the levels of apoptosis-related proteins, indicating a dose-dependent manner. Further, we explored the effect of fucoidan on MAPK pathway by determining the protein levels of p38, ERK, JNK, and their phosphorylated status. Our results showed that all the levels of p-p38, p-ERK, and p-JNK were significantly decreased by administration of fucoidan in a dose-independent way (p<0.05 for F80 and p<0.01 for F160) (Fig. 5C, 5D). The data suggested that fucoidan played a protective role in cerebral IRI might be by inhibition of MAPK signaling pathway.
Fig. 5.
Effects of fucoidan on apoptosis-related proteins and MAPK pathway. (A, B) The effect of fucoidan on apoptosis-related proteins, (C, D) The effect of fucoidan on MAPK pathway related proteins. IRI: ischemia-reperfusion injury, S: saline, F80: 80 mg/kg fucoidan, F160: 160 mg/kg fucoidan, MAPK: mitogen-activated protein kinase, Bcl: B-cell lymphoma, ERK: extracellular signal-regulated kinase, JNK: p-c-Jun N-terminal kinase. *p<0.05 compared to the IRI+S group, **p<0.01 compared to the IRI+S group.
DISCUSSION
In the present study, we explored the effect of fucoidan on cerebral IRI. The results showed that administration of fucoidan significantly decreased the neurological deficit scores and infarct volume. Also, fucoidan statistically changed the levels of inflammation-associated cytokines (IL-1β, IL-6, MPO, and TNF-α), oxidative stress-related protein SOD, and MDA, apoptosis-related proteins (p-p53, Bax and Bcl-2). These data indicated that fucoidan potently protected the brain from IRI injury and this protection may be mediated by MAPK signaling pathway.
Fucoidan is a complex of sulfated polysaccharide found in several different species of brown seaweed, such as bladder wrack and kelp. Fucoidan contains a large proportion of L-fucose and sulfate that are significantly contributed to its potential health benefits. Fucoidan has been reported a broad spectrum of biological activities and is used medicinally for a wide variety of health purposes. Many studies have showed that fucoidan showed a protective role in IRI. For example, Bojakowski and co-workers found that administration of fucoidan significantly increased postischemic renal blood flow and is beneficial to renal IRI (Bojakowski et al., 2001). Li et al. (2011) revealed that the administration of fucoidan could regulate the inflammation response by inactivation of nuclear factor (NF)-κB inactivation in IRI-induced myocardial damage. Omata and co-workers suggested fucoidan exerted a protective role in myocardial IRI by blockade of P-selectin-mediated neutrophil rolling on the vessel wall (Omata et al., 1997). Moreover, accumulating evidence has suggested that fucoidan has neuroprotective actions (Gao et al., 2012; Jin et al., 2013a, 2013b, 2014). Although the relatively large size of fucoidan precludes penetration of blood brain barrier (BBB), systemic administration of fucoidan has been reported to be effective in maintaining brain function in vivo and in vitro in a number of ways. Jhamandas et al. (2005) suggested that fucoidan blocked β-amyloid protein (Aβ)-induced decrease in whole-cell currents in basal forebrain neurons and showed neuroprotective effects against Aβ-induced neurotoxicity in basal forebrain neuronal cultures. Cui et al. (2010) found that fucoidan was able to protect against lipopolysaccharide (LPS)-induced nitric oxide (NO) production by microglia. Besides, Fucoidan is an inhibitor of Aβ binding to the type A macrophage scavenger receptor (SR-A) (Deane et al., 2003), and blocking the SR-A by fucoidan presents neuro-protective effects. For example, Li et al. (2004) demonstrated that Fucus vesiculosis fucoidan (40 ng/mL) suppressed uptake of fragmented DNA (fDNA) by adjacent activated microglia in neuronal nuclei in Alzheimer brain. Therefore, we speculated that fucoidan might play a protective role in cerebral IRI.
To ensure the speculation, we first administered different doses of fucoidan to the rats for continuous 7 days before the cerebral IRI was induced. After 24 h of reputation, the neurological deficit scores and infarct volume were evaluated. The data showed that pre-treatment with fucoidan significantly reduced both the neurological deficit scores and infarct volume compared to the rats received saline. Also, with higher dose of fucoidan, better outcome was observed. Our results indicated that fucoidan might play a protective role in cerebral IRI. To explore the underlying mechanisms, we focused on the anti-inflammatory, antioxidant and anti-apoptotic effects of fucoidan.
Inflammatory responses are characterized by hyper-expression of inflammatory cytokines and neutrophil infiltration into ischemic brain after injury, which play a vital role in the pathogenesis of cerebral ischemic damage (Wang et al., 2007; Terao et al., 2008). The inflammatory process is driven by various cytokines, such as IL-1β, IL-6 and TNF-α (Yamasaki et al., 1995; Loddick et al., 1998; Nilupul Perera et al., 2006). MPO is neutrophil-derived enzyme and a marker of neutrophil activation, which is considered as a biochemical marker for tissue invasion or content of neutrophils (Calcagni and Elenkov, 2006). It has been reported that fucoidan significantly reduced the expression levels of IL-1β, IL-1 and TNF-α during lipopolysaccharide (LPS)-induced inflammation in RAW264.7 cells (Kim et al., 2012). Also, MPO activity was significantly decreased by administration of fucoidin after rat heart IRI (Omata et al., 1997; Li et al., 2011; Chen et al., 2013). In our study, fucoidan also significantly decreased the levels of IL-1β, IL-1 and TNF-α and MPO activity in cerebral IRI with a dose-dependent manner, indicating an anti-inflammatory effect. Antioxidants, such as SOD, play critical roles in reversing the pathological damage caused by IRI (Paller et al., 1984). Chen et al. (2013) found that fucoidin corrected the abnormal levels of MPO, MDA and SOD induced by IRI. Our results also found that pre-treatment with fucoidin statistically decreased the levels of SOD and MDA, suggesting an antioxidant effect. Apoptosis induced by extrinsic pathway or intrinsic pathway has been well involved in cerebral IRI (Broughton et al., 2009). The Bcl-2 family members play significant roles in the mitochondria-dependent intrinsic pathway of apoptosis. Overexpression of Bcl-2 enhances cell survival by inhibiting apoptosis, whereas Bax, a member of the Bcl-2 family proteins, promotes apoptosis and counter plays the protective effect of Bcl-2 (Broughton et al., 2009). In addition, p53, the tumor-suppressor transcription factor, stops the cell cycle and triggers programmed cell death by promoting the expression of pro-apoptotic protein and suppressing the expression of anti-apoptotic protein (Speidel, 2010). Our data showed that fucoidin statistically reduced the levels of p-p53 and Bax but elevated Bcl-2, exerting a protective effect on inhibition of brain cells. Our results were similar with the researches of Boo and Chen. Boo et al. (2011) found that fucoidan reduced Bcl-2 expression, but increased Bax expression in a dose-dependent fashion in A549 human lung carcinoma cells. Chen et al. (2013) observed that pre-treatment with fucoidin remarkably inhibited the phosphorylation of p53, leading to a protective effect on myocardial cells. Moreover, the effects of fucoidin on a verity of human diseases have been reported to be involved in many signaling pathways, such as MAPK pathway (Do et al., 2010; Chen et al., 2013). Additionally, MAPK pathway is responsible for cerebral IRI (Kovalska et al., 2012). JNK and p38 are mainly activated through environmental stresses, such as oxidative stress, and inflammatory cytokine, while ERK is normally involved in cell proliferation and growth (Johnson and Lapadat, 2002). Activation of MAPKs stimulates transcription factors that regulate multiple genes involved in the survival or death of cells, and thus the transcription factors play significant roles in pharmacological intervention and drug development. When the rats were exposed to MCAO in our study, a drastic and rapid increase in phosphorylation of JNK, p38 and ERK was found. Pre-treatment with fucoidin resulted in decreased phosphorylation of all three molecules. Therefore, fucoidin inhibited the activation of MAPK pathway.
In conclusion, our results suggest that fucoidin plays a protective role in cerebral IRI, and these effects might be through inhibition of MAPK pathway.
Footnotes
CONFLICT OF INTEREST
The authors report no conflict of interest.
REFERENCES
- Bojakowski K, Abramczyk P, Bojakowska M, Zwolinska A, Przybylski J, Gaciong Z. Fucoidan improves the renal blood flow in the early stage of renal ischemia/reperfusion injury in the rat. J Physiol Pharmacol. 2001;52:137–143. [PubMed] [Google Scholar]
- Boo HJ, Hyun JH, Kim SC, Kang JI, Kim MK, Kim SY, Cho H, Yoo ES, Kang HK. Fucoidan from Undaria pinnatifida induces apoptosis in A549 human lung carcinoma cells. Phytother Res. 2011;25:1082–1086. doi: 10.1002/ptr.3489. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- Chang Y, Hsiao G, Chen SH, Chen YC, Lin JH, Lin KH, Chou DS, Sheu JR. Tetramethylpyrazine suppresses HIF-1α, TNF-α, and activated caspase-3 expression in middle cerebral artery occlusion-induced brain ischemia in rats. Acta Pharmacol Sin. 2007;28:327–333. doi: 10.1111/j.1745-7254.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang W, Zhang Q, Li F, Lei T, Luo D, Zhou H, Yang B. Low molecular weight fucoidan against renal ischemia-reperfusion injury via inhibition of the MAPK signaling pathway. PLoS ONE. 2013;8:e56224. doi: 10.1371/journal.pone.0056224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YQ, Zhang LJ, Zhang T, Luo DZ, Jia YJ, Guo ZX, Zhang QB, Wang X, Wang XM. Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide-activated primary microglia. Clin Exp Pharmacol Physiol. 2010;37:422–428. doi: 10.1111/j.1440-1681.2009.05314.x. [DOI] [PubMed] [Google Scholar]
- Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, Usov AI, Ustyuzhanina NE, Grachev AA, Sanderson CJ, Kelly M, Rabinovich GA, Iacobelli S, Nifantiev NE, Consorzio Interuniversitario Nazionale per la Bio-Oncologia. Italy A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Do H, Pyo S, Sohn EH. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-α- and IFN-γ-stimulated C6 glioma cells. J Nutr Biochem. 2010;21:671–679. doi: 10.1016/j.jnutbio.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Duan Q, Wang X, Wang Z, Lu T, Han Y, He S. Role of mitochondria in neuron apoptosis during ischemia-reperfusion injury. J Huazhong Univ Sci Technol Med Sci. 2004;24:441–444. doi: 10.1007/BF02831103. [DOI] [PubMed] [Google Scholar]
- Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Gao Y, Dong C, Yin J, Shen J, Tian J, Li C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell Mol Neurobiol. 2012;32:523–529. doi: 10.1007/s10571-011-9792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean WC, Spellman SR, Nussbaum ES, Low WC. Reperfusion injury after focal cerebral ischemia: the role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43:1382–1396. doi: 10.1097/00006123-199812000-00076. discussion 1396–1397. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Wie MB, Harris K, MacTavish D, Kar S. Fucoidan inhibits cellular and neurotoxic effects of β-amyloid (Aβ) in rat cholinergic basal forebrain neurons. Eur J Neurosci. 2005;21:2649–2659. doi: 10.1111/j.1460-9568.2005.04111.x. [DOI] [PubMed] [Google Scholar]
- Jin W, Wang J, Jiang H, Song N, Zhang W, Zhang Q. The neuroprotective activities of heteropolysaccharides extracted from Saccharina japonica. Carbohydr Polym. 2013a;97:116–120. doi: 10.1016/j.carbpol.2013.04.055. [DOI] [PubMed] [Google Scholar]
- Jin W, Zhang W, Wang J, Yao J, Xie E, Liu D, Duan D, Zhang Q. A study of neuroprotective and antioxidant activities of heteropolysaccharides from six Sargassum species. Int J Biol Macromol. 2014;67:336–342. doi: 10.1016/j.ijbiomac.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Jin W, Zhang W, Wang J, Zhang Q. The neuroprotective activities and antioxidant activities of the polysaccharides from Saccharina japonica. Int J Biol Macromol. 2013b;58:240–244. doi: 10.1016/j.ijbiomac.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Yoon KY, Lee BY. Low molecular weight fucoidan from the sporophyll of Undaria pinnatifida suppresses inflammation by promoting the inhibition of mitogen-activated protein kinases and oxidative stress in RAW264.7 cells. Fitoterapia. 2012;83:1628–1635. doi: 10.1016/j.fitote.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Kovalska M, Kovalska L, Pavlikova M, Janickova M, Mikuskova K, Adamkov M, Kaplan P, Tatarkova Z, Lehotsky J. Intracellular signaling MAPK pathway after cerebral ischemia-reperfusion injury. Neurochem Res. 2012;37:1568–1577. doi: 10.1007/s11064-012-0752-y. [DOI] [PubMed] [Google Scholar]
- Koyanagi S, Tanigawa N, Nakagawa H, Soeda S, Shimeno H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem Pharmacol. 2003;65:173–179. doi: 10.1016/S0006-2952(02)01478-8. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Gao Y, Xing Y, Zhu H, Shen J, Tian J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia-reperfusion injury in rats via regulating the inflammation response. Food Chem Toxicol. 2011;49:2090–2095. doi: 10.1016/j.fct.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Li M, Qu YZ, Zhao ZW, Wu SX, Liu YY, Wei XY, Gao L, Gao GD. Astragaloside IV protects against focal cerebral ischemia/reperfusion injury correlating to suppression of neutrophils adhesion-related molecules. Neurochem Int. 2012;60:458–465. doi: 10.1016/j.neuint.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu L, Liu D, Woodward S, Barger SW, Mrak RE, Griffin WS. Microglial activation by uptake of fDNA via a scavenger receptor. J Neuroimmunol. 2004;147:50–55. doi: 10.1016/j.jneuroim.2003.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Tamauchi H, Hashimoto M, Nakano T. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida. In Vivo. 2003;17:245–249. [PubMed] [Google Scholar]
- Muralikrishna Adibhatla R, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol. 2008;37:7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- Nilupul Perera M, Ma HK, Arakawa S, Howells DW, Markus R, Rowe CC, Donnan GA. Inflammation following stroke. J Clin Neurosci. 2006;13:1–8. doi: 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Omata M, Matsui N, Inomata N, Ohno T. Protective effects of polysaccharide fucoidin on myocardial ischemia-reperfusion injury in rats. J Cardiovasc Pharmacol. 1997;30:717–724. doi: 10.1097/00005344-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokofjeva MM, Imbs TI, Shevchenko NM, Spirin PV, Horn S, Fehse B, Zvyagintseva TN, Prassolov VS. Fucoidans as potential inhibitors of HIV-1. Mar. Drugs. 2013;11:3000–3014. doi: 10.3390/md11083000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speidel D. Transcription-independent p53 apoptosis: an alternative route to death. Trends Cell Biol. 2010;20:14–24. doi: 10.1016/j.tcb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke. 2008;39:943–950. doi: 10.1161/STROKEAHA.107.494542. [DOI] [PubMed] [Google Scholar]
- Thuy TT, Ly BM, Van TT, Quang V, Tu HC, Zheng Y, Seguin-Devaux C, Mi B, Ai U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr Polym. 2015;115:122–128. doi: 10.1016/j.carbpol.2014.08.068. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Q, Zhang Z, Song H, Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol. 2010;46:6–12. doi: 10.1016/j.ijbiomac.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15:1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–681. doi: 10.1161/01.STR.26.4.676. [DOI] [PubMed] [Google Scholar]
- Yi JH, Park SW, Kapadia R, Vemuganti R. Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem Int. 2007;50:1014–1027. doi: 10.1016/j.neuint.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ma Y, Zhou Y, Liu Z, Wang K, Chen G. Effects of magnesium sulfate on neuron apoptosis and expression of caspase-3, bax and bcl-2 after cerebral ischemia-reperfusion injury. Chin Med J. 2003;116:1532–1534. [PubMed] [Google Scholar]