Abstract
Background
Placebo-controlled and open-label studies have demonstrated the safety and efficacy of daily oral preexposure prophylaxis (PrEP) in preventing HIV infection, but data are limited on real-world PrEP use.
Methods
We conducted a cohort study from July 2012 through June 2015 of Kaiser Permanente Northern California members initiating PrEP. We assessed pharmacy refill adherence and discontinuation, decreases in estimated glomerular filtration rate (eGFR), and sexually transmitted infection (STI)/HIV incidence.
Results
Overall, 972 individuals initiated PrEP, accumulating 850 person-years of PrEP use. Mean adherence was 92% overall. Black race/ethnicity [adjusted risk ratio (aRR) 3.0; 95% confidence interval: 1.7 to 5.1, P < 0.001], higher copayments (aRR 2.0; 1.2 to 3.3, P = 0.005), and smoking (aRR 1.6; 1.1 to 2.3, P = 0.025) were associated with <80% adherence. PrEP was discontinued by 219 (22.5%); female sex (aRR 2.6; 1.5 to 4.6, P < 0.001) and drug/alcohol abuse (aRR 1.8; 1.3 to 2.6, P = 0.002) were associated with discontinuation. Among 909 with follow-up creatinine testing, 141 (15.5%) had an eGFR <70 mL·min−1·1.73 m−2 and 5 (0.6%) stopped PrEP because of low eGFR. Quarterly STI positivity was high and increased over time for rectal chlamydia (P < 0.001) and urethral gonorrhea (P = 0.012). No HIV seroconversions occurred during PrEP use; however, 2 occurred in individuals who discontinued PrEP after losing insurance coverage.
Conclusions
PrEP adherence was high in clinical practice, consistent with the lack of HIV seroconversions during PrEP use. Discontinuation because of renal toxicity was rare. STI screening every 6 months, as recommended by current guidelines, may be inadequate. Strategies are needed to increase PrEP access during gaps in insurance coverage.
Keywords: preexposure prophylaxis (PrEP), HIV, sexually transmitted infections (STI), renal insufficiency, medication adherence
INTRODUCTION
The efficacy of daily oral preexposure prophylaxis (PrEP) using tenofovir/emtricitabine (TDF/FTC) for prevention of HIV infection was demonstrated in randomized placebo-controlled trials,1–3 and TDF/FTC was approved by the U.S. Food and Drug Administration (FDA) for use as PrEP in 2012.4 Although results from open-label PrEP studies have been promising,5,6 concerns remain about implementation, including low adherence,7 increased risk behavior8 and sexually transmitted infections (STIs),9 and drug toxicity.10
We previously found no new HIV infections among men who have sex with men (MSM) initiating PrEP in a large integrated health care system in San Francisco,11 yet little is known about other important outcomes among PrEP users in routine clinical practice, including adherence, discontinuation, and renal safety. Adherence may be lower when PrEP and adherence support are delivered using a telemedicine approach.12 The cost of the medication may also introduce a barrier to PrEP adherence and continuation which was not present in research studies in which participants received medication at no cost.13,14 Finally, PrEP users in clinical practice may be older or have more comorbidities than participants in trials or demonstration projects, and may therefore be at increased risk of adverse events.10
We assessed adherence, discontinuation, renal safety, and HIV seroconversions among individuals initiating PrEP within Kaiser Permanente Northern California (KPNC). Given the high incidence of STIs we previously reported,11,15 we also evaluated STI testing frequency and quarterly positivity to inform optimal STI screening guidelines for PrEP users.
METHODS
Study Design, Setting, and Population
KPNC is a large integrated health care system that provides comprehensive medical services to 3.9 million members, corresponding to approximately 46% of insured individuals from the surrounding population (R. Fong, Kaiser Permanente Market Strategy and Analysis, written communication, April 2016). At KPNC, primary care or other providers refer members to specialized PrEP clinicians after risk assessment or patient-initiated request. Creatinine and HIV testing are required, and STI testing recommended, at least quarterly during PrEP use. These tests are available at KPNC laboratories on a drop-in basis, and as often as monthly, for most members. Refills of TDF/FTC can be requested online. Adherence counseling is provided in person at baseline. For most PrEP users, follow-up visits are conducted through telephone or secure email messages, along with telemedicine adherence support as needed (eg, for individuals who are late in picking up a refill or conducting HIV or creatinine testing).
Our study population included all KPNC members initiating TDF/FTC for PrEP from July 1, 2012 (the month of FDA approval) through December 31, 2014. Follow-up was from the first dispensing of TDF/FTC until the earliest of PrEP discontinuation, health plan disenrollment, HIV seroconversion, death, or end of study (June 30, 2015).
The institutional review board at KPNC approved this study with a waiver of written informed consent.
Study Measurements
Demographic and Clinical Characteristics
We extracted data from the clinical and administrative databases that comprise KPNC’s electronic health record (EHR), including age, legal sex, race/ethnicity, laboratory tests and results, pharmacy fills, dates of health plan enrollment, and member copayments for TDF/FTC. We also extracted inpatient and outpatient clinical diagnoses, including drug/alcohol abuse (International Classification of Disease Codes, version 9 [ICD-9]: 291, 292, 303–305.0, 305.2–305.5), smoking/tobacco use (ICD-9: 305.1, V15, V65, 649, internal social history codes), hypertension (ICD-9: 401–405 or antihypertension medication use), and diabetes (KPNC diabetes registry16).
Adherence and Discontinuation
We estimated adherence to PrEP as the medication possession ratio (MPR) using pharmacy refill data among individuals with more than 1 fill. MPR was calculated by subtracting days without TDF/FTC in possession from total days between first fill and end of follow-up, and dividing by total days of follow-up.17 Although 60% adherence (ie, taking at least 4 of 7 doses per week) may be sufficient to achieve high PrEP efficacy,18 an MPR below 80% may indicate that an individual needs additional support to maintain prevention-effective adherence19; thus, we defined low adherence as an MPR of <80%. We defined discontinuation as ≥ 120 days without TDF/FTC in possession.
Renal Safety
Our indicator of impaired renal function was an estimated glomerular filtration rate (eGFR) <70 mL·min−1·1.73 m−2, based on the Chronic Kidney Disease Epidemiology Collaboration equation,20,21 which provides a more accurate estimation of GFR compared with that of Cockcroft–Gault.22 We used an eGFR cutoff of <70 to allow for comparison with recent PrEP studies,23 and because this threshold acts as an early warning to clinicians that patients may need additional monitoring. Two clinicians (coauthors J.E.V. and D.P.N.) conducted independent chart reviews of individuals who had an eGFR <70 mL·min−1·1.73 m−2 and discontinued PrEP to determine whether PrEP was stopped for the duration of follow-up because of concerns about renal toxicity.
STI Testing and Infections
As part of the KPNC PrEP program, testing for urethral, pharyngeal, and rectal chlamydia and gonorrhea is conducted using nucleic acid amplification tests. Testing at each anatomic site is offered for both males and females, if indicated based on sexual exposures. Self-collection of throat and rectal swabs is available for most PrEP users. Serologic testing for syphilis is conducted using a rapid plasma reagin test and a treponemal IgG and IgM antibody test. We defined history of syphilis by positive antibody or reactive rapid plasma reagin tests, whereas syphilis at baseline and during follow-up was defined by receipt of treatment (ie, benzathine penicillin by intramuscular injection).
HIV Seroconversions
HIV seroconversions were identified using the KPNC HIV registry, which includes all known HIV/AIDS cases since the early 1980s, with HIV status confirmed by review of medical charts or medical center case lists. We assessed HIV seroconversions (1) during PrEP use and (2) after PrEP discontinuation among individuals who either remained KPNC members or reenrolled in the health plan after a gap in membership. A clinician (coauthor J.E.V.) conducted chart reviews of HIV seroconversions after discontinuation to identify reasons these individuals discontinued PrEP.
Statistical Analysis
Variables for analyses included age at PrEP initiation (<30, 30–39, 40–49, and ≥50 years); sex; race/ethnicity (White, Black, Hispanic, Asian/Pacific Islander, and other/ unknown); socioeconomic status (SES) as defined by median household income (<$50,000, ≥$50,000 USD) and the proportion without a high school diploma (≤20% and >20%) in the census block; average monthly copayment for TDF/FTC (≤$50, >$50 USD); current smoking (yes/no); eGFR at baseline (<90, ≥90 mL·min−1·1.73 m−2 in the 6 months before PrEP initiation); STI at baseline (yes/no in the 2 months before through 2 weeks after PrEP initiation); and history of STI, drug/alcohol abuse, hypertension, and diabetes (yes/no in the previous 2 years).
We used Poisson regression with robust variance to estimate unadjusted and adjusted risk ratios24 (RRs) for factors associated with (1) <80% adherence, (2) discontinuation, and (3) eGFR <70 mL·min−1·1.73 m−2 during follow-up. Multivariable models included variables for age, sex, race/ ethnicity, SES, monthly TDF/FTC copayment, smoking, baseline eGFR <90 mL·min−1·1.73 m−2, baseline STI, drug/alcohol abuse, hypertension, and diabetes. We plotted a Kaplan–Meier curve of the cumulative incidence of eGFR <70 mL·min−1·1.73 m−2, using the log-rank test for differences by age and baseline eGFR. We then computed the median number of STI tests per person-year, the cumulative incidence of STIs using Kaplan–Meier analysis, and STI positivity by quarter during the first year, with χ2 tests for trends in positivity over time.
Analyses were conducted in SAS 9.3 (Cary, NC) and Stata/SE 10.1 (College Station, TX). Statistical tests were 2-sided unless otherwise indicated, and statistical significance was defined as P < 0.05.
RESULTS
Study Population
The study population included 972 individuals initiating PrEP, with a total of 850 person-years of PrEP use and a mean of 0.9 years per person (range <0.1 to 2.9; Table 1). The mean age at PrEP initiation was 37.5 years (range 18–68), and 952 (97.9%) of PrEP users were male, with only 20 (2.1%) females. Of the 912 (93.8%) with known race/ ethnicity, most were White (69.6%), followed by Hispanic (12.2%), Asian (10.3%), Black (4.3%), and others (3.6%). Nearly one-quarter (23.3%) were current smokers and 6.3% had a history of drug/alcohol abuse. One-third (34.2%) had an STI documented at KPNC in the previous 2 years, and 15.9% had an STI at baseline. At the time of PrEP initiation, 32.0% had an eGFR <90 mL·min−1·1.73 m−2, and 10.8% and 3.0% had a history of hypertension and diabetes, respectively.
TABLE 1.
Characteristics of Individuals Initiating Preexposure Prophylaxis
| No. individuals | 972 |
| Person-years (mean/subject, range) | 850 (0.9, <0.1–2.9) |
| Status at end of study follow-up, % | |
| Continued to receive TDF/FTC | 69.7 |
| Discontinued TDF/FTC | 22.5 |
| Disenrolled from health plan | 7.8 |
| Age, mean (SD, range) | 37.5 (10.1, 18–68) |
| Male, % | 97.9 |
| Race/ethnicity, % among known | |
| White | 69.6 |
| Hispanic | 12.2 |
| Asian/Pacific Islander | 10.3 |
| Black | 4.3 |
| Other | 3.6 |
| Unknown race/ethnicity, % | 6.2 |
| % without high school diploma in census block, mean (SD) | 11.5 (11.1) |
| Median household income in census block, USD (IQR) | 74,094 (52,273–99,231) |
| Monthly TDF/FTC copayment in USD, mean (SD) | 34 (66) |
| Current smoking, % | 23.3 |
| eGFR <90 mL·min−1·1.73 m−2 at baseline, % | 32.0 |
| STI at baseline, % | 15.9 |
| STI in the previous 2 yrs, % | 34.2 |
| Gonorrhea, % | 13.0 |
| Chlamydia, % | 14.6 |
| Syphilis, % | 13.2 |
| Drug/alcohol abuse in the previous 2 yrs, % | 6.3 |
| Hypertension in the previous 2 yrs, % | 10.8 |
| Diabetes in the previous 2 yrs, % | 3.0 |
Adherence and Discontinuation
Among 915 individuals with >1 fill, the mean adherence as measured by MPR was 92% [median 97%, inter-quartile range (IQR) 90%–100%], with 118 (12.9%) having <80% adherence. Unadjusted and adjusted RRs for factors associated with <80% adherence are shown in Table 2. In multivariable analysis, Blacks compared with Whites [RR 3.0; 95% confidence interval (CI): 1.7 to 5.1, P < 0.001], individuals with higher TDF/FTC copayments (RR 2.0; 95% CI: 1.2 to 3.3, P = 0.005), and individuals who smoked (RR 1.6; 95% CI: 1.1 to 2.3, P = 0.025) were more likely to have <80% adherence.
TABLE 2.
Factors Associated With Low Adherence, Discontinuation, and Decreased eGFR During Preexposure Prophylaxis Use
| Adherence <80%
|
Discontinuation
|
eGFR <70 mL·min−1·1.73 m−2
|
||||
|---|---|---|---|---|---|---|
| Unadjusted RR (95% CI) | Adjusted RR (95% CI) | Unadjusted RR (95% CI) | Adjusted RR (95% CI) | Unadjusted RR (95% CI) | Adjusted RR (95% CI) | |
| Age <30 (ref. ≥50) | 1.7 (1.0 to 2.9) | 1.6 (0.8 to 3.3) | 1.3 (0.9 to 1.9) | 1.1 (0.7 to 1.9) | 0.1 (0.0 to 0.1) | 0.2 (0.1 to 0.5) |
| Age 30–39 (ref. ≥50) | 1.1 (0.6 to 1.8) | 1.2 (0.6 to 2.4) | 1.1 (0.8 to 1.7) | 1.2 (0.7 to 1.9) | 0.2 (0.1 to 0.3) | 0.5 (0.3 to 0.8) |
| Age 40–49 (ref. ≥50) | 0.6 (0.3 to 1.1) | 0.6 (0.3 to 1.3) | 1.0 (0.7 to 1.6) | 1.1 (0.7 to 1.7) | 0.5 (0.4 to 0.7) | 0.6 (0.4 to 0.9) |
| Females (ref. males) | 3.8 (2.1 to 6.7) | 2.2 (0.9 to 5.4) | 2.8 (1.9 to 4.0) | 2.6 (1.5 to 4.6) | NE | NE |
| Black (ref. White) | 2.8 (1.7 to 4.8) | 3.0 (1.7 to 5.1) | 1.2 (0.7 to 2.1) | 1.3 (0.7 to 2.4) | 0.8 (0.4 to 1.9) | 1.0 (0.5 to 2.2) |
| Hispanic (ref. White) | 1.5 (1.0 to 2.5) | 1.4 (0.8 to 2.5) | 1.5 (1.1 to 2.0) | 1.4 (0.9 to 1.9) | 0.3 (0.1 to 0.6) | 0.5 (0.2 to 1.1) |
| Asian/Pacific Islander (ref. White) | 0.5 (0.2 to 1.2) | 0.6 (0.2 to 1.4) | 0.8 (0.5 to 1.3) | 0.9 (0.5 to 1.5) | 0.8 (0.5 to 1.3) | 1.2 (0.7 to 2.3) |
| Other/unknown (ref. White) | 1.5 (0.9 to 2.5) | 1.5 (0.8 to 2.6) | 1.1 (0.7 to 1.6) | 1.0 (0.7 to 1.6) | 0.5 (0.3 to 1.0) | 0.6 (0.3 to 1.2) |
| Median household income <$50,000 | 1.1 (0.8 to 1.7) | 1.0 (0.7 to 1.6) | 1.3 (1.0 to 1.7) | 1.2 (0.9 to 1.6) | 0.8 (0.5 to 1.2) | 1.0 (0.7 to 1.5) |
| >20% without high school diploma | 1.1 (0.7 to 1.7) | 1.1 (0.7 to 1.7) | 1.0 (0.8 to 1.4) | 0.8 (0.6 to 1.2) | 0.9 (0.6 to 1.4) | 1.2 (0.8 to 1.7) |
| Monthly TDF/FTC copayment >$50 | 1.7 (1.1 to 2.7) | 2.0 (1.2 to 3.3) | 1.4 (1.0 to 2.0) | 1.3 (0.9 to 1.9) | 1.3 (0.9 to 2.1) | 1.1 (0.7 to 1.7) |
| Current smoking | 1.5 (1.0 to 2.1) | 1.6 (1.1 to 2.3) | 1.3 (1.1 to 1.8) | 1.3 (1.0 to 1.7) | 1.1 (0.8 to 1.5) | 1.1 (0.8 to 1.6) |
| eGFR <90 mL·min−1·1.73 m−2 at baseline | 0.6 (0.4 to 0.9) | 0.7 (0.4 to 1.2) | 0.8 (0.6 to 1.1) | 0.9 (0.7 to 1.2) | 10.3 (6.7 to 15.8) | 7.1 (4.3 to 11.6) |
| STI at baseline | 1.1 (0.7 to 1.8) | 1.0 (0.6 to 1.7) | 1.1 (0.8 to 1.5) | 1.0 (0.7 to 1.5) | 0.7 (0.5 to 1.2) | 0.9 (0.5 to 1.4) |
| History of drug/alcohol abuse | 1.2 (0.7 to 2.3) | 0.8 (0.4 to 1.7) | 2.0 (1.5 to 2.8) | 1.8 (1.3 to 2.6) | 1.3 (0.7 to 2.2) | 1.4 (0.9 to 2.1) |
| History of hypertension | 1.1 (0.7 to 1.9) | 1.4 (0.8 to 2.6) | 1.1 (0.8 to 1.6) | 1.1 (0.7 to 1.8) | 2.1 (1.5 to 3.0) | 1.0 (0.7 to 1.4) |
| History of diabetes | 0.9 (0.3 to 2.7) | 0.9 (0.3 to 3.3) | 1.1 (0.6 to 2.1) | 1.0 (0.5 to 2.3) | 2.2 (1.2 to 4.0) | 1.7 (1.0 to 2.8) |
RRs and P-values were obtained from Poisson regression with robust variance, with adjusted models including all variables listed. Bolded estimates were statistically significant at P < 0.05. Sex was not included in creatinine models because no females had decreased eGFR.
NE, not estimable.
At the end of follow-up, 677 (69.7%) continued to receive PrEP at KPNC, 219 (22.5%) discontinued, and 76 (7.8%) left the health plan. Of the 219 who discontinued, 38 (17.4%) restarted PrEP before the end of follow-up. In multivariable analysis, females (RR 2.6; 95% CI: 1.5 to 4.6, P < 0.001) and individuals with a history of drug/alcohol abuse (RR 1.8; 95% CI: 1.3 to 2.6, P = 0.002) were more likely to discontinue, with a borderline association for individuals who smoked (RR 1.3; 95% CI: 1.0 to 1.7, P = 0.055).
Renal Safety
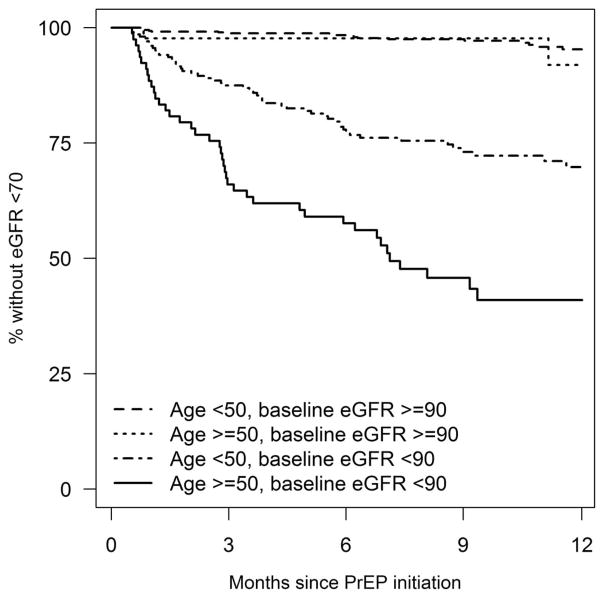
Among the 909 (93.5%) individuals with follow-up creatinine testing, 141 (15.5%) had an eGFR <70 mL·min−1·1.73 m−2, and 5 (0.6%) stopped PrEP for the duration of follow-up because of concerns about renal toxicity. The risk of eGFR <70 mL·min−1·1.73 m−2 differed by age and baseline eGFR (P < 0.001), with a cumulative incidence (95% CI) after 6 months ranging from 1.6% (0.9%–3.1%) among PrEP users aged <50 with baseline eGFR ≥90 mL·min−1·1.73 m−2 to 46.6% (36.4%–58.0%) among PrEP users aged ≥50 with baseline eGFR <90 mL·min−1·1.73 m−2 (Fig. 1). In multivariable analysis, younger age was associated with a reduced risk of eGFR <70 mL·min−1·1.73 m−2 during follow-up, with RRs (95% CI) of 0.2 for age <30 (0.1–0.5, P = 0.001), 0.5 for age 30–39 (0.3–0.8, P = 0.002), and 0.6 for age 40–49 (0.4–0.9, P = 0.007) compared with age ≥50 years. Baseline eGFR <90 mL·min−1·1.73 m−2 was associated with an increased risk of eGFR <70 mL·min−1·1.73 m−2 during follow-up (RR 7.1; 95% CI: 4.3 to 11.6, P < 0.001), with a borderline increased risk for individuals with diabetes (RR 1.7; 95% CI: 1.0 to 2.8, P = 0.063).
FIGURE 1.
Cumulative incidence of eGFR <70 mL·min−1·1.73 m−2 during the first year of preexposure prophylaxis use. Differences across strata of age and baseline eGFR were statistically significant at P < 0.001 by log-rank test.
STI Testing and Infections
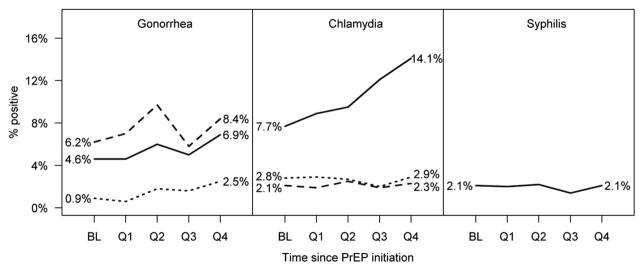
The median number of STI tests per person-year was 4.5 (IQR 2.6–6.4) for urethral gonorrhea/chlamydia, 3.6 (IQR 0–6.0) for rectal gonorrhea/chlamydia, 3.8 (IQR 0–6.0) for pharyngeal gonorrhea/chlamydia, and 4.9 (IQR 2.8–6.4) for syphilis. Of the 972 PrEP users, 342 were diagnosed with ≥1 STI during follow-up; of those, 173 had multiple STIs (range 2–19), for a total of 771 STIs diagnosed (90.7 per 100 person-years). The mean days between repeat STIs was 123, with 80.0% of repeat infections occurring <180 days apart. After 12 months of PrEP use, the cumulative incidence (95% CI) was 41.9% (38.1%–46.0%) for any STI, 26.7% (23.3%–30.5%) for any rectal STI, 26.3% (23.0%–29.9%) for chlamydia, 22.9% (19.7%–26.5%) for gonorrhea, and 7.3% (5.6%–9.5%) for syphilis. STI positivity increased from baseline through the first year for urethral gonorrhea (0.9%–2.5%, P = 0.012) and rectal chlamydia (7.7%–14.1%, P < 0.001), whereas all other STIs/ sites remained stable (Fig. 2).
FIGURE 2.
STIs at baseline (BL) and during the first year of preexposure prophylaxis use. For gonorrhea and chlamydia, solid, dashed, and dotted lines represent rectal, pharyngeal, and urethral infections, respectively, among those tested. For syphilis, the line represents treatment with benzathine penicillin among individuals at baseline and with any follow-up during each quarter. Increases were statistically significant for urethral gonorrhea (P = 0.012) and rectal chlamydia (P < 0.001).
HIV Seroconversions
There were no HIV seroconversions during 850 person-years of ongoing PrEP use (upper limit of one-sided 97.5% CI: 0.4%). However, 2 HIV seroconversions were identified after discontinuation of PrEP. One was a Black MSM aged <30 who received 1 TDF/FTC fill before losing insurance coverage. He later reestablished care at KPNC, having been diagnosed with acute HIV infection 6 months after discontinuing PrEP. The other was a Hispanic MSM aged <30 who received PrEP for 3 months before losing insurance coverage. Seven months later, he reenrolled in the health plan and contacted his physician to reinitiate PrEP. Initiation was delayed because of an acute viral syndrome that required inpatient admission, at which time he was diagnosed with acute HIV infection.
DISCUSSION
In the largest study to date of PrEP use in clinical practice, we observed no HIV infections during 850 person-years of ongoing PrEP use. Adherence was 92% overall; however, Black race/ethnicity, higher TDF/FTC copayments, and smoking were associated with lower adherence. Women and individuals with a history of drug/alcohol abuse were more likely to discontinue PrEP, but discontinuation because of renal toxicity was rare. PrEP users at KPNC accessed STI testing 4 to 5 times per year, with high STI positivity at baseline and quarterly throughout follow-up, suggesting that current recommendations to screen for STIs every 6 months may be inadequate. Finally, we observed 2 HIV seroconversions after PrEP discontinuation, highlighting the need to support continuation of PrEP throughout periods of HIV risk and across health care systems.
Using primarily telemedicine adherence support, we observed higher adherence than that seen in placebo-controlled1–3 or open-label5,6 PrEP studies. Higher adherence in clinical practice may be attributable to the absence of a placebo, the now well-established efficacy of PrEP, or a growing normalization of PrEP with increased use in the community.11,25 Notably, higher copayments were associated with lower adherence, indicating that cost is a barrier to consistent PrEP use and may exacerbate disparities even in an insured population. We also found that smoking, which may be a marker of recreational drug and alcohol use,26 was associated with lower adherence. Although drug and alcohol use were not associated with lower adherence in the Demo Project,6 there was a trend toward lower adherence with higher alcohol use in the open-label extension of the iPrEx study.5 The relationship between drug and alcohol use and adherence should be further explored in settings where substance use is directly measured. Finally, consistent with the Demo Project,6 we found that Black race/ethnicity was associated with lower adherence, a finding that is of particular concern given the disproportionately high risk of HIV acquisition in this group.27–29
Most PrEP users at KPNC continued to use PrEP throughout follow-up, and of those who discontinued, nearly 1 in 5 restarted. Unlike antiretroviral therapy for HIV treatment, PrEP is taken during periods of HIV risk rather than indefinitely; however, drug/alcohol abuse was associated with discontinuation, suggesting reasons for discontinuation other than reduced HIV risk. Furthermore, we identified 2 seroconversions after PrEP discontinuation, both in young men, 1 Black and 1 Hispanic, who became HIV-infected during gaps in insurance coverage. Strategies are needed to facilitate linkage to PrEP programs in other clinical settings and navigation of medication assistance programs30 for PrEP users who lose or switch insurance coverage.
Consistent with previous PrEP studies,6,31 we found that decreases in eGFR occurred but rarely resulted in long-term discontinuation of PrEP. Older age and lower eGFR at baseline were associated with decreases in eGFR during follow-up; consistent with recent findings from the Demo Project,32 we also observed a borderline increased risk for individuals with diabetes, a group excluded from PrEP trials.1,2 These risk factors for renal toxicity could inform discussions about initiation or continuation of PrEP, especially during periods of relatively low HIV risk. Incidence of decreased eGFR was very low among PrEP users with eGFR ≥90 mL·min−1·1.73 m−2 at baseline, suggesting that less frequent creatinine monitoring may be appropriate for these individuals.
STIs were common at baseline and throughout follow-up, as observed in previous PrEP studies,6,15,33,34 indicating an ongoing risk for HIV acquisition and highlighting the importance of STI education among individuals initiating PrEP. There were increases over time in urethral gonorrhea and rectal chlamydia positivity, which may reflect loss to follow-up or decreased STI testing among low-risk individuals, risk compensation, or secular trends in the community.35 Although current guidelines recommend STI screening every 6 months,36 STI positivity was high during each quarter in the first year of follow-up, reaching 14% for rectal chlamydia in 1 quarter. Furthermore, repeat STIs were common and most occurred fewer than 6 months apart. Given that rectal and pharyngeal STIs are likely to be asymptomatic,37 many of these infections would have remained undetected for months if screening had been conducted only twice per year. These findings from this largely male cohort of PrEP users, along with recent analyses of other PrEP cohorts,38,39 suggest that screening for STIs among PrEP users at 6-month intervals may delay the diagnosis and treatment of a substantial number of STIs, resulting in increased STI-related morbidity and STI transmission.
There are several limitations to our study. First, it was not feasible to assess levels of TDF/FTC in blood or hair, the gold standard for measurement of PrEP adherence. However, pharmacy refill-based adherence has been successfully used as a measure of adherence to antiretroviral therapy, predicting virologic response, AIDS, and death among HIV-infected individuals,40,41 and the MPR was more strongly correlated with TDF/FTC detection in blood compared with other adherence measures in the randomized iPrEx study.42 Second, we could not identify brief interruptions of PrEP using pharmacy refill data, including those related to decreased eGFR, and it was not feasible to identify these interruptions using chart review. However, we were able to use pharmacy data to identify long-term discontinuations of PrEP, and chart review of this subset revealed several discontinuations attributable to renal toxicity. Third, we defined new syphilis cases as individuals who were treated with benzathine penicillin; if some were treated presumptively because of exposure to syphilis, we may have overestimated syphilis prevalence and incidence. Fourth, we could not identify PrEP users who received reimbursement from a copayment assistance program30; thus, we may have underestimated the strength of the association between higher copayments and lower adherence. Fifth, we were not able to assess gender of sex partners or transgender identity using EHR data. However, using clinician-collected data, we previously found 99% MSM and only 1 transgender man in the subset of the KPNC cohort that initiated PrEP in San Francisco.11 Finally, although KPNC provides care to nearly half of insured individuals in the surrounding area, our cohort was largely male, older, White, and high SES, thus limiting the generalizability of our results to uninsured, female, younger, non-White, and lower-SES individuals at risk of HIV infection.
Our study also has several strengths. First, to our knowledge, this is the largest study of PrEP use in clinical practice. Second, this is the first study to evaluate adherence, discontinuation, renal safety, and the role of TDF/FTC copayments and loss of insurance coverage during PrEP implementation. Finally, we leveraged KPNC’s rich EHR to identify key risk factors, laboratory tests and results, and comorbidities among all individuals initiating TDF/FTC for PrEP, an approach that can be used to monitor long-term outcomes among PrEP users at KPNC and in similar clinical settings.
In summary, we found no new HIV infections among 972 active PrEP users in a large integrated health care system. PrEP is being accessed by a primarily male, White, and older population, indicating that outreach is needed to others at risk for HIV infection, including females, Blacks, and younger individuals. Adherence is high, but strategies are needed to increase affordability, facilitate PrEP access during gaps in insurance coverage, and support adherence and continuation among Blacks, females, and individuals with drug/alcohol abuse disorders. Finally, PrEP users access STI testing frequently and have high quarterly STI positivity, suggesting that STI screening every 6 months, as recommended by current guidelines,36 may be insufficient to prevent delays in treatment and ongoing transmission. Given no HIV seroconversions during ongoing PrEP use and excellent adherence and safety, our findings support the expanded implementation of PrEP.
Acknowledgments
Supported by a Kaiser Permanente Northern California Community Benefit grant to J.L.M.
Footnotes
Presented in part at the 23rd Conference on Retroviruses and Opportunistic Infections, February 25, 2016, Boston, MA.
J.L.M. has received research grant support from Merck. M.J.S. has received research grant support from Pfizer and Merck. The remaining authors have no conflicts of interest to disclose.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. [Accessed January 2, 2014];FDA Approves First Drug for Reducing the Risk of Sexually Acquired HIV Infection. 2012 Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm.
- 5.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2015;176:75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cremin I, Hallett TB. Estimating the range of potential epidemiological impact of pre-exposure prophylaxis: run-away success or run-away failure? AIDS. 2015;29:733–738. doi: 10.1097/QAD.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 8.Myers JE, Sepkowitz KA. A pill for HIV prevention: deja vu all over again? Clin Infect Dis. 2013;56:1604–1612. doi: 10.1093/cid/cit085. [DOI] [PubMed] [Google Scholar]
- 9.Scott HM, Klausner JD. Sexually transmitted infections and pre-exposure prophylaxis: challenges and opportunities among men who have sex with men in the US. AIDS Res Ther. 2016;13:5. doi: 10.1186/s12981-016-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siemieniuk RA, Bogoch II. Preexposure prophylaxis for HIV infection. N Engl J Med. 2015;372:1767–1768. doi: 10.1056/NEJMc1502749. [DOI] [PubMed] [Google Scholar]
- 11.Volk JE, Marcus JL, Phengrasamy T, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61:1601–1603. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus JL, Volk JE, Pinder J, et al. Successful implementation of HIV preexposure prophylaxis: lessons learned from three clinical settings. Curr HIV/AIDS Rep. 2016;13:116–124. doi: 10.1007/s11904-016-0308-x. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Figueroa RE, Kapadia F, Barton SC, et al. Acceptability of PrEP uptake among racially/ethnically diverse young men who have sex with men: the P18 Study. AIDS Educ Prev. 2015;27:112–125. doi: 10.1521/aeap.2015.27.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auerbach JD, Kinsky S, Brown G, et al. Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (PrEP) use among US women at risk of acquiring HIV. AIDS Patient Care STDS. 2015;29:102–110. doi: 10.1089/apc.2014.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volk JE, Marcus JL, Phengrasamy T, et al. Incident hepatitis C virus infections among users of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;60:1729–1729. doi: 10.1093/cid/civ129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karter AJ, Ferrara A, Liu JY, et al. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 17.Steiner JF, Koepsell TD, Fihn SD, et al. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26:814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haberer JE, Bangsberg DR, Baeten JM, et al. Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. AIDS. 2015;29:1277–1285. doi: 10.1097/QAD.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313:837–846. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michels WM, Grootendorst DC, Verduijn M, et al. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5:1003–1009. doi: 10.2215/CJN.06870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi M, Glidden DV, Liu AY, et al. Higher cumulative TFV/FTC levels in PrEP associated with decline in renal function. 23rd Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. February 22–25, 2016. [Google Scholar]
- 24.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Flash C, Landovitz R, Giler RM, et al. Two years of Truvada for pre-exposure prophylaxis utilization in the US. J Int AIDS Soc. 2014;17(4 suppl 3):19730. doi: 10.7448/IAS.17.4.19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daskalopoulou M, Rodger A, Phillips AN, et al. Recreational drug use, polydrug use, and sexual behaviour in HIV-diagnosed men who have sex with men in the UK: results from the cross-sectional ASTRA study. Lancet HIV. 2014;1:e22–e31. doi: 10.1016/S2352-3018(14)70001-3. [DOI] [PubMed] [Google Scholar]
- 27.Vital signs: HIV infection, testing, and risk behaviors among youths—United States. MMWR Morb Mortal Wkly Rep. 2012;61:971–976. [PubMed] [Google Scholar]
- 28.Disparities in diagnoses of HIV infection between blacks/African Americans and other racial/ethnic populations–37 states, 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:93–98. [PubMed] [Google Scholar]
- 29.Hess K, Hu X, Lansky A, et al. Estimating the lifetime risk of a diagnosis of HIV infection in the United States. 23rd Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. February 22–25, 2016. [Google Scholar]
- 30.Gilead Sciences, Inc. [Accessed July 9, 2015];Truvada for PrEP Medication Assistance Program. Available at: http://www.gilead.com/responsibility/us-patient-access/truvada%20for%20prep%20medication%20assistance%20program.
- 31.Solomon MM, Lama JR, Glidden DV, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014;28:851–859. doi: 10.1097/QAD.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu AY, Vittinghoff E, Anderson PL, et al. Changes in renal function associated with TDF/FTC PrEP use in the U.S. Demo Project. 23rd Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. February 22–25, 2016. [Google Scholar]
- 33.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 35.Chen YH, Snowden JM, McFarland W, et al. Pre-exposure prophylaxis (PrEP) use, seroadaptation, and sexual behavior among men who have sex with men, San Francisco, 2004–2014. AIDS Behav. 2016 doi: 10.1007/s10461-016-1357-2. [DOI] [PubMed] [Google Scholar]
- 36. [Accessed December 30, 2014];Preexposure Prophylaxis for the Prevention of HIV Infection in the United States—2014: A Clinical Practice Guideline. 2014 Available at: http://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf.
- 37.Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005;41:67–74. doi: 10.1086/430704. [DOI] [PubMed] [Google Scholar]
- 38.Cohen SE, Vittinghoff E, Philip SS, et al. Quarterly STI screening optimizes STI detection among PrEP users in the demo project. 23rd Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. February 22–25, 2016. [Google Scholar]
- 39.Golub SA, Pena S, Boonrai K, et al. STI data from community-based PrEP implementation suggest changes to CDC guidelines. 23rd Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. February 22–25, 2016. [Google Scholar]
- 40.Gross R, Yip B, Lo Re V, III, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194:1108–1114. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- 41.Kitahata MM, Reed SD, Dillingham PW, et al. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. Int J STD AIDS. 2004;15:803–810. doi: 10.1258/0956462042563666. [DOI] [PubMed] [Google Scholar]
- 42.Amico KR, Marcus JL, McMahan V, et al. Study product adherence measurement in the iPrEx placebo-controlled trial: concordance with drug detection. J Acquir Immune Defic Syndr. 2014;66:530–537. doi: 10.1097/QAI.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]