Abstract
30-60% of patients receiving methadone for opioid use disorder (OUD) actively use cocaine. Cocaine use disorder (CUD) has no FDA-approved pharmacological treatment; existing psychosocial treatments are inadequate. Oxytocin, a social neuropeptide, has preclinical promise as an adjunctive treatment for both OUD and CUD. Twenty-two individuals receiving methadone for OUD with co-occurring CUD were randomized to receive oxytocin or placebo intranasally 40 IU twice daily for two weeks. A priori aims were feasibility and safety. Exploratory effectiveness aims included laboratory-based measures of drug craving, drug-related implicit cognition, and drug use. High retention rates (93.5%), the absence of study-related adverse events, and the fact that oxytocin was well tolerated in this population support the feasibility of larger trials. Two weeks of oxytocin (but not placebo) significantly reduced cocaine craving at day 15 compared to baseline (mean change±SD: OT=−0.23±0.19, p=0.004; PL=−0.16±0.29, p=0.114). For heroin craving, the placebo group reported a trend-level increase over time while the oxytocin group remained unchanged - with medium to large effect sizes between the groups (Cohen's d=0.71-0.90). Oxytocin led to a significant switch from implicit self-association with drugs to implicitly associating drugs with others (mean change±SD: 0.25±0.35, p=0.037) and a trend-level reduction in self-reported cocaine use over time (Z=−1.78, p=0.075). Furthermore, oxytocin significantly increased the accuracy of self-reported cocaine use when correlated with quantitative urine levels of cocaine metabolite. This proof-of-concept study provides promising early evidence that oxytocin may be an effective adjunct to the treatment of co-occurring CUD and OUD. Further investigation with larger trials is warranted.
Keywords: cocaine, social, addiction, methadone, oxytocin
INTRODUCTION
Opioid agonist treatment, including methadone maintenance therapy (MMT), is the mainstay for the treatment of opioid use disorder (OUD). MMT is associated with positive treatment outcomes, including reductions in illicit opioid craving and use and improved social functioning (Bart, 2012). Despite its effectiveness, roughly 30-60% of patients receiving MMT actively use cocaine (Weinstock J, 2010), which is associated with worse treatment outcomes (e.g., more than double the rate of relapse to heroin) (White et al, 2014). Those using both cocaine and heroin have mortality rates 14.3 times higher than the general population (de la Fuente, 2014). Moreover, patients often initiate or increase cocaine use after enrolling in MMT clinics (Chaisson RE, 1989). Aside from the influence of behavioral contagion within a clinic setting, increased cocaine use upon starting MMT may result from a synergistic effect of opioids and stimulants on striatal dopamine release (Lan, 2009). In fact, MMT patients report a subjective enhancement in the positive effects of cocaine use compared to non-MMT controls (Preston KL, 1996). No medications are currently FDA-approved for the treatment of cocaine use disorder (CUD); thus, psychosocial interventions remain the predominant treatment modality. Unfortunately, psychosocial treatments have low retention rates (e.g., 30%) (Siqueland et al, 2002). In sum, co-occurring OUD and CUD is a highly meaningful clinical problem in patients receiving MMT, and current treatment options do not adequately address the complex needs of this population.
Oxytocin (OT) is a hypothalamo-pituitary neuropeptide well known for its role in social cognition and behavior (Lieberwirth and Wang, 2014; Meyer-Lindenberg A, 2011). Endogenous OT levels are lower in cocaine using individuals (Light, 2004), and lower OT levels in patients receiving MMT are correlated with greater novelty seeking, which is a predictor of addiction-related behavior (Lin, 2015). OT administration has demonstrated myriad anti-addiction effects in animal models of substance dependence (Sarnyai Z, 2014; Carson et al, 2013). For example, OT dose-dependently attenuates opioid and cocaine tolerance and opioid withdrawal reactions. This is important because tolerance and withdrawal are key drivers of physiological drug dependence. Behaviorally, OT administration reduces stress-induced opioid seeking (Zanos et al, 2014), cue-induced cocaine seeking (Bentzley BS, 2014; Morales-Rivera et al, 2014), cocaine-induced stereotyped behavior (Carson et al, 2013), and opioid and cocaine self-administration (Sarnyai Z, 2014) in rodents. Thus, OT functions at multiple levels to diminish the effects of both opioids and psychostimulants and, ultimately, reduce their consumption, making it a prime candidate for the treatment of co-occurring OUD and CUD.
There is a growing interest in the investigation of OT's effects on substance use disorders in humans (Carson et al, 2013; Sarnyai Z, 2014; Stauffer and Woolley, 2014; Tops et al, 2014). OT administration has been shown to reduce alcohol withdrawal and craving in patients experiencing acute detoxification (Pedersen et al, 2013) and to decrease stress-induced marijuana craving in dependent individuals (McRae-Clark et al, 2013). The only published study of OT administration in human subjects with CUD (n=23) recruited cocaine-abstinent participants from a mandatory inpatient treatment program (Lee et al, 2014) and employed a single dose, double blind, placebo-controlled, crossover design to measure cue-reactivity to cocaine-related video clips. The authors found that OT removed the effect of state anger on several measures of cue-reactivity. They also found a seemingly paradoxical increase in baseline self-reported urge to use cocaine after administration of OT compared to placebo; however, there was no effect of OT on cue-induced urge to use.
No studies to our knowledge have investigated OT's effects on patients actively using cocaine, a population notoriously challenging to retain in treatment and research (Siqueland et al, 2002), particularly crack cocaine users (Rowan-Szal et al, 2000). We recruited active cocaine users engaged voluntarily in a harm reduction treatment model in the setting of an MMT clinic, where they were being treated for concomitant OUD. Intranasal OT administration has been found to be extremely safe and well-tolerated in healthy (MacDonald et al, 2011) and multiple patient populations (Hofmann et al, 2015). However, the dramatic OT-induced loss of opioid tolerance seen in animal studies presents safety concerns for those receiving a steady daily dose of MMT, which could theoretically lead to fatal overdose if a similar reduction in opioid tolerance were to occur in humans. Prior to the current study, our research group conducted a preliminary, single-dose, placebo-controlled, crossover study of intranasal OT 40 International Units (IU) in patients receiving MMT (Woolley, personal communication) and determined that this dosage was safe with no obvious acute effects on opioid tolerance.
The current study is a proof-of-concept, randomized, double-blind, placebo-controlled trial of OT administered intranasally twice daily for two weeks to male and female patients with CUD receiving MMT for OUD. The a priori goals of this pilot study were to: 1) determine the feasibility of our novel protocol, which utilizes the structure of an MMT clinic to recruit active crack cocaine users and retain them in a longitudinal clinical trial, and 2) to assess the safety of repeated intranasal OT dosing in patients receiving a steady daily dose of opioid agonist (i.e., MMT) (Clinicaltrials.gov #NCT02028533). Moreover, laboratory-based measures of drug craving (Everitt, 2014) and drug-related implicit cognitions (Marhe et al, 2013) predict real-world addictive behavior. Therefore, in addition to our primary outcomes of feasibility and safety, we also aimed to gather preliminary effect size data for OT's influence on measures of 3a) cue-induced drug craving, 3b) drug-related implicit cognitions, and 3c) drug use.
METHOD
Recruitment and Participants
The Committee on Human Research at the University of California, San Francisco approved all study protocols. Participants were recruited from the San Francisco General Hospital Opiate Treatment Outpatient Program (OTOP). All participants had a history of intravenous heroin use, were on a stable dose of methadone for at least two weeks, had at least one routine clinic-administered urine toxicology screen positive for cocaine in the past month, reported to clinic daily for their methadone dose (i.e., no take-home doses), and had no more than three clinic absences in the previous month. We excluded participants with cardiovascular disease, a history of traumatic brain injury, renal dialysis, observable pathology of the nasal mucosa, or urine toxicology screens positive for heroin or methamphetamine in the prior thirty days. No participants were taking hormones or 5HT1a agonists/antagonists, as these agents could hypothetically alter OT levels (Thompson et al, 2007). Female participants with a positive pregnancy test or a history of premenstrual dysphoric disorder were excluded. All female participants used a non-hormone-based form of birth control or reported sexual abstinence. Given the comorbidity of substance use disorders and other psychiatric symptoms, taking psychotropic medications was not an exclusion criteria provided the patient did not have a primary Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis I diagnosis other than OUD or CUD upon enrollment in the study.
Overview
During a 90-minute initial evaluation, informed written consent was obtained followed by administration of the Structured Clinical Interview for DSM-IV Disorders – Clinical Trials Version (SCID-4-CT), adapted to our study criteria, and a brief physical exam that included an assessment of the nasal mucosa. The severity of addiction to heroin and cocaine was estimated by determining the number of days of use in the past thirty days and the number of years the substance was used at least three times per week in the participant's lifetime (Cacciola, 2007). Participants were randomized, using gender as a stratum, to receive either OT or placebo.
Study participation included brief daily visits with study staff for twenty-one consecutive days. Participants received OT 40 IU, or matched placebo, (Abbotts Compounding Pharmacy, Berkeley) intranasally twice daily for the first two weeks. This was followed by a washout week of daily safety assessments without any study drug. Study staff trained on proper spray application (Guastella et al, 2013) administered each AM dose of the study drug immediately after participants received their daily observed methadone dose from OTOP clinic staff. After being trained on proper self-administration, participants were given a PM dose of OT or placebo to self-administer 8-10 hours following their AM dose. Each PM dose was individually bottled, and participants were incentivized to return each bottle the following day for weighing (adherence monitoring). Participants received $5 for daily assessments if the PM bottle from the previous day was returned – empty or full – but were only given $3 for daily assessments if no bottle was returned. Participants received $5 for each daily visit during the final washout week. Participants were paid $15 for the initial evaluation; an extra $10 for completing additional tasks (see below) on days 1, 15, and 21; and a $20 completion bonus. All compensation was in the form of cash.
Measures
1) Feasibility
Of the 369 patients receiving MMT at the OTOP clinic at the beginning of our six-week recruitment period, 107 (29%) had a documented routine urine toxicology screen positive for cocaine in the previous 30 days. To measure the feasibility of conducting larger longitudinal studies within this population, we recorded the number of patients recruited and screened for study participation. We also tracked study retention and adherence to daily participation within the three-week study protocol.
2) Safety
Participants were assessed daily for safety of the intervention. Clinical measures monitoring for acute reduction in opioid tolerance were collected, including: respiratory rate (a sensitive sign of opioid toxicity), pupil size, heart rate, blood pressure, and temperature. We also asked participants to report any adverse events.
Visual Analog Scale – Effects of Drug (VAS-ED)
Participants completed a daily computerized questionnaire assessing subjective drug effects. “Drug” was defined as any substance recently used, including: illicit drugs, MMT, or the study drug. The following questions were asked: 1)“Do you feel any drug effects?”, 2)“Does the drug have any good effects?”, 3)“Does the drug have any bad effects?”, 4)“Do you like the drug?”, 5)“Does the drug make you feel sick?”, 6)“How high are you?”. Answers were marked on a 10-cm visual analog scale (VAS) and recorded as a proportion of the full line (range = 0-1). In our preliminary study, we found that a single dose of OT given to participants receiving MMT for OUD (but not actively using any illicit substances) led to a significant increase in ratings of “bad effects” and feeling “high” (Woolley, personal communication).
3) Effectiveness Measures
Computerized measures, created on PsychoPy v1.80.03, of drug craving/urge to use and drug-related implicit cognition were conducted on day 1, prior to any study drug administration (baseline), and repeated on day 15, after two weeks of study drug administration, and day 21, after the washout week.
3a) Craving & Urge Paradigm
To measure cue-induced craving we employed the following paradigm, which was performed separately using both cocaine and heroin-related cues. Participants were asked “How much are you currently craving [drug]?” and “How high is your urge to use [drug] currently?” and marked their response on a 10-cm VAS, which was recorded as a proportion of the full line (range = 0-1). We collected VAS measurements (in the following order): 1) prior to any drug cue exposure, 2) immediately following exposure to a 1-minute video of publically-available documentary footage depicting [drug] use, and 3) following a 3-minute video of neutral footage. A 3-minute period of neutral stimuli was chosen to allow time for cue-induced craving to subside between tasks (Garavan et al, 2000).
3b) Implicit Association Test (IAT)
Positive drug-related implicit cognitions are linked to relapse (Marhe, 2013), and there is a growing interest in the development of treatment options that target drug-related implicit cognitions (Rooke, 2008). To measure drug-related implicit cognitions, we used a modified IAT aimed to assess implicit self-identification with drugs. Recent theories suggest that identity change in recovery is socially negotiated (Best, 2016), thus OT may enhance this process. Images of [drug] paraphernalia versus neutral hardware tools were used as concept discriminations, and words implying “self” (e.g., mine, myself) versus “other” (e.g., theirs, others) were used as attribute discriminations. The IAT was administered separately using both cocaine and heroin-related images. Participants were instructed to categorize images and words as quickly as possible while making as few mistakes as possible. IAT scores are derived from response latencies – assuming that participants respond more rapidly and make fewer mistakes when the concept and attribute sharing the same key are more strongly implicitly associated (Greenwald et al, 2003).
3c) Drug Use
Self-Reported
Participants reported daily on quantity and route of administration of any illicit drug use in the past 24 hours. Crack cocaine use was reported in dollar amounts. Study staff were trained to conduct all interviews using an adapted supportive clinical management approach (Carroll et al, 1994). This approach emphasizes confidentiality, rapport-building, and using empathy and non-judgment without providing any active ingredients of drug counseling.
Quantitative
Urine samples were collected at baseline and subsequently every four days. A liquid chromatography tandem mass spectrometry method described previously was used to quantitate the level of benzoylecgonine, the primary cocaine metabolite, in each urine sample (Lynch et al, 2011). This level was then normalized to the concentration of creatinine. Creatinine was measured on a Siemens ADVIA 1800 system using an FDA-approved enzymatic creatininase method according to manufacturer's instructions. The calibration for this method is traceable to SRM967 and correlated with the NIST liquid chromatography isotope-dilution mass spectrometry method. The half-life of benzoylecgonine is 6.6 hours; therefore, the minimum window of detection in urine is approximately 2 days (7 half-lives). It is generally accepted that this window of detection for benzoylecgonine is longer in chronic crack cocaine users.
Analysis Plan
Standard descriptive statistics were used to summarize the demographic and clinical data at baseline and to assess feasibility and basic safety aims. Student's t-tests were used to compare the two groups regarding adherence and safety measures. Normality was assessed using the Shapiro-Wilk test. A mixed-effects ANOVA model was estimated and tested using the within-subjects factor of time (baseline, day 15, day 21) and the between-subjects factor of drug (OT, placebo) as independent variables and, separately, measures of cocaine/heroin craving, urge, and implicit association as dependent variables. Similarly, mixed-effects ANOVA was estimated and tested for VAS-ED and urine BE, although the within-subject factor of time was defined as (baseline, day 5, day 9, day 13, day 17, and day 21) and (daily measurements for days 1-7 and 8-14), respectively. Within-subject differences were further explored separately for each study drug group (OT versus placebo) using a repeated measures ANOVA. Within-group differences for self-reported drug use, which was not normally distributed, were calculated using a Wilcoxon signed-rank test. Mean daily self-reported cocaine use was collapsed for days 2-14 and days 15-21, and differences from baseline (day 1) were calculated for each. Due to the exploratory nature of our effectiveness aims and our small sample size, we also report Cohen's d effect sizes for between-group differences of all measures to aid in power analyses of future studies. Figure values are shown as group means and standard error of the mean, and significant or trend-level within-subject changes are marked. Pearson's correlation coefficients were calculated for urine BE levels and mean self-reported use from the four days prior to each BE level. A Fisher r-to-z transformation was used to assess the significance of the difference in correlation coefficients between the oxytocin and placebo groups at each timepoint, and two-tailed p-values were determined.
RESULTS
Participants
Participants were predominantly African American with a mean age of 49.5 years. They received a mean daily methadone dose of 101.4mg and used an average of $28 worth of crack cocaine daily. Eight of the 22 participants were taking one or more psychoactive medications. Participants were prescribed these medications by their regular clinicians prior to study entry and were on a stable dose that did not change through the course of the study. For a summary of demographics and baseline measurements see Table 1.
Table 1.
Demographic and baseline characteristics for completed subjects.
| Oxytocin (n = 11) | Placebo (n = 11) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Male | 6 | 55 | 5 | 45 |
| Caucasian | 4 | 36 | 1 | 9 |
| African American | 5 | 45 | 7 | 64 |
| Hispanic | 0 | 0 | 2 | 18 |
| Other | 2 | 18 | 1 | 9 |
| Psychoactive Medications: | ||||
| SSRI | 2 | 18 | 1 | 9 |
| Trazodone | 1 | 9 | 1 | 9 |
| Mirtazapine | 0 | 0 | 1 | 9 |
| Misc. Prescribed Opioid | 1 | 9 | 3 | 27 |
| Mean | SD | Mean | SD | |
|---|---|---|---|---|
| Age (years) | 49.3 | 9.67 | 49.6 | 9.63 |
| Education (years) | 12.6 | 2.4 | 11.1 | 1.8 |
| Methadone (mg/day) | 105.5 | 27.7 | 97.3 | 23.6 |
| Heroin, 30-day* | 0.02 | 0.05 | 0.01 | 0.02 |
| Heroin, lifetime† | 0.27 | 0.17 | 0.31 | 0.16 |
| Cocaine, 30-day* | 0.81 | 0.28 | 0.92 | 0.19 |
| Cocaine, lifetime† | 0.25 | 0.17 | 0.49 | 0.23 |
| Baseline Measures | Mean | SD | Mean | SD |
|---|---|---|---|---|
| Cocaine Use, Self-Report ($) | 24.5 | 28.2 | 31.5 | 25.9 |
| Cocaine Use, BE (ng/mg) | 70.66 | 49.12 | 68.44 | 61.8 |
| Cocaine Craving | 0.49 | 0.22 | 0.46 | 0.23 |
| Cocaine Urge to Use | 0.48 | 0.24 | 0.50 | 0.20 |
| Heroin Craving | 0.11 | 0.17 | 0.08 | 0.08 |
| Heroin Urge to Use | 0.15 | 0.28 | 0.06 | 0.06 |
| IAT score | −0.16 | 0.46 | −0.28 | 0.23 |
number of days of drug use/past 30 days
number of years using at least three times per week/patient's age.
SSRI = selective serotonin reuptake inhibitor, SD = standard deviation, BE = benzoylecgonine.
1) Feasibility
A total of 78 patients were recruited and screened for participation (Figure 1). 23 participants (12 men) were enrolled. One male participant was unable to adequately complete tasks due to neurocognitive deficits; he was removed from the study on day five and not included in analysis. 22 participants completed full study participation for a retention rate of 95.7%. The 22 participants completing the study collectively demonstrated 93.5% daily attendance over the twenty-one day study period with no difference between study groups (mean±SD: OT 19.73±1.27, Placebo 19.55±1.57, p=0.77). The daily rate of incentivized return of nasal spray bottles from the previous day's PM dose was 98.05% with no difference between study groups (mean±SD: OT 13.64±0.50, Placebo 13.82±0.41, p=0.36).
Figure 1.
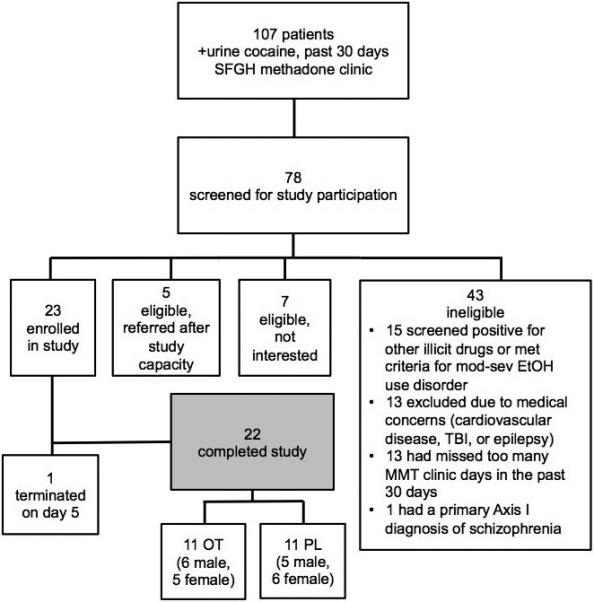
Enrollment flow-chart. SFGH = San Francisco General Hospital, EtOH = alcohol, TBI = traumatic brain injury, MMT = methadone maintenance treatment, OT = oxytocin, PL = placebo.
2) Safety
There were no study-related adverse events reported. There were no significant differences in safety measures between the two groups, including respiratory rate (mean±SD: OT 14.63±0.75, Placebo 14.58±0.92, p=0.93). One participant's (female, OT group) methadone dose was increased during study participation in order to address the emergence of opioid withdrawal symptoms triggered by the initiation of rifampin prescribed for a surgery-related skin infection. All other participants’ methadone dose remained stable throughout study participation. There was a trend-level between-group effect for the VAS-ED rating “Do you feel any drug effects?” (F1,3=8.26, p=0.06), with the OT group reporting less overall “drug effects” (between-group Cohen's d effect size=0.60). There were no significant effects for the other VAS-ED ratings.
3) Effectiveness Measures
3a) Craving & Urge to Use
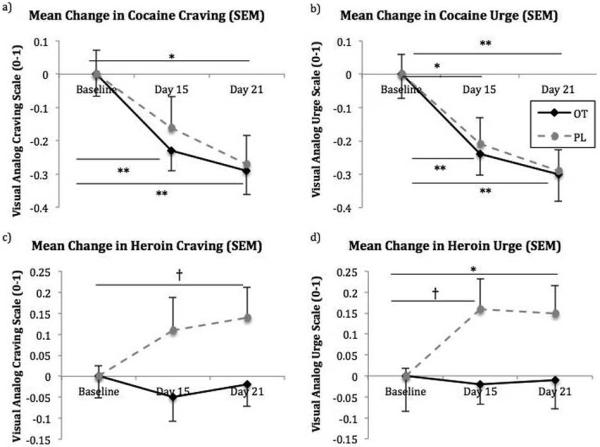
Participants did not report a significant change in craving or urge to use cocaine or heroin in response to our drug-cue videos on any of the testing days. Therefore, measures from the testing paradigm were collapsed into mean ratings for both craving and urge for each of the three testing days. There was a significant mean effect of time for cocaine craving (F2,36=15.32, p<0.001), but no significant effect of drug (F1,18<0.001, p=0.98) or a time*drug interaction (F2,36=0.23, p=0.80). Cohen's d effect sizes measuring between-group differences for change in cocaine craving from baseline were 0.29 and 0.09 between the two groups at days 15 and 21, respectively. There was a significant mean effect of time for urge to use cocaine (F2,36=20.16, p<0.001), but no significant effects of drug (F1,18=0.10, p=0.75) or time*drug interactions (F2,36=0.09, p=0.92). Cohen's d effect sizes measuring between-group differences for change in urge to use cocaine from baseline were 0.13 and 0.04 between the two groups at days 15 and 21, respectively. Both groups demonstrated significant within-group changes in cocaine craving (OT: F2,18=10.37, p=0.001; Placebo: F2,18=5.94, p=0.01) and urge to use (OT: F2,18=9.98, p=0.001; Placebo: F2,18=10.28, p=0.001) (Figure 2a,b).
Figure 2.

Changes in a) cocaine craving, b) urge to use cocaine, c) heroin craving, and d) urge to use heroin as measured by a visual-analog scale recorded as a number between 0 and 1. Baseline measures for each group were set to zero. SEM = standard error of the mean, OT = oxytocin, PL = placebo. †p < 0.10, *p < 0.05, and **p < 0.01.
For heroin craving, there were no significant mean effects of time (F2,38=0.62, p=0.55) or drug (F1,19=2.12, p=0.16) nor a significant time*drug interaction (F2,38=1.64, p=0.21). Cohen's d effect sizes measuring between-group differences for change in heroin craving from baseline were 0.71 and 0.77 at days 15 and 21, respectively (Figure 2c). For urge to use heroin, there were no significant mean effects of time (F2,38=1.31, p=0.28) or drug (F1,19=0.03, p=0.87) nor a significant time*drug interaction (F2,38=2.11, p=0.14). Cohen's d effect sizes measuring between-group differences for change in urge to use heroin from baseline were 0.90 and 0.73 at days 15 and 21, respectively. There were trend-level within-group increases in urge to use heroin in the placebo group (F2,20=2.70, p=0.09), but no significant changes in the OT group (F2,18=.08, p=0.92) (Figure 2d).
3b) IAT
There were no significant mean effects of time (F2,34=1.17, p=.32) or drug (F1,17=2.00, p=.18), nor a significant time*drug interaction (F2,34=.80, p=.46) on implicit associations of cocaine images with “self” versus “other” words. In the OT group, there was a significant switch from associating cocaine images with “self” words to associating cocaine images with “other” words at day 21 compared to baseline (F1,10=5.77, p=.04). The placebo group did not change significantly in self-association with cocaine at this time point (F1,8=.25, p=.63). Cohen's d effect size measuring between-group differences for change in IAT from baseline was .58 at day 21. Due to a computer error, baseline IAT scores for heroin were only collected for four participants; thus, IAT change scores for heroin were not analyzed.
3c) Cocaine Use
The OT group showed a trend-level reduction in self-reported cocaine use for both the Study Drug Days (SDD, days 1-14) and the Washout Week (WW, days 15-21) compared to baseline (SDD: Z=−1.78, p=0.08; WW: Z=−1.68, p=0.09), while the placebo group reported no significant change (SDD: Z=−1.17, p=0.24; WW: Z=−1.48, p=0.14). Cohen's d effect sizes measuring change in self-reported cocaine use between the two groups were 0.31 and 0.28 for SDD and WW, respectively (Figure 3a). There were no significant mean effects for time (F5,90=1.09, p=0.37) or drug (F1,18=.001, p=0.97) nor a significant time*drug interaction (F5,90=1.03, p=0.41) for urine BE levels. Cohen's d effect sizes measuring between-group differences in BE changes from baseline were .19 and .23 at days 15 and 21, respectively (Figure 3b). Self-reported cocaine use significantly correlated with subsequent urine BE levels in the OT group for urine sample timepoints 3, 4, and 5. The placebo group did not demonstrate any significant correlations. There was a trend-level difference in correlation coefficients between the two groups at urine sample timepoint 3 (z=1.82, p=0.07) and a significant difference at urine sample timepoint 4 (z=2.11, p=0.03). Between-group differences in correlation coefficents at the other urine sample timepoints were not significant (Figure 3c).
Figure 3.
Changes in a) self-reported cocaine use and b) urine cocaine metabolite levels. BE = benzoylecgonine. c) Correlation values (y-axis) for urine BE levels collected every four days (x-axis) and average self-reported cocaine use for the four days prior to each urine collection. Significant correlations existed for the third (r = 0.72), fourth (r = 0.76), and fifth (r = 0.70) urine samples in the oxytocin group. OT = oxytocin, PL = placebo. †p < 0.10, *p < 0.05 (also represented by the dotted line in c), and **p < 0.01.
DISCUSSION
Our recruitment, retention, and adherence rates support the feasibility of conducting a larger randomized controlled trial of intranasal OT's effects on OUD and CUD in the setting of an MMT clinic. We are the first to demonstrate that OT administration is safe and tolerable for patients receiving MMT; specifically, we found no evidence of reduction in opioid tolerance as a result of OT 40 IU administered intranasally twice daily for two weeks. In fact, we found that participants reported feeling less overall “drug effects” when receiving OT versus placebo, while we would expect the opposite if patients were losing opioid tolerance in the setting of a stable daily dose of MMT. Furthermore, the exploratory measures included in our proof-of-concept study uncovered promising preliminary support for OT's effects on the symptoms of OUD and CUD. While most differences did not reach statistical significance, and much larger trials would be needed to validate the effect sizes found, there is a consistent pattern of more positive outcomes in the OT group compared to the placebo group (see Figures 2 & 3). With a strong preclinical evidence base and growing clinical interest, continued early-stage human studies of intranasal OT's effects on the symptoms of OUD and CUD are warranted.
OT has been found to reduce opioid and cocaine-related cue-reactivity in numerous animal studies (Bentzley BS, 2014; Morales-Rivera et al, 2014); however, we were unable to assess acute cue-reactivity because our paradigm failed to elicit self-reported increases in cue-induced craving or urge to use cocaine or heroin. Given OT's known role in ameliorating stress reactivity (Cardoso, 2014), future studies of this population should include stress-induced craving paradigms, which likely involve more robust physiological responses than video cues of drugs (Mantsch et al, 2015) and may more effectively highlight OT's effects on drug craving in humans (e.g., McRae et al.'s, 2013). Additionally, the Cocaine Craving Questionnaire may be a more effective self-report measure of cocaine craving than VAS ratings (Heinz et al, 2006). Both groups in our study reported significant reductions in overall cocaine craving and urge to use over the course of the study (Figure 2a,b), while measures of heroin craving and urge to use increased over time in the placebo group and remained stable in the OT group (Figure 2c,d) with large between-group effect sizes. Despite not reaching statistical significance, the directionality of the signals detected in response to OT versus placebo on both cocaine and heroin measures is promising.
Thomas Insel (2003) hypothesized that drugs of abuse hijack a motivational reward system evolved for social interaction, and social context has been deemed highly relevant to addiction treatment outcomes (Alexander and Hadaway, 1982; Robins et al, 2010). Sarnyai (2014) hypothesized that OT administration may help shift attentional bias toward adaptive social reward at the expense of conditioned drug-related reward. However, the availability of adaptive social reward in populations with substance use disorders may be highly limited, leaving substances of abuse as the most salient coping strategy when faced with stressors (Stauffer and Woolley, 2014). This may help explain why participants reported an increased urge to use cocaine in response to OT administration in Lee et al's (2014) study of inpatients with CUD. The social context of this study was court-mandated treatment following incarceration and forced abstinence from cocaine for an average of 28 months. Our study, on the other hand, is the first to examine OT's effects in active cocaine users engaged in voluntary addiction treatment. Aside from routine engagement with clinical providers, participants had daily contact with study staff who explicitly emphasized a supportive approach and focused on individual rapport with participants. This positive social context may have facilitated OT's trust- and cooperation-boosting effects (Lieberwirth et al, 2014; Meyer-Lindenberg A, 2011) and led to our finding of more honest cocaine use self-reporting in the OT group (Figure 3(c)), although this hypothesis requires further exploration to rule out a direct OT-induced reduction in participant defensiveness or enhancement of cognition leading to more accurate reporting. In further support of the hypothesis that OT shifts attentional bias, participants receiving OT switched implicit association of drugs with “self” to drugs with “others” by the end of our study period. To our knowledge, this is the first evidence implying a pharmacological change in any implicit association. Despite the disparity in social context, direct comparison of our study with Lee et al's (2014) study is limited due to differences in our study designs (e.g., repeated versus single OT dosing, between-subjects versus crossover design, use of slightly different paradigms and measures). Nonetheless, our results may not generalize beyond patients engaged in the structure of existing psychosocial treatment and frequent supportive contact with staff. Social context has repeatedly been shown to be an important moderating influence on the effects of OT administration (Bartz et al, 2011; Declerck et al, 2010; Grillon et al, 2013), and future studies of substance use disorder populations should factor this into their design.
Like the extant studies of OT's effects in substance use disorders in humans, analyses were limited by our small sample size. For example, it is likely that moderating factors exist for the effectiveness of OT in ameliorating the symptoms of substance use disorders (e.g., gender, OT receptor variation, exposure to early life stress) (Bisagno, 2014; Myers, 2014); however, our sample was too small for such subanalyses. Additionally, animal studies of OT and addiction often demonstrate inverted U-shaped dose response curves. Currently, the most effective dosage of intranasal OT in humans is unknown. Our study utilized a standard dosage from the field, but tiered dosing studies should begin to target the most effective dose range. Lastly, our results may not be generalizable to patients with either OUD or CUD in isolation. However, there is an alarming prevalence of cocaine use in real-world populations of patients receiving MMT, and concomitant OUD and CUD leads to poorer treatment outcomes and higher mortality rates. Furthermore, this challenging clinical problem is often overlooked due to a lack of viable treatment options. Therefore, we feel this is a worthwhile target population, particularly given the evidence of OT's non-specific effects on a wide variety of substances of abuse.
In conclusion, OT is a safe and feasible candidate to address the dire need for innovative treatment options for substance use disorders. Much larger trials will be needed to determine the efficacy of intranasal OT administration as an adjunctive treatment for co-occurring OUD and CUD prevalent in MMT clinics.
Acknowledgments
This project was supported by the San Francisco Treatment Research Center at the University of California, San Francisco via grant number P50 DA009253 from the National Institute on Drug Abuse. Additional support was provided by the National Institute of Mental Health via grant number R25 MH060482.
Footnotes
DECLARATION OF INTEREST STATEMENT
The authors have no conflicts of interest related to this project to report.
REFERENCES
- Alexander BK, Hadaway PF. Opiate addiction: The case for an adaptive orientation. Psychological bulletin. 1982;92(2):367. [PubMed] [Google Scholar]
- Bart G. Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis. 2012;31(3):207–225. doi: 10.1080/10550887.2012.694598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in cognitive sciences. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bentzley BSJT, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rate. Proc Natl Acad Sci USA. 2014;111(32):11822–11827. doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best D, Beckwith M, Haslam C, Haslam SA, Jetten J, Mawson E, Lubman DI. Overcoming alcohol and other drug addiction as a process of social identity transition: the social identity model of recovery (SIMOR). Addiction Research and Theory. 2016;24(2):111–123. [Google Scholar]
- Bisagno V, Cadet JL. Stress, sex, and addiction: potential roles of corticotropin-releasing factor, oxytocin, and arginine-vasopressin. Behav Pharmacol. 2014;25(5-6):445–57. doi: 10.1097/FBP.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG. Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug Alcohol Depend. 2007;87(2-3):297–302. doi: 10.1016/j.drugalcdep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Kingdon D, Ellenbogen MA. A meta-analytic review of the impact of intranasal oxytocin administration on cortisol concentrations during laboratory tasks: moderation by method and mental health. Psychoneuroendocrinology. 2014;49:161–70. doi: 10.1016/j.psyneuen.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, et al. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Archives of general psychiatry. 1994;51(3):177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Carson DS, Guastella AJ, Taylor ER, McGregor IS. A brief history of oxytocin and its role in modulating psychostimulant effects. Journal of psychopharmacology. 2013;27(3):231–247. doi: 10.1177/0269881112473788. [DOI] [PubMed] [Google Scholar]
- Chaisson REBP, Osmond D, Brodie B, Sande MA, Moss AR. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA : the journal of the American Medical Association. 1989;261(4):561–565. [PubMed] [Google Scholar]
- Chini B, Leonzino M, Braida D, Sala M. Learning about oxytocin: pharmacologic and behavioral issues. Biological psychiatry. 2014;76(5):360–366. doi: 10.1016/j.biopsych.2013.08.029. [DOI] [PubMed] [Google Scholar]
- de la Fuente L, Molist G, Espelt A, Barrio G, Guitart A, Bravo MJ, Brugal MT. Mortality risk factors and excess mortality in a cohort of cocaine users admitted to drug treatment in Spain. Journal of substance abuse treatment. 2014;46(2):219–226. doi: 10.1016/j.jsat.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Hormones and behavior. 2010;57(3):368–374. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE, et al. Oxytocin facilitates the extinction of conditioned fear in humans. Biological psychiatry. 2014;78(3):194–202. doi: 10.1016/j.biopsych.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories–indications for novel treatments of addiction. European Journal of Neuroscience. 2014;40(1):2163–2182. doi: 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. An improved scoring algorithm. Journal of personality and social psychology. 2003;85(2):197. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Grillon C, Krimsky M, Charney D, Vytal K, Ernst M, Cornwell B. Oxytocin increases anxiety to unpredictable threat. Molecular psychiatry. 2013;18(9):958. doi: 10.1038/mp.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella A, Hickie IB, McGuinness MM, Otis MWE, Disinger HM, Chan HK, et al. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38(5):612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Heinz AJ, Epstein DH, Schroeder JR, Singleton EG, Heishman SJ, Preston KL. Heroin and cocaine craving and use during treatment: measurement validation and potential relationships. Journal of substance abuse treatment. 2006;31(4):355–364. doi: 10.1016/j.jsat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Fang A, Brager DN. Effect of intranasal oxytocin administration on psychiatric symptoms: A meta-analysis of placebo-controlled studies. Psychiatry research. 2015;228(3):708–714. doi: 10.1016/j.psychres.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Insel T. Is social attachment an addictive disorder? Physiology & behavior. 2003;79(3):351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of neuroscience. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan K-C, Chang AC, Liu S-H, Ho IK, Lin-Shiau S-Y. Enhancing effects of morphine on methamphetamine-induced reinforcing behavior and its association with dopamine release and metabolism in mice. Journal of Neurochemistry. 2009;109:382–392. doi: 10.1111/j.1471-4159.2009.05998.x. [DOI] [PubMed] [Google Scholar]
- Lee MR, Glassman M, King-Casas B, Kelly DL, Stein EA, Schroeder J, et al. Complexity of oxytocin's effects in a chronic cocaine dependent population. European Neuropsychopharmacology. 2014;24(9):1483–1491. doi: 10.1016/j.euroneuro.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. Social bonding: regulation by neuropeptides. Frontiers in neuroscience. 2014;8:171. doi: 10.3389/fnins.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KL, Dominy SS, Graf J, Kral AH. Detection of levamisole exposure in cocaine users by liquid chromatography-tandem mass spectrometry. Journal of analytical toxicology. 2011;35(3):176–178. doi: 10.1093/anatox/35.3.176. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36(8):1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.142. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R, Waters AJ, van de Wetering BJ, Franken IH. Implicit and explicit drug-related cognitions during detoxification treatment are associated with drug relapse: an ecological momentary assessment study. Journal of consulting and clinical psychology. 2013;81(1):1–12. doi: 10.1037/a0030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Moran-Santa Maria M, Brady KT. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology. 2013;228(4):623–631. doi: 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A DG, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Morales-Rivera A, Hernández-Burgos MM, Martínez-Rivera A, Pérez-Colón J, Rivera R, Montalvo J, et al. Anxiolytic effects of oxytocin in cue-induced cocaine seeking behavior in rats. Psychopharmacology. 2014;231(21):4145–4155. doi: 10.1007/s00213-014-3553-y. [DOI] [PubMed] [Google Scholar]
- Myers AJ, Williams L, Gatt JM, McAuley-Clark EZ, Dobson-Stone C, Schofield PR, Nemeroff CB. Variation in the oxytocin receptor gene is associated with increased risk for anxiety, stress and depression in individuals with a history of exposure to early life stress. J Psychiatr Res. 2014;59:93–100. doi: 10.1016/j.jpsychires.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, et al. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcoholism: Clinical and Experimental Research. 2013;37(3):484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(26):6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KLSJ, Strain EC, Bigelow GE. Enhancement of cocaine's abuse liability in methadone maintenance patients. Psychopharmacology. 1996;123:15–25. doi: 10.1007/BF02246276. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Hesselbrock M, Wish E. Vietnam veterans three years after Vietnam: How our study changed our view of heroin. The American Journal on Addictions. 2010;19(3):203–211. doi: 10.1111/j.1521-0391.2010.00046.x. [DOI] [PubMed] [Google Scholar]
- Rooke SE, Hine DW, Thorsteinsson EB. Implicit cognition and substance use: A meta-analysis. Addictive Behaviors. 2008;33(10):1314–1328. doi: 10.1016/j.addbeh.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Rowan-Szal GA, Joe GW, Simpson DD. Treatment retention of crack and cocaine users in a national sample of long term residential clients. Addiction Research and Theory. 2000;8(1):51–64. [Google Scholar]
- Sarnyai ZKG. Oxytocin in learning and addiction: From early discoveries to the present. Pharmacology, Biochemistry and Behavior. 2014;119:3–9. doi: 10.1016/j.pbb.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Siqueland L, Crits-Christoph P, Gallop R, Barber JP, Griffin ML, Thase ME, et al. Retention in psychosocial treatment of cocaine dependence: predictors and impact on outcome. The American Journal on Addictions. 2002;11(1):24–40. doi: 10.1080/10550490252801611. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. International Journal of Neuropsychopharmacology. 2013;16(2):255–260. doi: 10.1017/S1461145712000533. [DOI] [PubMed] [Google Scholar]
- Stauffer CS, Woolley JD. Can we bottle psychosocial treatments for addiction? the role of oxytocin. The Journal of clinical psychiatry. 2014;75(9):1028. doi: 10.4088/JCP.14ac09437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M, Callaghan P, Hunt G, Cornish J, McGregor I. A role for oxytocin and 5-HT 1A receptors in the prosocial effects of 3, 4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146(2):509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Tops M, Koole SL, IJzerman H, Buisman-Pijlman FT. Why social attachment and oxytocin protect against addiction and stress: insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacology Biochemistry and Behavior. 2014;119:39–48. doi: 10.1016/j.pbb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Weinstock JRC, Petry NM. Contingency management for cocaine use in methadone maintenance patients: When does abstinence happen? Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2010;24(2):282–291. doi: 10.1037/a0017542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WL, Campbell MD, Spencer RD, Hoffman HA, Crissman B, DuPont RL. Patterns of abstinence or continued drug use among methadone maintenance patients and their relation to treatment retention. Journal of psychoactive drugs. 2014;46(2):114–122. doi: 10.1080/02791072.2014.901587. [DOI] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky-Sommerer R, et al. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(4):855–865. doi: 10.1038/npp.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]


