Abstract
Background
Several studies have described the differences in electromyographic activity and histological changes of paravertebral muscles in patients with adolescent idiopathic scoliosis (AIS). However, there is little knowledge about the muscle volumetric and fatty infiltration imbalance of patients with AIS.
Material/Methods
Thirty-four patients with AIS were evaluated with standardized anteroposterior (AP) and lateral standing films for the location and direction of the apex of scoliosis, coronal Cobb angle, apex vertebra translation, and thoracic kyphosis; and with magnetic resonance imaging (MRI) scan of the spine at the level of T4–L1. The muscle volume and fatty infiltration rate of bilateral deep paravertebral muscles at the level of upper end, apex, and lower end vertebra were measured.
Results
All patients had major thoracic curve with apex of curves on the right side. The muscle volume on the convex side was larger relative to the concave side at the three levels, while the fatty infiltration rate was significantly higher on the concave side. The difference index of the muscle volume was significantly larger at the apex vertebra level than at the upper end vertebra level (p=0.002) or lower end vertebra level (p<0.001). The difference index of muscle volume correlated with apex vertebra translation (r=−0.749, p=0.032), and the difference index of fatty involution correlated with apex vertebra translation (r=0.727, p=0.041) and Cobb angle (r=0.866, p=0.005).
Conclusions
Our findings demonstrated significant imbalance of muscle volume and fatty infiltration in deep paravertebral muscles of AIS patients. Moreover, these changes affected different vertebra levels, with the most imbalance of muscle volume at the apex vertebra. We interpreted this as morphological changes corresponding with known altered muscle function of AIS.
MeSH Keywords: Magnetic Resonance Imaging, Muscular Atrophy, Paraspinal Muscles, Scoliosis
Background
Adolescent idiopathic scoliosis (AIS) is the most common pediatric musculoskeletal disorder that affects the spine and is characterized by a three-dimensional deformity of the spine, and altering coronal and sagittal profiles. Affected individuals are at risk for increasing deformity until growth ceases [1]. Brace treatment is the most common non-operative intervention for the prevention of curve progression [2], but the rate of treatment success is only 72% [3]. Curves larger than 50 degrees usually indicate the need for surgery. Numerous studies have proposed a broad variety of theories to elucidate the etiologies and pathogenesis behind AIS, including genetic predisposition [4], neuromuscular dysfunction [5], and hormonal and environmental factors [6].
Trunk muscle imbalance is one of the most important factors in the onset and progression of AIS [7]. Authors have postulated a muscle balancing/tuning theory to explain the mechanism of muscle imbalance causing progression of AIS [8]. Moreover, there are some interesting study findings that have shown that some AIS risk loci, identified by genetic study, occurred in regions near or within genes associated with muscle biogenesis [9,10]. Since many authors focused on the differences in electromyographic activity [11,12] and histological changes [13] of paravertebral muscle in patients with AIS, there is limited research on muscle volumetric and fatty infiltration imbalance of patients with AIS. Whether the changes of muscular morphology occur in all of the levels of vertebrae involved in the major curve remains unresolved.
The objective of this study was to measure the muscular volume and fatty infiltration rate of paravertebral muscles in different vertebra levels of AIS patients and to analyze the relationship between the degree of imbalance and several radiographic parameters, such as the apical vertebral translation and coronal Cobb angle, which would provide a better understanding of mechanisms underlying the development and progression of AIS.
Material and Methods
This was a prospective cross-sectional study on 34 consecutive AIS patients with primary thoracic scoliosis (Lenke I–IV) identified at the Forth Spine Department of Shanghai Changzheng Hospital during the period from January 2014 to September 2016, following institutional review board approval. Inclusion criteria were as follows: coronal thoracic Cobb angle was over 40° or there was an increase of more than 5° per year. Patients were excluded from the study if had identified Lenke V, VI or other types of scoliosis (congenital, neurological, etc.); received previous treatment for AIS; had a history of spine surgery; or had other pathological conditions involving the spine or paravertebral muscle.
Demographic information was obtained from the patients’ electronic medical records. Standardized anteroposterior (AP) and lateral standing films were obtained. On each AP radiograph the following parameters were measured besides main thoracic coronal Cobb angle: the thoracic apical vertebral translation (AVT), which is the distance between the apical thoracic vertebra of the curve and the C7 vertebra plumb line; the coronal balance (CB), which is the distance between the C7 vertebra plumb line and the central sacral vertical line; the thoracic kyphosis (TK) was measured on lateral radiographs for each patient and was defined as the angle between the superior endplate of T4 and the inferior endplate of T12.
The MRI system used in this study was a 3.0 tesla imaging system (Achieva Nova Dual; Philips Medical Systems, Hamburg, Germany). A sagittal sequence was performed with the following sequences: T1-weighted turbo spin echo SE (TR 550 ms, TE 12 ms) and T2-weighted turbo SE (TR 4,000 ms, TE 120 ms). With the same sequences, transverse images were acquired at the level from T4–L1, where the major thoracic scoliosis curvature in AIS patients is usually located, with each slice of 4 mm section thickness, a 180×180 mm field of view, and a 512×512 matrix per level.
To explore the imbalance of the paravertebral muscles, the muscle volume of deep paravertebral muscles at the level of the apical vertebra, upper end vertebra, and lower end vertebra was calculated. Because the paravertebral muscle is too small in the thoracic region to measure, and it is difficult to isolate one specific muscle from the other, we grouped the deepest muscle layer of the thoracic spine, including the thoracic multifidus, semispinalis, and rotator muscles, and referred to them collectively as the deep thoracic paravertebral muscle.
Transverse images were parallel to the vertebra at the apex, but not at the level of upper end or lower end vertebra because of the Cobb angle, which could influence the calculation of the muscle volume at these levels. Thus we made the normalized curves as mentioned by Zoalbi et al. [14]. After normalization, the bilateral cross-sectional area (CSA) of the deep paravertebral muscles were measured by outlining the fascial boundary of the muscle manually using the Image J ver.1.3 software. The CSAs of eight consecutives slices in T1 sequence, approximately the thickness of a vertebra, were used to get a volume associated with each vertebral level. The muscle volume was obtained by multiplication of its CSA with the slice thickness. The fatty infiltration rate was measured using a pseudo-coloring technique as mentioned by Lee et al. [15].
To facilitate the correlation test of the degree of muscle imbalance with radiographic parameters, the difference indexes of muscle volume and fatty infiltration rate were calculated as follows:
Statistical analysis
Continuous data were summarized as means with standard deviation (SD). For pair-wise comparisons between the convex and concave side, either a one-sample t-test or one-sample Wilcoxon test was used. One-way ANOVA test or SNK test were used for comparison of difference index of muscle volume and fatty infiltration rate within different levels. Pearson correlation test was used to analysis the correlation between the difference index and the Cobb angle, age, TK, AVT, and CB. Values of p less than 0.05 were considered statistically significant.
Results
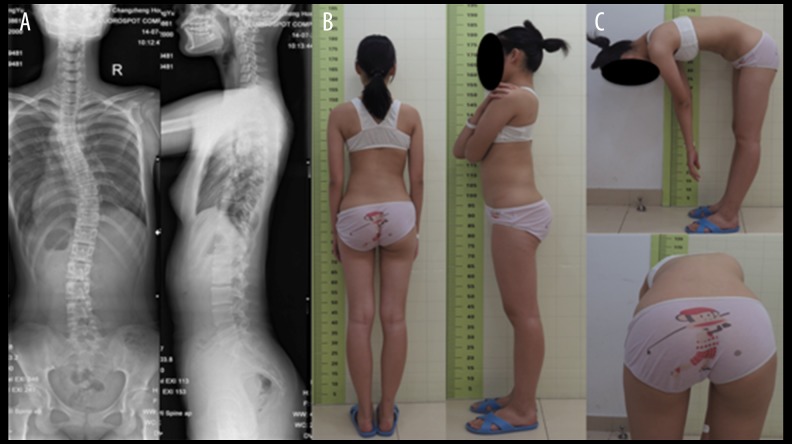
A total of 34 patients (28 females, 6 males) with an average age of 14.3 years (range 11–17 years) were included in this study. Based on Lenke’s classification, there were 18 type I, 7 type II, 6 type III, and 3 type IV (Figure 1). All patients demonstrated thoracic convexity to the right and the mean Cobb angle of the major thoracic curve in coronal plane was 48.2° (range from 28.3° to 65.8°). The upper end vertebrae involved in the major thoracic curve ranged from T4–T7, while the lower end vertebrae ranged from T9–L1 (Table 1).
Figure 1.
(A) PA radiograph of a 16-year-old girl with AIS showing a major thoracic curve of 43.5 degree. (B) Lateral radiograph showing thoracic hypokyphosis. (C) Clinical photograph of the same patient showing right thoracic prominence and slight right trunk shift.
Table 1.
Distribution of the upper end and lower end vertebrae of the primary thoracic curves.
| Upper end vertebrae | Lower end vertebrae | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T4 | T5 | T6 | T7 | T9 | T10 | T11 | T12 | L1 | |
| I | 7 | 5 | 3 | 3 | 3 | 4 | 5 | 3 | 3 |
| II | 0 | 1 | 6 | 0 | 0 | 0 | 1 | 2 | 4 |
| III | 3 | 2 | 0 | 1 | 1 | 2 | 2 | 0 | 1 |
| IV | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
I, II, III and IV – Lenke classification. Upper end vertebrae ranged from T4–T7. Lower end vertebrae ranged from T9–L1.
For both sides, the muscle volume increased from upper end vertebra to lower end vertebra. And the muscle volume was significantly larger on the convex side rather than that on the concave side at the three levels studied (Table 2, Figure 2). The fatty infiltration rate showed a trend of increasing in the lower level vertebra. Different from the result of the muscle volume, significantly higher fatty infiltration rate was found on the concave side of the scoliosis curve (Table 3).
Table 2.
Muscle volume of paravertebral muscle in concave and convex side in AIS patients at different levels.
| Mean of muscle volumeconcave (mm3) | Mean of muscle volumeconvex (mm3) | Mean of muscle volume index (%) | p Value | |
|---|---|---|---|---|
| Upper end vertebra | 2171.9±76.3 | 2303.9±77.6 | 0.95±0.06 | 0.000* |
| Apical vertebra | 2406.4±108.5 | 2474.4±102.4 | 0.99±0.02 | 0.011* |
| Lower end vertebra | 4574.5±112.1 | 4404.9±119.2 | 0.93±0.06 | 0.002* |
Significant if p<0.05.
Figure 2.
Example of a T1-weighted axial magnetic resonance image from the same patient in Figure 1 at the level of the upper end vertebra (A), the apex vertebra (B) and the lower end vertebra (C) used for calculating the cross-sectional area of the multifidus and spinal erectors.
Table 3.
Fatty infiltration rate of paravertebral muscle in concave and convex side in AIS patients at different levels.
| Mean of fatty infiltration rateconcave (%) | Mean of fatty infiltration rateconvex (%) | Mean of fatty infiltration rate index (%) | p Value | |
|---|---|---|---|---|
| Upper end vertebra | 10.8±5.4 | 7.7±5.9 | 1.02±1.51 | 0.008* |
| Apical vertebra | 17.5±7.5 | 13.5±7.4 | 0.93±0.77 | 0.044* |
| Lower end vertebra | 26.7±8.7 | 21.3±6.9 | 0.89±0.49 | 0.005* |
Significant if p<0.05.
To find the differences in degree of the muscle imbalance in the three levels, we compared the difference index of the muscle volume and fatty infiltration rate (Table 4). The difference index of muscle volume was significantly larger at the apex of the curvature level than at the upper end vertebra level or lower end vertebra level (p=0.002 and p=0.000, respectively). The difference index of muscle volume was not significant statistically between the upper end and lower end vertebra level. However, the difference index of fatty infiltration rate was similar within the three levels.
Table 4.
Comparison of the difference index of muscle volume and fatty infiltration rate within different levels.
| Difference index of muscle volume | Difference index of fatty infiltration rate | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% confidence interval of the difference | 95% confidence interval of the difference | |||||||
| Mean | Upper | Lower | p Value | Mean | Upper | Lower | p Value | |
| Upper vs. apex | −0.0402 | −0.0156 | −0.0648 | 0.002* | 0.0896 | −0.4014 | −0.5805 | 0.718 |
| Upper vs. lower | 0.0174 | 0.0072 | −0.0421 | 0.163 | 0.1314 | 0.3596 | −0.6223 | 0.597 |
| Apex vs. lower | 0.0576 | 0.0823 | 0.0330 | 0.000* | 0.0418 | 0.5328 | −0.4492 | 0.866 |
Upper – upper end vertebra; Apex – apex vertebra; Lower – lower end vertebra; Upper vs. apex – compare the difference index of the upper end vertebra level with that of apex vertebra level with SNK test;
significant if p<0.05.
In the correlation test, the difference index of muscle volume at the apex level was negatively correlated with the apex vertebra translation (r=−0.749, p=0.032), but not with age, Cobb angle, thoracic kyphosis, or coronal balance (Table 5). The difference index of fatty infiltration rate was positively correlated with the apex vertebra translation (r=0.727, p=0.041) and Cobb angle (r=0.866, p=0.005).
Table 5.
Correlation Analysis of the difference index of muscle volume and fatty infiltration rate at the apical level with age, Cobb angle, apex vertebra translation, coronal balance, and thoracic kyphosis.
| Difference index of muscle volume | Difference index of fatty infiltration rate | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age | −0.112 | 0.792 | 0.605 | 0.112 |
| Cobb angle | −0.210 | 0.618 | 0.866 | 0.005* |
| AVT | −0.749 | 0.032* | 0.727 | 0.041* |
| CB | −0.668 | 0.070 | 0.293 | 0.482 |
| TK | −0.278 | 0.505 | −0.571 | 0.140 |
AVT – apex vertebra translation; CB – coronal balance; TK – thoracic kyphosis; r – Pearson correlation coefficient;
significant if p<0.05.
Discussion
The main finding of this study was that a magnetic resonance imaging of the paravertebral muscles revealed significant asymmetry in muscle volume and fatty infiltration with larger volume on the convexity of the scoliotic curves and higher fatty infiltration rate on the concave side. The muscle imbalance occurred not only at the apex of the AIS major curve, but also at the level of upper end and lower end vertebra, implying that the changes observed were universal at all levels of vertebrae involved in the major curve.
Fidler and Jowett [16] measured the length of multifidus muscle at the apex in IS patients during operation and found the multifidus muscle was shorter on the convex side. They explained this as a theory of primary muscular imbalance causing the spinal deformity, in which the muscle on the convex side with higher proportion of “slow twitch” fibers contracts and shortens as the deformity is produced. But they only included one cadaveric spine and two IS patients. Using MRI, Chan et al. [17] found hyperintense signal change on the concave side of the apex of the curve and thought that the concave side muscles were the morphologically abnormal ones. Some researchers have demonstrated the difference in the cross-sectional area (CSA) of paravertebral muscles in patients with degenerative lumbar scoliosis (DLS) [18]. The limitation of CSA is that the CSA itself is not adequate to represent the functional status of muscle due to the three-dimensional nature of scoliosis. Recently, Zapata et al. [19] measured the paravertebral muscle thickness by means of ultrasound imaging and showed significant differences in the muscle thickness on the concave side in mild curves of AIS. But none of the parameters mentioned above could capture the entire muscle asymmetry. So we evaluated the muscular volume to better present the changes of muscle morphology [20].
Findings related to muscle volume have been reported by Saka [21] who have observed increased muscle volume on the convex side in one idiopathic scoliosis patient. In another study, Zoalbi et al. [14] analyzed the MR images of 17 AIS patients with 25 scoliotic curves and indicated a larger muscle volume on the convex side at the apex level, but on some curves only. Because scoliosis developed in various regions (thoracic/thoracolumbar/lumbar) could have different impacts on the paravertebral muscle, we included patients with primary thoracic curves to minimize the deviations. In this study, the volume of paravertebral muscles on the convex side was larger relative to those on the concave side at the three levels.
Acaroglu et al. [22] found that tissue calmodulin concentrations of paravertebral muscles were significantly higher on the convex side in AIS patients. Calmodulin regulates the contractile properties of muscle cells, and its change in concentration implied a potential effect of paravertebral muscles on the progression of scoliosis. Stetkarova et al. [23] found increased proportion of type I muscle fiber on the convexity, which correlated significantly with higher progression of AIS curves. In addition, a larger EMG activity on the convex side at the apex level has been reported [24]. These changes were mostly interpreted as secondary adaption to higher load demand by the muscles on the convex side in AIS. Our findings showed an imbalanced muscle volume with predominance on the convexity at the apex level. The onset of spinal deformity in patients with AIS coincides with pubertal growth spurt. Normal myogenesis, as in childhood growth, is established by extensive hypertrophy of the muscle fibers, which requires the continuous activation, proliferation, and differentiation of satellite cells into new myonuclei [25]. We assumed for our study that when skeletal muscle on the convex side was stretched, satellite cells were activated to enter the cell cycle and contributed to the muscle hypertrophy.
On the other hand, Martinez et al. [26] found that different muscle groups including upper and lower limb muscles in AIS patients had function impairment, and they defined AIS as a primary and systemic muscle disorder which might lead to both spinal deformity and muscle dysfunction. In our study, paravertebral muscle imbalance was generalized among different levels in AIS patients, which supports the hypothesis that systemic factors may play an important role in the muscle changes associated with AIS, such as hormone [27] and inflammatory factors [28].
This study also revealed that the fatty infiltration rate was significantly higher on the concave side, supporting the load asymmetry theory aforementioned, as muscle atrophy was associated with increased fatty involution. Based on Wajchenberg’s [29] histochemical analysis of paravertebral rotator muscles from patients with AIS, fatty involution was considered to be significantly higher on the concavity of the curve, which was similar to our results. Recently, Stetkarova et al. [23] demonstrated that muscles of both sides of the curve in AIS were affected – the convex side by the increased proportion of type I fibers and the concave side by the lower proportion of type I fibers. The declined number of type I fibers and increased fatty involution on the concave side could be a result of muscle atrophy and degeneration. Moreover, our correlation test suggested that the difference index of muscle volume correlated significantly with apex vertebra translation, while the difference index of fatty involution correlated significantly with apex vertebra translation and the Cobb angle.
The rationale for many approaches to conservative management of scoliosis during skeletal growth assumes an important role of the paravertebral muscles in deformity progression. The compensatory role of the paravertebral muscle is substantiated by the partial straightening of the spinal column of AIS during nighttime using electric muscle stimulation [30]. Tamoxifen, a calmodulin antagonist, has been reported to be effective in decreasing the magnitude of the scoliosis in animal models [31]. Recently, physiotherapy treatment through straightening the paravertebral muscles has appeared advisable in children with the scoliosis angle of 10–25° [32]. Our study demonstrated the important role of paravertebral muscles on the progression of scoliosis, and implied that better muscle “re-straightening” programs could be developed to improve prognosis of progressive curves.
Conclusions
Our findings demonstrated a significant asymmetry in muscle volume and fatty infiltration with larger volume on the convex side of the scoliotic curves and higher fatty infiltration rate on the concave side. These changes might be morphological changes corresponding with known altered muscle function of AIS, and be important in understanding the pathogenesis as well as mechanisms of progression.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Hresko MT. Clinical practice. Idiopathic scoliosis in adolescents. New Engl J Med. 2013;368(9):834–41. doi: 10.1056/NEJMcp1209063. [DOI] [PubMed] [Google Scholar]
- 2.Misterska E, Glowacki M, Harasymczuk J. Brace and deformity-related stress level in females with adolescent idiopathic scoliosis based on the Bad Sobernheim Stress Questionnaires. Med Sci Monit. 2011;17(2):CR83–90. doi: 10.12659/MSM.881392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein SL, Dolan LA, Wright JG, Dobbs MB. Effects of bracing in adolescents with idiopathic scoliosis. New Engl J Med. 2013;369(16):1512–21. doi: 10.1056/NEJMoa1307337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi Y, Kou I, Takahashi A, et al. A genome-wide association study identifies common variants near LBX1 associated with adolescent idiopathic scoliosis. Nat Genet. 2011;43(12):1237–40. doi: 10.1038/ng.974. [DOI] [PubMed] [Google Scholar]
- 5.Wajchenberg M, Astur N, Kanas M, Martins DE. Adolescent idiopathic scoliosis: Current concepts on neurological and muscular etiologies. Scoliosis Spinal Disord. 2016;11:4. doi: 10.1186/s13013-016-0066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burwell RG, Dangerfield PH, Moulton A, Grivas TB. Adolescent idiopathic scoliosis (AIS), environment, exposome and epigenetics: A molecular perspective of postnatal normal spinal growth and the etiopathogenesis of AIS with consideration of a network approach and possible implications for medical therapy. Scoliosis. 2011;6(1):26. doi: 10.1186/1748-7161-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modi HN, Suh SW, Yang JH, et al. Spontaneous regression of curve in immature idiopathic scoliosis – does spinal column play a role to balance? An observation with literature review. J Orthop Surg Res. 2010;5:80. doi: 10.1186/1749-799X-5-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong C. Mechanism of right thoracic adolescent idiopathic scoliosis at risk for progression; A unifying pathway of development by normal growth and imbalance. Scoliosis. 2015;10:2. doi: 10.1186/s13013-015-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S, Londono D, Eckalbar WL, et al. A PAX1 enhancer locus is associated with susceptibility to idiopathic scoliosis in females. Nat Commun. 2015;6:6452. doi: 10.1038/ncomms7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Z, Tang NL, Xu L, et al. Genome-wide association study identifies new susceptibility loci for adolescent idiopathic scoliosis in Chinese girls. Nat Commun. 2015;6:8355. doi: 10.1038/ncomms9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farahpour N, Ghasemi S, Allard P, Saba MS. Electromyographic responses of erector spinae and lower limb’s muscles to dynamic postural perturbations in patients with adolescent idiopathic scoliosis. J Electromyogr Kinesiol. 2014;24(5):645–51. doi: 10.1016/j.jelekin.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Avikainen VJ, Rezasoltani A, Kauhanen HA. Asymmetry of paraspinal EMG-time characteristics in idiopathic scoliosis. J Spin Disord. 1999;12(1):61–67. [PubMed] [Google Scholar]
- 13.Ford DM, Bagnall KM, Clements CA, McFadden KD. Muscle spindles in the paraspinal musculature of patients with adolescent idiopathic scoliosis. Spine. 1988;13(5):461–65. doi: 10.1097/00007632-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Zoabli G, Mathieu PA, Aubin CE. Back muscles biometry in adolescent idiopathic scoliosis. Spine J. 2007;7(3):338–44. doi: 10.1016/j.spinee.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Lee JC, Cha JG, Kim Y, et al. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: Comparison with the normal controls. Spine. 2008;33(3):318–25. doi: 10.1097/BRS.0b013e318162458f. [DOI] [PubMed] [Google Scholar]
- 16.Fidler MW, Jowett RL. Muscle imbalance in the aetiology of scoliosis. J Bone Joint Surg Br. 1976;58(2):200–1. doi: 10.1302/0301-620X.58B2.932082. [DOI] [PubMed] [Google Scholar]
- 17.Chan YL, Cheng JC, Guo X, et al. MRI evaluation of multifidus muscles in adolescent idiopathic scoliosis. Pediatr Radiol. 1999;29(5):360–63. doi: 10.1007/s002470050607. [DOI] [PubMed] [Google Scholar]
- 18.Shafaq N, Suzuki A, Matsumura A, et al. Asymmetric degeneration of paravertebral muscles in patients with degenerative lumbar scoliosis. Spine. 2012;37(16):1398–406. doi: 10.1097/BRS.0b013e31824c767e. [DOI] [PubMed] [Google Scholar]
- 19.Zapata KA, Wang-Price SS, Sucato DJ, Dempsey-Robertson M. Ultrasonographic measurements of paraspinal muscle thickness in adolescent idiopathic scoliosis: A comparison and reliability study. Pediatr Phys Ther. 2015;27(2):119–25. doi: 10.1097/PEP.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 20.Zoabli G, Mathieu PA, Aubin C. Magnetic resonance imaging of the erector spinae muscles in Duchenne muscular dystrophy: Implication for scoliotic deformities. Scoliosis. 2008;3:21. doi: 10.1186/1748-7161-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saka K. [Biomechanical analysis of scoliosis and back muscles using CT evaluation and the finite element method]. Nihon Seikeigeka Gakkai Zasshi. 1987;61(4):299–310. [in Japanese] [PubMed] [Google Scholar]
- 22.Acaroglu E, Akel I, Alanay A, et al. Comparison of the melatonin and calmodulin in paravertebral muscle and platelets of patients with or without adolescent idiopathic scoliosis. Spine. 2009;34(18):E659–63. doi: 10.1097/BRS.0b013e3181a3c7a2. [DOI] [PubMed] [Google Scholar]
- 23.Stetkarova I, Zamecnik J, Bocek V, et al. Electrophysiological and histological changes of paraspinal muscles in adolescent idiopathic scoliosis. Eur Spine J. 2016;25(10):3146–53. doi: 10.1007/s00586-016-4628-8. [DOI] [PubMed] [Google Scholar]
- 24.Cheung J, Veldhuizen AG, Halberts JP, et al. Geometric and electromyographic assessments in the evaluation of curve progression in idiopathic scoliosis. Spine. 2006;31(3):322–29. doi: 10.1097/01.brs.0000197155.68983.d8. [DOI] [PubMed] [Google Scholar]
- 25.Tatsumi R. Mechano-biology of skeletal muscle hypertrophy and regeneration: Possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim Sci J. 2010;81(1):11–20. doi: 10.1111/j.1740-0929.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Llorens J, Ramirez M, Colomina MJ, et al. Muscle dysfunction and exercise limitation in adolescent idiopathic scoliosis. Eur Respir J. 2010;36(2):393–400. doi: 10.1183/09031936.00025509. [DOI] [PubMed] [Google Scholar]
- 27.Qiu Y, Wu L, Wang B, et al. Asymmetric expression of melatonin receptor mRNA in bilateral paravertebral muscles in adolescent idiopathic scoliosis. Spine. 2007;32(6):667–72. doi: 10.1097/01.brs.0000257536.34431.96. [DOI] [PubMed] [Google Scholar]
- 28.Samaan MC, Missiuna P, Peterson D, Thabane L. Understanding the role of the immune system in adolescent idiopathic scoliosis: Immunometabolic CONnections to Scoliosis (ICONS) study protocol. BMJ Open. 2016;6(7):e011812. doi: 10.1136/bmjopen-2016-011812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wajchenberg M, Martins DE, de Luciano RP, et al. Histochemical analysis of paraspinal rotator muscles from patients with adolescent idiopathic scoliosis: A cross-sectional study. Medicine. 2015;94(8):e598. doi: 10.1097/MD.0000000000000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axelgaard J. Transcutaneous electrical muscle stimulation for the treatment of progressive spinal curvature deformities. Int Rehabil Med. 1984;6(1):31–46. doi: 10.3109/09638288409166968. [DOI] [PubMed] [Google Scholar]
- 31.Akel I, Kocak O, Bozkurt G, et al. The effect of calmodulin antagonists on experimental scoliosis: A pinealectomized chicken model. Spine. 2009;34(6):533–38. doi: 10.1097/BRS.0b013e31818be0b1. [DOI] [PubMed] [Google Scholar]
- 32.Bialek M. Conservative treatment of idiopathic scoliosis according to FITS concept: presentation of the method and preliminary, short term radiological and clinical results based on SOSORT and SRS criteria. Scoliosis. 2011;6:25. doi: 10.1186/1748-7161-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]