Abstract
Introduction
One of the most dangerous dermatologic emergencies is Stevens-Johnson Syndrome (SJS)/toxic epidermal necrolysis (TEN). Although a rare disease, it can often lead to significant mortality.
Case Presentation
In this case report, we present a 77-year-old man who developed a sloughing rash that was secondary to a nonsteroidal anti-inflammatory drug. In addition to the recommended supportive care, the patient was treated with etanercept, a new, less commonly used intervention.
Discussion
We provide a brief review of SJS/TEN. Nonsteroidal anti-inflammatory drugs are a rare cause of SJS/TEN, and additionally, the use of biologics is a novel treatment modality for SJS/TEN.
INTRODUCTION
Stevens-Johnson Syndrome (SJS)/toxic epidermal necrolysis (TEN) is a rare manifestation of a drug reaction. Sulfonamide antibiotics are often implicated as the source of SJS/TEN.1–3 However, more than 100 drugs have been reported to potentially cause SJS/TEN.1–3 Patients with SJS/TEN have a high mortality rate. The mainstay treatment of SJS/TEN is supportive care, but there exist other treatment options that can often be controversial.
CASE REPORT
A 77-year-old man with a history of diabetes mellitus type 2, hypertension, hyperlipidemia, and coronary artery disease presented to the Emergency Department (ED) with a 1-day history of rash. The patient reported a fever 2 days earlier, which had resolved by the time he presented to the ED. The patient reported no new medications but did take 2 tablets of naproxen 2 days earlier in the setting of the fever and cough. The patient had seen the dermatologist earlier that day, and SJS was considered among other diagnoses. Other than the rash, the patient appeared to be healthy, and a biopsy was scheduled for the next day. Routine laboratory tests were ordered and revealed a significantly elevated serum glucose of 283 mg/dL (normal range, 60–159 mg/dL). Other pertinent laboratory test values included a normal white blood cell count of 4.4 K/μL (normal range, 3.5–12.5 K/μL) with a differential showing elevated eosinophils of 10% (normal range, 0%–6%), normal serum creatinine of 1.09 mg/dL (normal range, ≤ 1.34 mg/dL), normal serum blood urea nitrogen of 24 mg/dL (normal range, 7–27 mg/dL), normal serum bicarbonate of 24 mEq/L (normal range, 24–33 mEq/L), and normal serum alanine aminotransferase of 27 U/L (normal range, 0–36 U/L). Given the elevated glucose and the concern for SJS, the patient was asked to present to the ED.
On presentation to the ED, the patient was in stable condition with these vital signs: heart rate, 69 beats/min; blood pressure, 134/67 mmHg; temperature, 98.4°F; respiratory rate, 19 breaths/min; and peripheral oxygen saturation, 99%. He was noted to have some sloughing on the left forearm (Figure 1), right neck (Figure 2), and scrotum with a positive Nikolsky sign. He also was found to have dusky skin on his back, right forearm, and lower extremities without sloughing (Figure 3). His skin was nontender. The patient’s conjunctiva and oral mucosa were normal. Laboratory test values were significant for an elevated lactate. Skin cultures were also obtained. Dermatology was consulted for the patient in the ED owing to concern for SJS both in the dermatologic clinic and in the ED along with his recent nonsteroidal anti-inflammatory drug (NSAID) use. The decision was made to treat with an antibiotic (intravenous nafcillin) for possible Staphylococcal scalded skin syndrome (SSSS) with an elevated lactate. Drug reaction with eosinophilia and systemic symptoms (DRESS) was also on the differential diagnosis. The patient was admitted for observation in consideration of the extent of involvement. The patient’s rash was biopsied and ultimately showed full-thickness epidermal necrosis with dyskeratotic keratinocytes and subepidermal clefting. The pathology report suggested TEN.
Figure 1.
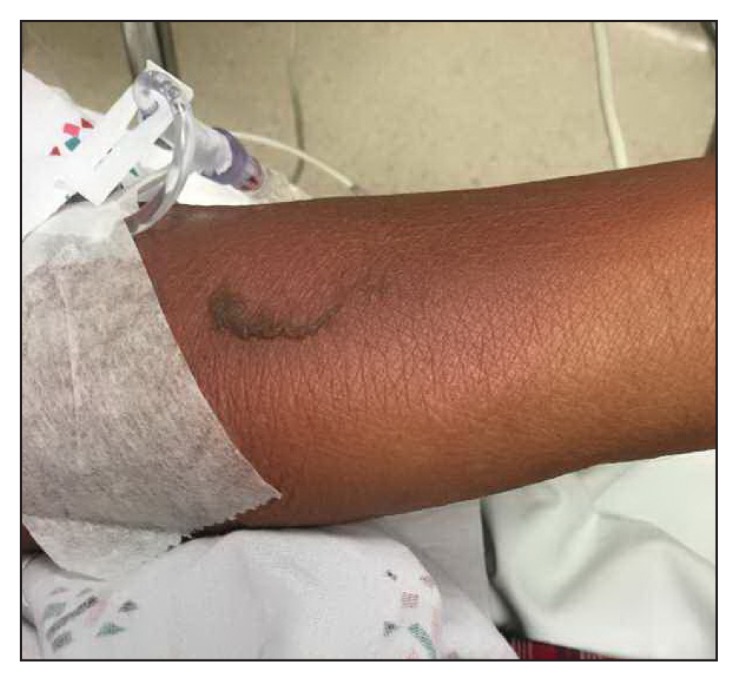
Patient’s left forearm showing sloughing of skin. Patient had a positive Nikolsky sign.
Figure 2.
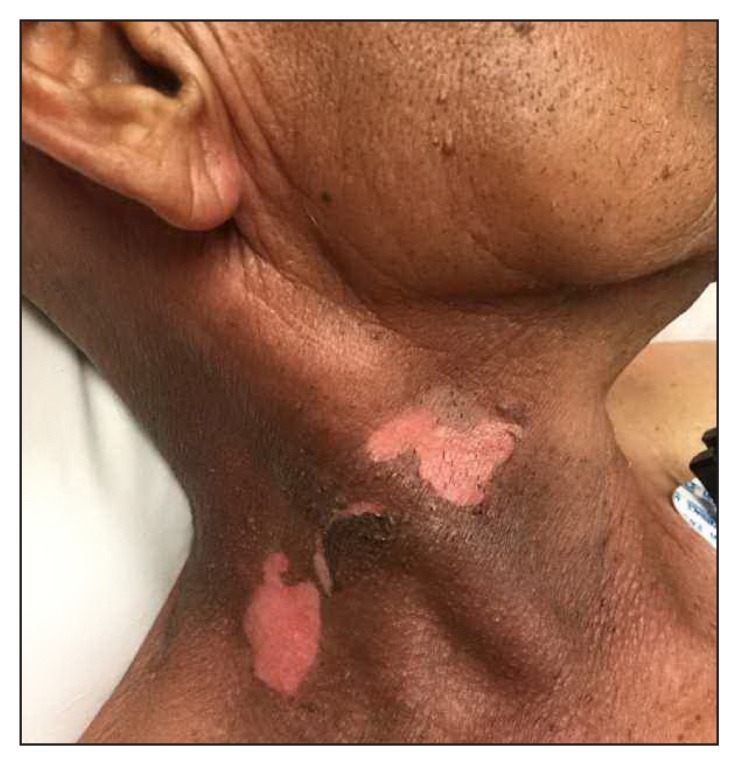
Patient’s neck showing sloughing of skin.
Figure 3.
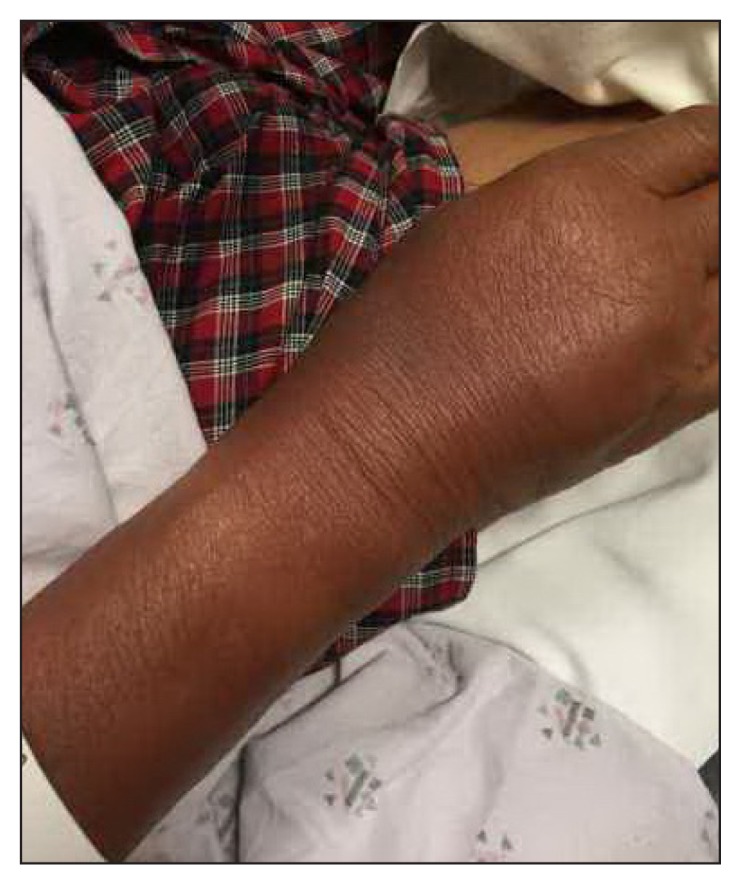
Patient’s right wrist is dusky but without sloughing.
With this diagnosis, the patient was given a single dose of etanercept for his TEN. Local wound care with vaseline was recommended. The patient’s wound cultures also grew Streptococcus agalactiae; thus, antibiotics were continued and the patient was transitioned from intravenous nafcillin to oral cephalexin.
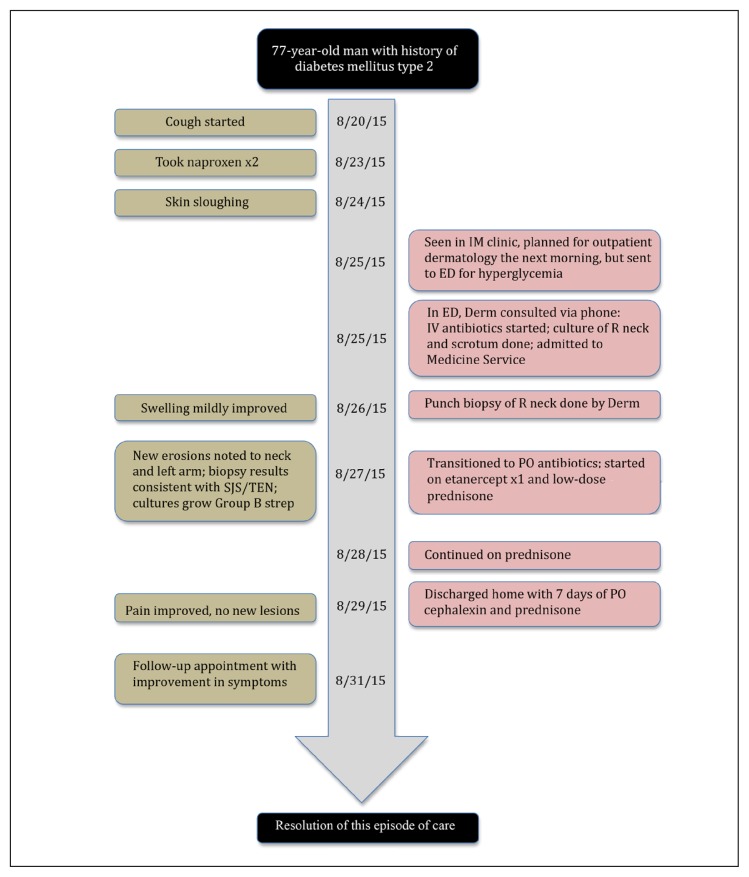
The patient was discharged on hospital day 5. He completed a 7-day course of cephalexin. At 3-month follow-up, the patient was doing well with resolution of the rash and no recurrence of symptoms. Figure 4 illustrates a timeline of the case. He has avoided all NSAIDs since discharge at the recommendation of his treatment team because this was the most likely trigger for his TEN.
Figure 4.
Timeline of the case.
Derm = Dermatology Department; ED = Emergency Department; IM = Internal Medicine IV = intravenous; PO = orally; R = right; SJS = Stevens-Johnson Syndrome; TEN = toxic epidermal necrolysis.
DISCUSSION
TEN is a term used to describe a severe exfoliative skin reaction resulting in greater than 30% body surface area of epidermal detachment.4 This is a part of a spectrum that includes SJS, which represents epidermal detachment of less than 10% body surface area.4,5 For 10% to 30% body surface area, there is an overlap of both SJS and TEN.4 SJS and TEN are quite rare, and the incidence is estimated to be 1 to 7 cases per million person-years and 0.4 to 1.5 cases per million person-years, respectively.4,6–10 Mortality for SJS and TEN are 1% to 3% and 30% to 50%, respectively.4,11–13 The pathogenesis of SJS/TEN is not completely understood, but several theories postulate either an “immunologic mechanism, reactive drug metabolites, or interactions between these two.”4
As in the patient presented, NSAID usage resulting in SJS/TEN has been reported.1 There have been four published case reports associated with naproxen.1,14–17 There were also four published cases of ibuprofen resulting in SJS/TEN, which also resulted in liver involvement.1,2,18–20 A review article by Ward et al1 reported that older patients, women, and patients within the first month of treatment with NSAIDS may have the highest risk for SJS/TEN. However, the overall risk of SJS/TEN secondary to NSAIDS is very low.1
There are more than 100 drugs that have been known to result in SJS/TEN.1 The most frequently cited cause is sulfonamides.1 Other drugs include, but are not limited to, cephalosporins, quinolones, corticosteroids, allopurinol, phenytoin, phenobarbital, and anti-inflammatories.4 Identification of the cause is usually dependent upon patient history and confirmation via skin biopsy.21 Patch testing has been shown to have a very limited role in identification of the cause of SJS/TEN spectrum.22
Treatment of SJS/TEN begins with immediate cessation of the inciting drug. This has reportedly resulted in reducing the mortality rate to 5%–11% in one study23 and 21% in another.24 Recurrence may happen if a patient is exposed to the same drug.25 Patients with significant skin loss should be treated as burn victims and with early transfer to a burn center. Given that our patient had limited total body surface area involvement, referral to the local burn center was not needed. Reduction in mortality has been shown with transfer of care to a burn center within 7 days: 29.8% reduction with transfer vs 51.4% without transfer.26 Patients with limited skin involvement and limited progression of the disease may be admitted to a hospital ward.27 Using the prognosis scoring system for SJS/TEN (SCORTEN, for SCORe of Toxic Epidermal Necrolysis; Figure 5),28 the patient scored a SCORTEN of 2 (age, elevated glucose), which has a predicted mortality rate of approximately 12.1%.28 Notably, hyperglycemia was one risk factor with a direct correlation with mortality in SJS/TEN.28
Figure 5.
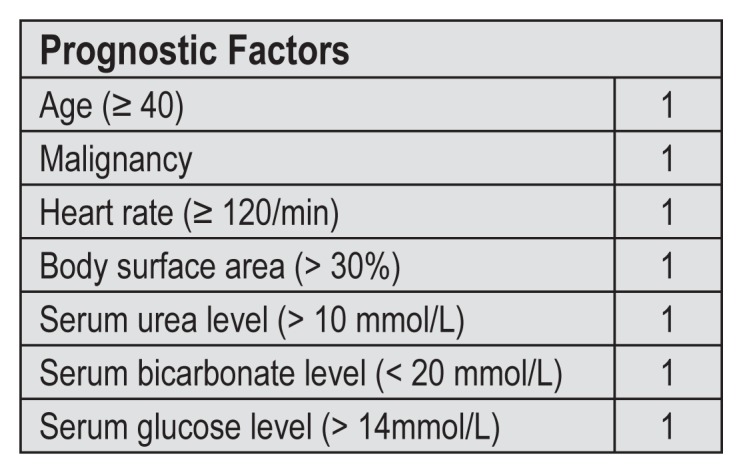
Severity of illness score called SCORTEN (SCORe of Toxic Epidermal Necrolysis).28
The use of steroids is quite controversial in SJS/TEN patients. There have been reports showing benefit, such as a reduction in duration of fever.4,29 Alternatively, another study showed an increase in mortality with the use of steroids: 66% in the group with steroids vs 33% in the group without steroids.30 A more novel approach to the treatment of TEN utilizes etanercept, a tumor necrosis factor-alpha antagonist. Some case reports have suggested that etanercept may help control TEN.31–33 As was used in our patient, only 1 dose of etanercept is usually necessary.31
CONCLUSION
Although SJS/TEN is a rare diagnosis, it is an important one to consider as a dermatologic emergency given the high morbidity and mortality associated with it. A cautious approach to SJS/TEN with treatment similar to that for burns may help to decrease morbidity and mortality. The treatment of SJS/TEN is often supportive, but as demonstrated in this case, the use of biologics such as etanercept may offer new potential treatment modalities. At this time, given the novelty of etanercept, we recommend consultation with dermatology before initiating biologics.
Acknowledgment
Mary Corrado, ELS, provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Ward KE, Archambault R, Mersfelder TL. Severe adverse skin reactions to nonsteroidal antiinflammatory drugs: A review of the literature. Am J Health Syst Pharm. 2010 Feb 1;67(3):206–13. doi: 10.2146/ajhp080603. DOI: https://doi.org/10.2146/ajhp080603. [DOI] [PubMed] [Google Scholar]
- 2.Morelli MS, O’Brien FX. Stevens-Johnson syndrome and cholestatic hepatitis. Dig Dis Sci. 2001 Nov;46(11):2385–8. doi: 10.1023/a:1012351231143. DOI: https://doi.org/10.1023/A:1012351231143. [DOI] [PubMed] [Google Scholar]
- 3.Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995 Dec 14;333(24):1600–7. doi: 10.1056/NEJM199512143332404. DOI: https://doi.org/10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 4.Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: A review. Crit Care Med. 2011 Jun;39(6):1521–32. doi: 10.1097/CCM.0b013e31821201ed. DOI: https://doi.org/10.1097/CCM.0b013e31821201ed. [DOI] [PubMed] [Google Scholar]
- 5.Stevens AM, Johnson FC. A new eruptive fever associated with stomatitis and ophthalmia: Report of two cases in children. Am J Dis Child. 1922 Dec;24(6):526–33. https://doi.org/10.1001/archpedi.1922.04120120077005. [Google Scholar]
- 6.Strom BL, Carson JL, Halpern AC, et al. A population-based study of Stevens-Johnson syndrome. Incidence and antecedent drug exposures. Arch Dermatol. 1991 Jun;127(6):831–8. DOI: https://doi.org/10.1001/archderm.1991.01680050075007. [PubMed] [Google Scholar]
- 7.Naldi L, Locati F, Marchesi L, Cainelli T. Incidence of toxic epidermal necrolysis in Italy. Arch Dermatol. 1990 Aug;126(8):1103–4. doi: 10.1001/archderm.1990.01670320127028. DOI: https://doi.org/10.1001/archderm.1990.01670320127028. [DOI] [PubMed] [Google Scholar]
- 8.Roujeau JC, Guillaume JC, Fabre JP, Penso D, Fléchet ML, Girre JP. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981–1985. Arch Dermatol. 1990 Jan;126(1):37–42. doi: 10.1001/archderm.126.1.37. DOI: https://doi.org/10.1001/archderm.126.1.37. [DOI] [PubMed] [Google Scholar]
- 9.Schöpf E, Stühmer A, Rzany B, Victor N, Zentgraf R, Kapp JF. Toxic epidermal necrolysis and Stevens-Johnson syndrome. An epidemiologic study from West Germany. Arch Dermatol. 1991 Jun;127(6):839–42. doi: 10.1001/archderm.1991.01680050083008. DOI: https://doi.org/10.1001/archderm.1991.01680050083008. [DOI] [PubMed] [Google Scholar]
- 10.Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990 Jan;126(1):43–7. DOI: https://doi.org/10.1001/archderm.1990.01670250049006. [PubMed] [Google Scholar]
- 11.Roujeau JC, Chosidow O, Saiag P, Guillaume JC. Toxic epidermal necrolysis (Lyell syndrome) J Am Acad Dermatol. 1990 Dec;23(6 Pt 1):1039–58. doi: 10.1016/0190-9622(90)70333-d. DOI: https://doi.org/10.1016/0190-9622(90)70333-d. [DOI] [PubMed] [Google Scholar]
- 12.Fritsch PO, Sidoroff A. Drug-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Am J Clin Dermatol. 2000 Nov-Dec;1(6):349–60. doi: 10.2165/00128071-200001060-00003. DOI: https://doi.org/10.2165/00128071-200001060-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kelly JP, Auquier A, Rzany B, et al. An international collaborative case-control study of severe cutaneous adverse reactions (SCAR). Design and methods. J Clin Epidemiol. 1995 Sep;48(9):1099–108. doi: 10.1016/0895-4356(95)00004-n. DOI: https://doi.org/10.1016/0895-4356(95)00004-n. [DOI] [PubMed] [Google Scholar]
- 14.Mansur AT, Aydingöz IA. A case of toxic epidermal necrolysis with lesions mostly on sun-exposed skin. Photodermatol Photoimmunol Photomed. 2005 Apr;21(2):100–2. doi: 10.1111/j.1600-0781.2005.00149.x. DOI: https://doi.org/10.1111/j.1600-0781.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- 15.Ting W, Stone MS, Racila D, Scofield RH, Sontheimer RD. Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus and the spectrum of the acute syndrome of apoptotic pan-epidermolysis (ASAP): A case report, concept review and proposal for new classification of lupus erythematosus vesiculobullous skin lesions. Lupus. 2004;13(12):941–50. doi: 10.1191/0961203304lu2037sa. DOI: https://doi.org/10.1191/0961203304lu2037sa. [DOI] [PubMed] [Google Scholar]
- 16.Barrera JE, Meyers AD, Hartford EC. Hypopharyngeal stenosis and dysphagia complicating toxic epidermal necrolysis. Arch Otolaryngol Head Neck Surg. 1998 Dec;124(12):1375–6. doi: 10.1001/archotol.124.12.1375. DOI: https://doi.org/10.1001/archotol.124.12.1375. [DOI] [PubMed] [Google Scholar]
- 17.Kalemoglu M, Keskin O. Naproxen sodium-induced toxic epidermal necrolysis: A case report and review of the literature. Eur Surg. 2005 Dec;37(6):343–7. DOI: https://doi.org/10.1007/s10353-005-0194-8. [Google Scholar]
- 18.Taghian M, Tran TA, Bresson-Hadni S, Menget A, Felix S, Jacquemin E. Acute vanishing bile duct syndrome after ibuprofen therapy in a child. J Pediatr. 2004 Aug;145(2):273–6. doi: 10.1016/j.jpeds.2004.05.027. DOI: https://doi.org/10.1016/j.peds.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava M, Perez-Atayde A, Jonas MM. Drug-associated actue-onset vanishing bile duct and Stevens-Johnson syndromes in a child. Gastroenterology. 1998 Sep;115(3):743–8. doi: 10.1016/s0016-5085(98)70154-4. DOI: https://doi.org/10.1016/S0016-5085(98)70154-4. [DOI] [PubMed] [Google Scholar]
- 20.Sternlieb P, Robinson RM. Stevens-Johnson syndrome plus toxic hepatitis due to ibuprofen. N Y State J Med. 1989 Jul;78(8):1239–43. [PubMed] [Google Scholar]
- 21.Rzany B, Hering O, Mockenhaupt M, et al. Histopathological and epidemiological characteristics of patients with erythema exudativum multiforme major, Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 1996 Jul;135(1):6–11. DOI: https://doi.org/10.1046/j.1365-2133.1996.d01-924.x. [PubMed] [Google Scholar]
- 22.Wolkenstein P, Chosidow O, Fléchet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996 Oct;35(4):234–6. doi: 10.1111/j.1600-0536.1996.tb02364.x. DOI: https://doi.org/10.1111/j.1600-0536.1996.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: Does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000 Mar;136(3):323–7. doi: 10.1001/archderm.136.3.323. DOI: https://doi.org/10.1001/archderm.136.3.323. [DOI] [PubMed] [Google Scholar]
- 24.Gerdts B, Vloemans AF, Kreis RW. Toxic epidermal necrolysis: 15 years’ experience in a Dutch burns centre. J Eur Acad Dermatol Venereol. 2007 Jul;21(6):781–8. doi: 10.1111/j.1468-3083.2006.02082.x. DOI: https://doi.org/10.1016/j.burns.2006.10.196. [DOI] [PubMed] [Google Scholar]
- 25.Finkelstein Y, Soon GS, Acuna P, et al. Recurrence and outcomes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Pediatrics. 2011 Oct;128(4):723–8. doi: 10.1542/peds.2010-3322. DOI: https://doi.org/10.1542/peds.2010-3322. [DOI] [PubMed] [Google Scholar]
- 26.Palmieri TL, Greenhalgh DG, Saffle JR, et al. A multicenter review of toxic epidermal necrolysis treated in US burn centers at the end of the twentieth century. J Burn Care Rehabil. 2002 Mar-Apr;23(2):87–96. doi: 10.1097/00004630-200203000-00004. DOI: https://doi.org/10.1097/00004630-200103002-00040. [DOI] [PubMed] [Google Scholar]
- 27.Valeyrie-Allanore L, Roujeau JC. Epidermal Necrolysis (Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis) In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick’s dermatology in general medicine. 8th ed. New York, NY: The McGraw-Hill Companies, Inc; 2012. [Google Scholar]
- 28.Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: A severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000 Aug;115(2):149–53. doi: 10.1046/j.1523-1747.2000.00061.x. DOI: https://doi.org/10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 29.Kakourou T, Klontza D, Soteropoulou F, Kattamis C. Corticosteroid treatment of erythema multiforme major (Stevens-Johnson syndrome) in children. Eur J Pediatr. 1997 Feb;156(2):90–3. doi: 10.1007/s004310050561. DOI: https://doi.org/10.1007/s004310050561. [DOI] [PubMed] [Google Scholar]
- 30.Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg. 1986 Nov;204(5):503–12. doi: 10.1097/00000658-198611000-00001. DOI: https://doi.org/10.1097/00000658-198611000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paradisi A, Abeni D, Bergamo F, Ricci F, Didona D, Didona B. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014 Aug;71(2):278–83. doi: 10.1016/j.jaad.2014.04.044. DOI: https://doi.org/10.1016/j.jaad.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 32.Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: Definition of a new model based on translational research. Int J Dermatol. 2009 Aug;48(8):913–4. doi: 10.1111/j.1365-4632.2008.04020.x. DOI: https://doi.org/10.1111/j.1365-4632.2008.04020.x. [DOI] [PubMed] [Google Scholar]
- 33.Gubinelli E, Canzona F, Tonanzi T, Raskovic D, Didona B. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009 Mar;36(3):150–3. doi: 10.1111/j.1346-8138.2009.00616.x. DOI: https://doi.org/10.1111/j.1346-8138.2009.00616.x. [DOI] [PubMed] [Google Scholar]