Abstract
Background
Although platinum-based chemotherapy is the most effective strategy for esophageal cancer, toxicity and drug resistance limit the dose administration and the application of chemotherapy. Capilliposide C (CPS-C) is isolated from the Chinese herb Lysimachia capillipes Hemsl and is approved to be effective against carcinomas. However, the activity of CPS-C against esophageal cancer remains unclear. The present study was conducted to assess the chemosensitizing effects of CPS-C for enhancing the therapeutic efficacy of oxaliplatin in esophageal squamous carcinoma cells and explore the underlying mechanism.
Material/Methods
Human esophageal squamous cell carcinoma (ESCC) TE-1 and TE-2 were used. Several in vitro and in vivo analyses were carried out, including MTT, Annexin V/PI, Western blot, and TUNEL and immunohistochemistry in a xenograft model.
Results
CPS-C significantly enhanced the proliferative inhibition and apoptotic effect of oxaliplatin in ESCC cells. Oxaliplatin combined with CPS-C decreased the expressions of PI3K, phospho-Akt, phospho-mTOR, Bcl-2, and Bcl-XL, and increased the expression of Bax and caspase-3 significantly compared to oxaliplatin-only treatment. Furthermore, in the ESCC xenograft model, CPS-C significantly enhanced the anti-cancer effects and apoptosis of oxaliplatin.
Conclusions
The results indicated that CPS-C enhanced the anti-proliferative and apoptotic effect of oxaliplatin by modulating the PI3K/Akt/mTOR pathway on ESCC in vitro and in vivo.
MeSH Keywords: Apoptosis, Esophageal Neoplasms, Phosphatidylinositol 3-Kinases, Saponins
Background
Esophageal cancer (EC) is one of the most rapidly growing causes of cancer mortality and remains the 8th most common cancer and the 6th most common cause of death from cancer worldwide [1]. EC is mainly classified into two types: squamous cell carcinoma (SCC) and esophageal adenocarcinoma (EAC). SCC is the main histological type of EC and accounts for more than 90% of all cases [2]. Despite the developments in the management of esophageal squamous cell carcinoma (ESCC), more than 60% patients will suffer tumor recurrence and systemic metastasis within 5 years. The overall median survival is 9 months (95% CI: 8.9–9.1), with an overall 5-year survival rate of 15.5%. Patients with SCC have worse survival outcomes than those with EAC [3]. Chemotherapy is the main treatment for metastatic diseases and can improve quality of life. Cisplatin and 5-FU are the most effective agents and have been extensively studied in ESCC for decades. However, they are also associated with significant toxicity. Several trials have suggested that oxaliplatin is equally or more effective but less toxic than cisplatin [4,5]. Oxaliplatin is another promising agent and is used with increasing frequency in ESCC [6–10]. Although platinum-based chemotherapy inhibits ESCC cell growth, it leads to severe side effects including nausea, vomiting, diarrhea, and suppression of the hemopoietic system. Therefore, less toxic treatments and more effective strategies are needed. Natural products are suitable alternatives as sensitizers, and increasing numbers of research studies have focused on agents extracted from natural resources [11–16].
Capilliposide C (CPS-C) (Figure 1), isolated from the Chinese herb Lysimachia capillipes Hemsl (L. capillipes), is a novel oleanane triterpenoid saponin and is approved to be effective against a variety of cancers, including gastric cancer, prostate cancer, non-small cell lung cancer, nasopharyngeal cancer, ovarian cancer, and so on[17–20]. As a natural plant extract, CPS-C has fewer side effects and its mechanism is related to inducing apoptosis, down-regulation of Bcl-2, JNK, and P38a/b, up-regulation of Bax, p-JNK, and p-P38, and increasing the PUMA-Bax pathway [17–20]. However, the mechanism underlying its anti-tumor effects in ESCC and how to obtain optimum therapeutic modality still remain unclear. Therefore, the aim of the present study was to investigate the synergism between CPS-C and oxaliplatin in ESCC and elucidate the underlying molecular mechanism. The results of our study suggested that CPS-C serves as a novel anti-tumor agent to sensitize ESCC cells to oxaliplatin and that the combination therapy of CPS-C and oxaliplatin may be beneficial for patients with ESCC.
Figure 1.
Chemical structure of CPS-C.
Material and Methods
Chemicals and reagents
CPS-C was obtained from Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College (Peking, China). Oxaliplatin was purchased from Sigma-Aldrich Co. (St. Louis, Missouri, USA). MTT was purchased from Sigma-Aldrich Co. (St. Louis, Missouri, USA). The Annexin V Apoptosis Detection kit was purchased from BD Biosciences (Franklin Lakes, New Jersey, USA). PI3 kinase p110α (C73F8) rabbit mAb (#4249), phospho-Akt (Ser473) (D9E) rabbit mAb (#4060), phospho-mTOR (Ser2448) (D9C2) rabbit mAb (#5536), phospho-mTOR (Ser2448) (49F9) rabbit mAb (IHC Specific) (#2976), Bcl-2 (124) mouse mAb (#15071), Bcl-xL (54H6) rabbit mAb (#2764), Bax (D2E11) rabbit mAb (#5023), and caspase-3 antibody (#9662) were purchased from Cell Signaling Technology (Danvers, Massachusetts, USA). Anti-GAPDH was purchased from Santa Cruz Biotechnology, Inc. (Dallas, Texas, USA). The In Situ Cell Death Detection Kit was purchased from Sigma-Aldrich Co. (St. Louis, Missouri, USA).
ESCC cell lines
Two cell lines (TE-1 and TE-2) that were established from a well or poorly differentiated human ESCC [21] and the human esophageal epithelial cell line Het-1A [22] were obtained from Shanghai Institute of Cell Biology (Shanghai, China). TE-1 and TE-2 cell lines were cultured in RPMI 1640 (GE Healthcare Life Sciences, Logan, Utah, USA) and Het-1A cells were cultured in BEGM (GE Healthcare Life Sciences, Logan, Utah, USA) supplemented with 10% fetal bovine serum (GE Healthcare Life Sciences, Logan, Utah, USA) at 37°C with humidified 5% CO2.
MTT assay
The antiproliferative effects of CPS-C and oxaliplatin were assessed using a MTT assay. TE-1 and TE-2 cells were seeded and then treated with CPS-C (from 0.0625 to 1 μg/mL). Absorbances at a wavelength of 450 nm as the OD value were detected, and dose-dependent curves were generated. Further, TE-1 and TE-2 cells were treated with oxaliplatin (from 1 to 256 μM) and CPS-C (1 μg/mL) to generate curves and calculate 50% inhibition concentration (IC50) values.
Apoptosis assay
Apoptosis was quantified using the Annexin V/PI method. TE-1 and TE-2 cells were treated with oxaliplatin (16 μM) with or without CPS-C (1 μg/mL). Cells were harvested, trypsinized, and washed with phosphate-buffered saline (PBS) after treatment for 48 h. Then the cells were stained with the Annexin V Apoptosis Detection kit prior to being detected by flow cytometry (Beckman Coulter, Inc., Brea, California, USA).
Western blot analysis
TE-1 and TE-2 cells were treated with oxaliplatin (16 μM) with or without CPS-C (1 μg/mL) for 48 h. Cells were lysed. and total protein was extracted by cell lysis buffer containing Tris-HCl and Triton X-100. Cell lysate proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose membranes (Millipore, Billerica, Massachusetts, USA). Membranes were blocked with 5% non-fat milk in TBST, and then incubated overnight at 4°C with primary antibodies (rabbit anti-rat PI3K, phospho-Akt, phospho-mTOR, Bcl-2, Bcl-xL, Bax, caspase-3 monoclonal antibodies, and mouse anti-rat GAPDH monoclonal antibody [1: 1,000]). Membranes then were reacted with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibodies for 30 min at room temperature. Proteins were visualized with enhanced chemiluminescence reagent and detected using ImageQuant LAS 4000 (Pittsburgh, Pennsylvania, USA).
Animal treatment
BALB/c nu/nu mice were obtained from and housed at the Laboratory Animal Center of Zhejiang University (Hangzhou, China).The animal care and experimental protocols were carried out according to the guidelines established by National Institutes of Health. The protocol was approved by the Hangzhou Cancer Hospital Research Ethics Committee. The mouse model of xenograft tumor was established by subcutaneously injecting TE-2 cells. Twenty-four tumor-bearing nude mice (male, aged 4 weeks, weighing 19–21 g) were randomly divided into four groups: control group (intraperitoneal injection with 0.1 mL of normal saline twice a week); CPS-C group (intraperitoneal injection with 0.1 mL of CPS-C [1 mg/kg] twice a week); oxaliplatin group (intraperitoneal injection with 0.1 mL of oxaliplatin [5 mg/kg] twice a week); and CPS-C + oxaliplatin group (intraperitoneal injection with 0.1 mL of CPS-C [1 mg/kg] and 0.1 ml oxaliplatin [5 mg/kg] twice a week). Tumor volume and weight were evaluated. and mice were sacrificed after 7 weeks. Tumor samples were obtained and fixed in 4% formalin fixative, and then were embedded in paraffin.
TUNEL analysis and immunohistochemistry
The terminal deoxynucleotidyl transferase-dUTP nick end labeling (TUNEL) method was used to detect apoptosis with an in situ cell death detection kit according to the manufacturer’s instructions. Reactions were examined under an Olympus BX61 fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Paraffin was removed from the paraffin sections by using xylene, 100% ethanol, 95% ethanol, 80% ethanol, and distilled water. After antigen retrieval by microwave heating in citrate buffer for a 5-min cycle, tissue sections were incubated with protein blocking solution and washed with PBS while switching from one step to other. The Expose Mouse and Rabbit Specific HRP/DAB kit was used to perform immunostaining according to the manufacturer’s instructions (Abcam, Cambridge, UK). Tissue sections then were incubated with primary antibodies for 1 h at ambient temperature, followed by incubation with HRP conjugate, and then were incubated with the DAB substrate for 10 min and counterstained with Mayer’s hematoxylin for 2 min.
Statistical analysis
Data were expressed as the mean ±SD. SPSS 19.0 (SPSS Inc., Chicago, Illinois, USA) was used to perform statistical analysis. Student’s t-test was used to analyze two groups, and one-way ANOVA was used to analyze multiple groups. P values <0.05 were considered statistically significant.
Results
CPS-C enhances proliferative inhibition effect of oxaliplatin in ESCC cells
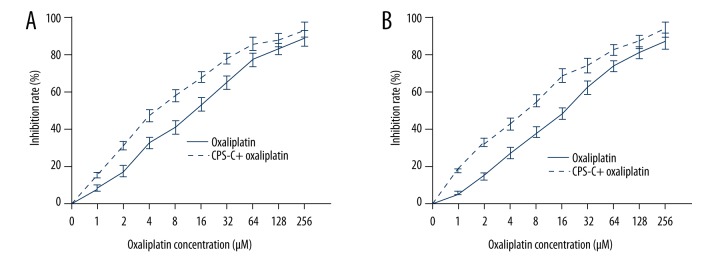
In order to explore the anti-tumor and chemosensitizing activities of CPS-C, the MTT assay was used to evaluate the effect of CPS-C in normal human esophageal epithelial cell line Het-1A and ESCC cell lines TE-1 and TE-2. The results showed that CPS-C did not have an anti-proliferative effect in esophageal epithelial cell line Het-1A with increasing concentrations. CPS-C also displayed no cytotoxicity in ESCC cell lines TE-1 and TE-2 at concentrations less than 1 μg/mL (Figure 2). TE-1 and TE-2, which were ESCC cell lines with different malignant potentials, were treated with CPS-C and oxaliplatin. The results showed that oxaliplatin (from 1 to 256 μM) inhibited the proliferation of ESCC cell lines in a dose-dependent manner (IC50 values for TE-1 and TE-2 cells at 48 h were 14.90±1.26 μM and 20.39±1.74 μM, respectively) and that the proliferative inhibition effect of oxaliplatin significantly increased when combined with CPS-C (1 μg/mL), with IC50 values decreasing to 5.43±0.63 μM and 6.64±0.91 μM for TE-1 and TE-2 cells at 48 h, respectively (Figure 3A, 3B).
Figure 2.
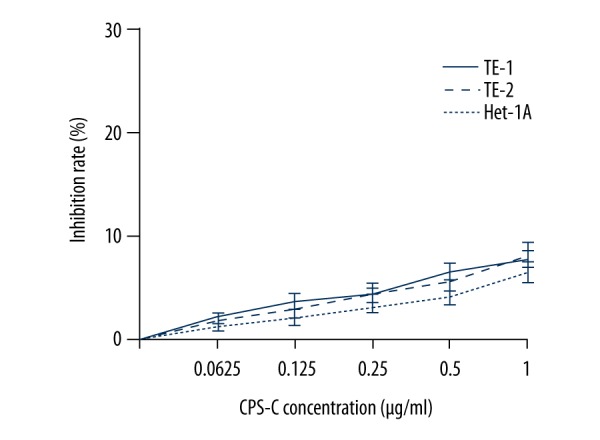
Anti-proliferative activity of CPS-C in normal human esophageal epithelial cell line Het-1A and ESCC cell lines TE-1 and TE-2.
Figure 3.
CPS-C enhances oxaliplatin sensitivity in ESCC cell lines TE-1 (A) and TE-2 (B), as determined by MTT assay. Inhibition rates were significantly increased in the CPS-C + oxaliplatin treatment group compared with the oxaliplatin group (P<0.01) at 48 h and were dose dependent.
CPS-C enhances apoptosis induced by oxaliplatin in ESCC cells
To evaluate the effect of CPS-C on apoptosis induced by oxaliplatin in ESCC cells, the Annexin V-FITC assay was performed. The results showed that oxaliplatin induced significant apoptosis compared to the control group (18.6±2.43% and 23.4±2.60% for TE-1 and TE-2 cells, respectively, vs. 2.83±0.91% and 3.17±1.14%) and that the apoptosis rates increased significantly when oxaliplatin was combined with CPS-C 50 μg/mL (33.7±2.98% and 37.4±3.15% for TE-1 and TE-2 cells, respectively) at 48 h (Figure 4).
Figure 4.
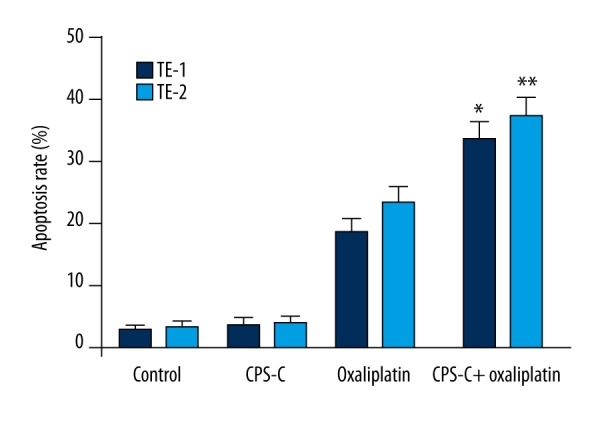
CPS-C enhances oxaliplatin-induced apoptosis in ESCC cell lines TE-1 and TE-2. *, ** Statistically significant difference (P<0.01) between the CPS-C + oxaliplatin treatment group and the oxaliplatin treatment group in TE-1 and TE-2, respectively.
CPS-C enhances proliferative inhibition and apoptosis effects of oxaliplatin by modulating the PI3K/Akt/mTOR pathway
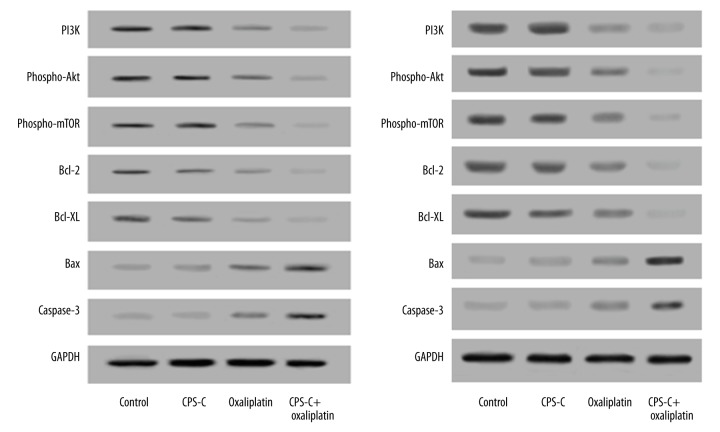
The expression levels of key regulator proteins in the PI3K/Akt/mTOR pathway including PI3K, phospho-Akt, phospho-mTOR, Bcl-2, Bcl-XL, Bax, and caspase-3 in ESCC cells treated by oxaliplatin with or without CPS-C for 48 h were detected by Western blotting. The results showed that oxaliplatin combined with CPS-C decreased the expressions of PI3K, phospho-Akt, phospho-mTOR, Bcl-2, and Bcl-XL, but increased the expression of Bax and caspase-3 significantly compared to oxaliplatin-only treatment, while no differences were observed in either the CPS-C group or the control group in TE-1 (Figure 5A) and TE-2 (Figure 5B) cells, respectively.
Figure 5.
CPS-C increases oxaliplatin-induced changes in protein levels of the PI3K/Akt/mTOR pathway in ESCC cell lines TE-1 (A) and TE-2 (B). Protein levels were detected using Western blotting analysis. Oxaliplatin combined with CPS-C decreased the expressions of PI3K, phospho-Akt, phospho-mTOR, Bcl-2, and Bcl-XL, but increased the expression of Bax and caspase-3 significantly compared to oxaliplatin-only treatment, while no differences were observed in either the CPS-C group or the control group (P>0.05).
CPS-C enhances the anti-cancer effects and apoptosis of oxaliplatin in the ESCC xenograft model
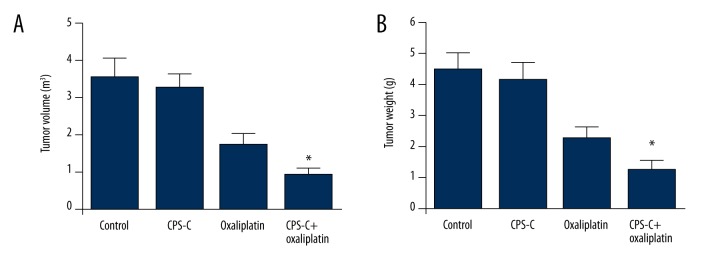
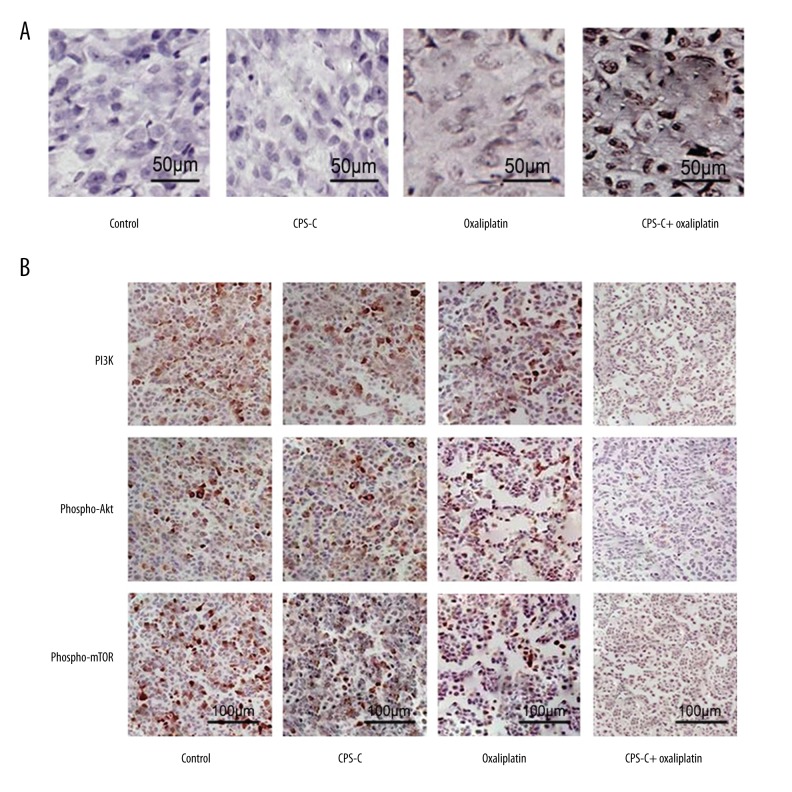
We further determined the activity of β-elemene combined with oxaliplatin in TE-2 cells in vivo. The results showed that tumor volumes and weight in the CPS-C + oxaliplatin group were reduced significantly compared to the oxaliplatin-only group, while no differences were observed in either the CPS-C group or the control group (Figure 6A, 6B). TUNEL was used to analyze the apoptosis induced by CPS-C and oxaliplatin in vivo. Positively labeled cells were counted to determine the distribution of apoptotic cells in random microscopic fields. The apoptotic index of the control group was 0.48±0.17 and that of the CPS-C group was 0.65±0.44, while the apoptotic index of the oxaliplatin group was 4.81±0.63 and that of the CPS-C + oxaliplatin group was 8.60±0.52. There was a statistically significant difference between the oxaliplatin group and the CPS-C + oxaliplatin group (P<0.01) (Figure 7A). Immunohistochemical analysis was performed to verify the molecular mechanism of CPS-C in sensitizing the anti-cancer activity of oxaliplatin through the PI3K/Akt/mTOR pathway in vivo. The expressions of PI3K, phospho-Akt, and phospho-mTOR were decreased significantly in the CPS-C + oxaliplatin group compared to the oxaliplatin-only group (Figure 7B).
Figure 6.
The antitumor effects of oxaliplatin combined with CPS-C on tumor growth in the xenograft model in nude mice of TE-2 cells. Tumor volumes (A) and weight (B) in the model mice. * Statistically significant difference (P<0.01) between the CPS-C + oxaliplatin group and oxaliplatin-only group. No differences were observed in either the CPS-C group or the control group (P>0.05).
Figure 7.
The effects of oxaliplatin combined with CPS-C on apoptosis and PI3K/Akt/mTOR protein expressions in tumor tissues. (A) Representative examples of TE-2 tumor xenografts stained with apoptosis by TUNEL assay (400× magnification). (B) Representative examples of immunohistochemistry staining for PI3K, phospho-Akt, and phospho-mTOR protein from TE-2 xenografts (400× magnification).
Discussion
Chemotherapy has been applied to treat EC for more than 30 years. Although novel targeted drugs have been shown to be active against EC, platinum-based regimens remain the most active standard protocol and the mainstay of typical combination chemotherapy. Cisplatin is the most effective agent for EC, but its high toxicity leads to limitations in dose administration. Further, its efficacy also is limited owing to the development of drug resistance in tumor cells. Oxaliplatin [(trans-R,R)1,2-diaminocyclohexaneoxalatoplatinum (II), C8H14N2O4Pt] is equally or more effective but less toxic than cisplatin, and is a promising agent for combination chemotherapy in treating ESCC. However, the side effects of chemotherapy and drug resistance still seriously limit its wide application.
CPS-C has been shown to have comprehensive anti-tumor effects on numerous cancer types and low toxicity in patients, although its molecular mechanism remains unclear. Recently, there has been increasing interest in this agent for treatment of carcinomas. Previous studies showed that CPS-C inhibited prostate cancer cell lines PC3 and DU145 by inducing apoptosis through activating the MAPK pathway, decreasing P38 and JNK, but increasing p-P38, p-JNK, and ROS [17]. Another study demonstrated that CPS-C inhibited and induced the apoptosis of CNE-2 cells via increasing the PUMA-Bax pathway [18]. Also, CPS-C exerts an anti-tumor effect on non-small cell lung cancer cell lines through inducing ROS generation, cell cycle arrest, and apoptosis [19].
In the present study, we evaluated the combination effect of CPS-C and oxaliplatin on ESCC in vitro and in vivo. The results indicated the pharmacologic advantage of CPS-C in enhancing oxaliplatin-induced cytotoxicity, but found little effect of CPS-C on ESCC and normal human esophageal epithelial cells. We investigated the mechanisms by which CPS-C increases oxaliplatin sensitivity in ESCC and found that CPS-C promoted the effect of oxaliplatin by inducing apoptosis significantly and modulating the PI3K/Akt/mTOR pathway. Apoptosis is one of the most important processes in cell proliferation and a key molecular mechanism of anticancer therapies. Therefore, modulation of apoptosis is critical for the pharmacological strategy of chemotherapeutic agents. The PI3K/Akt/mTOR signaling pathway is involved in cell proliferation, differentiation, survival, apoptosis, and metastasis [23,24]. Overexpression of PI3K and the PI3K signaling pathway may cause cancer [25,26]. Akt is also a key regulator of cell growth that can regulate antiapoptotic proteins and cell proliferation [27]. Akt increases the phosphorylation of Bax and inactivates Bad, leading to inhibition of apoptosis [28]. mTOR increases protein translation by activation of the Akt and the Akt/mTOR signaling pathway through a multistep mechanism [29]. The Bcl-2 family including anti-apoptotic proteins, such as Bcl-2, Bcl-xL, and p-Bad, and proapoptotic proteins, such as Bad and Bax, is the main controller and regulates mitochondria-dependent apoptosis [30]. The Bcl-2/Bax ratio is considered to be a critical factor for resistance to apoptosis [31]. Caspases are cysteine proteases that mediate apoptotic cell death, and they are activated during apoptosis. Caspase-3, which is a downstream caspase, represents the most important member in the caspase family. Caspase-3 activates Bax and then releases cytochrome C, leading to the cleavage of caspase-9 [32]. Interaction between caspase-9 and caspase-3, caspase-6, and caspase-7 activates the latter capases, and finally cell apoptosis [33]. Our results showed that CPS-C promoted oxaliplatin-induced apoptosis by decreasing the expressions of PI3K, phospho-Akt, phospho-mTOR, Bcl-2, and Bcl-XL and increasing the expression of Bax and caspase-3.
Conclusions
We found that CPS-C enhanced the anti-proliferative and apoptotic effect of oxaliplatin by modulating the PI3K/Akt/mTOR pathway on ESCC in vitro and in vivo. This therapeutically effective combination is a novel, safe, and practical form of chemotherapy and results in a more powerful anti-ESCC effect.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Source of support: This study was supported by a grant from the National Natural Science Foundation of China (grant no. 81303274)
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Higuchi K, Koizumi W, Tanabe S, et al. Current management of esophageal squamous-cell carcinoma in japan and other countries. Gastrointest Cancer Res. 2009;3:153–61. [PMC free article] [PubMed] [Google Scholar]
- 3.Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in united states adults from 1973 to 2009: A seer database analysis. J Gastroenterol Hepatol. 2016;31:1141–46. doi: 10.1111/jgh.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362:858–59. doi: 10.1056/NEJMc0911925. [DOI] [PubMed] [Google Scholar]
- 5.Montagnani F, Turrisi G, Marinozzi C, et al. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: A systematic review and meta-analysis. Gastric Cancer. 2011;14:50–55. doi: 10.1007/s10120-011-0007-7. [DOI] [PubMed] [Google Scholar]
- 6.Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: Trio-013/logic – a randomized phase iii trial. J Clin Oncol. 2016;34:443–51. doi: 10.1200/JCO.2015.62.6598. [DOI] [PubMed] [Google Scholar]
- 7.Petrioli R, Francini E, Roviello F, et al. Treatment of advanced oesophagogastric cancer with folfox-4 regimen followed by leucovorin/bolus and continuous infusion 5-fu as maintenance chemotherapy in patients aged ≥75 years with impaired performance status. J Geriatr Oncol. 2015;6:380–86. doi: 10.1016/j.jgo.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Yoon DH, Jang G, Kim JH, et al. Randomized phase 2 trial of s1 and oxaliplatin-based chemoradiotherapy with or without induction chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2015;91:489–96. doi: 10.1016/j.ijrobp.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Seetharamu N, Melamed J, Miller G, et al. Complete pathological response in a patient with metastatic esophageal cancer treated with a regimen of capecitabine, oxaliplatin and docetaxel: A case report. J Gastrointest Cancer. 2014;45(Suppl 1):108–11. doi: 10.1007/s12029-013-9575-6. [DOI] [PubMed] [Google Scholar]
- 10.Stein A, Arnold D, Thuss-Patience PC, et al. Docetaxel, oxaliplatin and capecitabine (tex regimen) in patients with metastatic gastric or gastro-esophageal cancer: Results of a multicenter phase i/ii study. Acta Oncol. 2014;53:392–98. doi: 10.3109/0284186X.2013.833346. [DOI] [PubMed] [Google Scholar]
- 11.Jiang H, Zhao P, Feng J, et al. Effect of paris saponin i on radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett. 2014;7:2059–64. doi: 10.3892/ol.2014.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang H, Zhao PJ, Su D, et al. Paris saponin i induces apoptosis via increasing the bax/bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol Med Rep. 2014;9:2265–72. doi: 10.3892/mmr.2014.2108. [DOI] [PubMed] [Google Scholar]
- 13.Zhao P, Jiang H, Su D, et al. Inhibition of cell proliferation by mild hyperthermia at 43 c with paris saponin i in the lung adenocarcinoma cell line pc-9. Mol Med Rep. 2015;11:327–32. doi: 10.3892/mmr.2014.2655. [DOI] [PubMed] [Google Scholar]
- 14.Zhao PJ, Song SC, Du LW, et al. Paris saponins enhance radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line by inducing apoptosis and g2/m cell cycle phase arrest. Mol Med Rep. 2016;13:2878–84. doi: 10.3892/mmr.2016.4865. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Jiang H, Li J, et al. Anticancer effects of paris saponins by apoptosis and pi3k/akt pathway in gefitinib-resistant non-small cell lung cancer. Med Sci Monit. 2016;22:1435–41. doi: 10.12659/MSM.898558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng RRY, Jiang H, Liu X, et al. Therapeutic potential of ginsenoside rg3 via inhibiting notch/hes1 pathway in lung cancer cells. Transl Cancer Res. 2016;5:464–69. [Google Scholar]
- 17.Li R, Zhang L, Chen D, et al. Capilliposide c derived from lysimachia capillipes hemsl inhibits growth of human prostate cancer pc3 cells by targeting caspase and mapk pathways. Int Urol Nephrol. 2014;46:1335–44. doi: 10.1007/s11255-013-0641-6. [DOI] [PubMed] [Google Scholar]
- 18.Hua Y, Hu Q, Piao Y, et al. Effect of capilliposide for induction apoptosis in human nasopharyngeal cancer cne-2 cells through up-regulating puma expression. J Cancer Res Ther. 2015;11(Suppl):C239–43. doi: 10.4103/0973-1482.170529. [DOI] [PubMed] [Google Scholar]
- 19.Fei ZH, Wu K, Chen YL, et al. Capilliposide isolated from lysimachia capillipes hemsl. Induces ros generation, cell cycle arrest, and apoptosis in human nonsmall cell lung cancer cell lines. Evid Based Complement Alternat Med. 2014;2014:497456. doi: 10.1155/2014/497456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Z, Huang M, Chen G, et al. Cell-based assays in combination with ultra-high performance liquid chromatography-quadrupole time of flight tandem mass spectrometry for screening bioactive capilliposide c metabolites generated by rat intestinal microflora. J Pharm Biomed Anal. 2016;119:130–38. doi: 10.1016/j.jpba.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Nishihira T, Kasai M, Mori S, et al. Characteristics of two cell lines (te-1 and te-2) derived from human squamous cell carcinoma of the esophagus. Gan. 1979;70:575–84. [PubMed] [Google Scholar]
- 22.Underwood TJ, Derouet MF, White MJ, et al. A comparison of primary oesophageal squamous epithelial cells with het-1a in organotypic culture. Biol Cell. 2010;102:635–44. doi: 10.1042/BC20100071. [DOI] [PubMed] [Google Scholar]
- 23.Street A, Macdonald A, Crowder K, Harris M. The hepatitis c virus ns5a protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J Biol Chem. 2004;279:12232–41. doi: 10.1074/jbc.M312245200. [DOI] [PubMed] [Google Scholar]
- 24.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic pi3k deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–29. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 25.Bachman KE, Argani P, Samuels Y, et al. The pik3ca gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–75. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 26.Lin JJ, Su JH, Tsai CC, et al. 11-epi-sinulariolide acetate reduces cell migration and invasion of human hepatocellular carcinoma by reducing the activation of erk1/2, p38mapk and fak/pi3k/akt/mtor signaling pathways. Mar Drugs. 2014;12:4783–98. doi: 10.3390/md12094783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan TO, Rittenhouse SE, Tsichlis PN. Akt/pkb and other d3 phosphoinositide-regulated kinases: Kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher JI, Huang DC. Controlling the cell death mediators bax and bak: Puzzles and conundrums. Cell Cycle. 2008;7:39–44. doi: 10.4161/cc.7.1.5178. [DOI] [PubMed] [Google Scholar]
- 29.Risberg K, Redalen KR, Sonstevold L, et al. Pro-survival responses to the dual inhibition of anti-apoptotic bcl-2 family proteins and mtor-mediated signaling in hypoxic colorectal carcinoma cells. BMC Cancer. 2016;16:531. doi: 10.1186/s12885-016-2600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shroff EH, Snyder C, Chandel NS. Bcl-2 family members regulate anoxia-induced cell death. Antioxid Redox Signal. 2007;9:1405–9. doi: 10.1089/ars.2007.1731. [DOI] [PubMed] [Google Scholar]
- 31.Reed JC, Miyashita T, Takayama S, et al. Bcl-2 family proteins: Regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996;60:23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Mirzayans R, Andrais B, Kumar P, Murray D. The growing complexity of cancer cell response to DNA-damaging agents: Caspase 3 mediates cell death or survival? Int J Mol Sci. 2016;17(5) doi: 10.3390/ijms17050708. pii: E708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linder M, Tschernig T. Vasculogenic mimicry: Possible role of effector caspase-3, caspase-6 and caspase-7. Ann Anat. 2016;204:114–17. doi: 10.1016/j.aanat.2015.11.007. [DOI] [PubMed] [Google Scholar]