Abstract
Cyclic tetrapyrroles are the active core of compounds with crucial roles in living systems, such as hemoglobin and chlorophyll, and in technology as photocatalysts and light absorbers for solar energy conversion. Zinc tetraphenylporphyrin (Zn-TPP) is a prototypical cyclic tetrapyrrole that has been intensely studied in past decades. Due to its importance for photo-chemical processes the optical properties are of particular interest and, accordingly, numerous studies have focused on light absorption and excited-state dynamics of Zn-TPP. Relaxation after photoexcitation in the Soret band involves internal conversion that is preceded by an ultrafast process. This relaxation process has been observed by several groups. Hitherto, it has not been established if it involves a higher lying “dark” state or vibrational relaxation in the excited S2 state. Here we combine high time resolution electronic and vibrational spectroscopy to show that this process constitutes vibrational relaxation in the anharmonic S2 potential.
Keywords: Porphyrinoids, ultrafast relaxation, excited state dynamics, ultrafast vibrational spectroscopy
Graphical Abstract
Porphyrins and other heterocyclic pyrroles are among the most ubiquitous photoactive molecules in nature, science and technology. They are at the center of research endeavors reaching from photodynamic cancer therapy,1 solar energy conversion2 to catalysis,3 and they are a core component of many new materials like metal-organic frameworks.4,5 Porphyrins are ideal candidates for these applications due to the combination of optical properties with their large versatility with respect to structural modifications like substitutions at the periphery and of the center metal atom. Accordingly, a large amount of literature is available covering all aspects of porphyrins including their ultrafast excited-state dynamics, a property that is essential in many applications mentioned above. Zn-tetraphenylphorphyrin (Zn-TPP) is a prototypical member of this group. It is among the most extensively studied metal-porphyrins and its excited-state dynamics is often used as a reference for more complex systems like molecular aggregates.6–9 While many aspects of Zn-TPP excited-state dynamics are well understood, one remaining question concerns the initial relaxation in the S2 state. Controversy exists if this process can be attribute to vibrational relaxation in the S2 state or if a second excited state ( ) is responsible.7,10,11 While it is in general assumed that this initial dynamics involves some kind of vibrational relaxation, hitherto, no direct measurement has been presented to confirm this assertion.7,12 This process was first observed by Gustavson’s group using time resolved fluorescence and it was assigned to vibrational relaxation (IVR).12 Later, Zewail’s group recovered the fast time constant in transient absorption measurements and assigned it to a distinct higher lying state above the S2 energy surface.10 Enescu et al. confirmed the existence of fast dynamics in or above the S2 state and assigned it to a relaxation of the coherently excited degenerate S2 state.11 The Steer group discussed both possibilities without giving an unambiguous assignment.13,14
Here we focus on an unequivocal assignment of the initial relaxation dynamics. We employed pump-degenerate four-wave mixing (pump-DFWM) to study vibrational dynamics in the first 100s of femtoseconds. The observed continuous shift of an inner ring stretching mode that is coupled to electronic excitation of the molecule in the S2 state is characteristic for vibrational relaxation in the anharmonic potential energy surface (PES). At the same time, the absence of coexisting modes excludes the presence of an additional state. Excitation wavelength dependent pump-DFWM and transient absorption (TA) measurement confirm the assignment.
Porphyrin excited-state dynamics are susceptible to complications stemming from the presence of molecular aggregates.15–17 To ensure the integrity of the prepared Zn-TPP solutions, careful measurements of the electronic spectrum were performed over a range of concentrations. Steady state absorption spectra show no sign of aggregation (Figure S3)18,19 Fluorescence excitation and emission spectra similarly provide no evidence of alteration to the electronic structure of the molecule, showing consistent spectral features down to a concentration of 0.1 μM. The high solubility of Zn-TPP in THF serves to mitigate aggregation.20 Measurements were performed with 50 μM for transient absorption and 100 μM for pump-DFWM. Further indication of sample purity was provided by liquid injection field desorption mass spectrometry, which showed no demetallated porphyrin or molecular substructures (Figure S5).
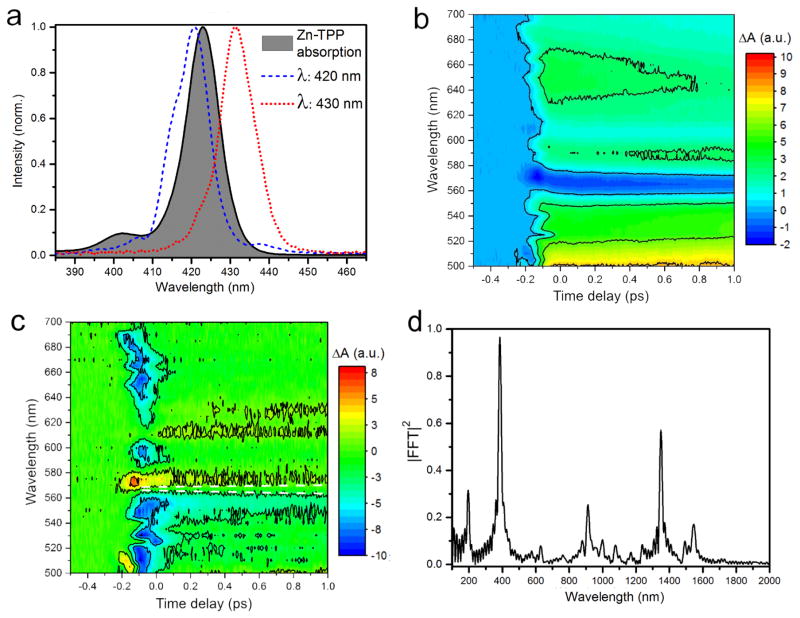
A ground state spectrum of Zn-TPP together with spectra of the excitation pulses is shown in Figure 1a. A transient absorption map after 420 nm excitation on the blue side of the Soret band is shown in Figure 1b. The map confirms the data that has been presented previously in Ref. 7. Individual kinetic traces at representative wavelength and fits are shown in Figure S6. excited-state absorption is observed across the visible spectrum, with negative contributions from ground-state bleach (GSB) of the Q band near 550 nm and 600 nm. The contributions have been assigned by global fitting to a rate model discussed in the supporting information and result in five time constants: τ1= 70 fs, initial relaxation in or above S2; τ2= 1.8 ps, S2 - S1 internal conversion (IC); τ3= 10 ps and τ4= 100 ps, vibrational relaxation in S1; τ5= 1.8 ns intersystem crossing to T1. Our measurements are in very good agreement with recent literature values. However, the fastest component, τ1, has been assigned based on order of magnitude arguments only.7 This component has been reported several times with time-constants of around 100 fs. It was attributed to S2 population dynamics, because it was not present after exciting the Q band and it was not observed in SE from the Q band. Direct S2 - S0 relaxation can also be excluded because GSB recovery was not observed on this time scale. It should be mentioned that at delay times shorter than 500 fs the signal between 510 nm and 540 nm stems predominantly from S2 absorption, since IC to S1 occurs on a 1 ps time scale. Accordingly, internal conversion from a dark state above S2, or relaxation inside the S2 PES have been discussed as possible explanations. The latter explanation raises the question how vibrational cooling leads to a rise time in the TA signal. It is not a priori clear why a hot S2 state should have weaker excited-state absorption compared to the cold state. In general one would expect a peak shift associated with cooling. However, the fact that this component manifests itself as a rise of the TA signal is often neglected and not discussed in literature. Hitherto no attempts have been made to study this component after excitation on the red side of the Soret band as suggested by Enescu et al.11 This is a straightforward approach to probe how the process depends on the presence of excess energy. Excitation of Zn-TPP with 430 nm gives rise to the TA map shown in Figure S7. The map is very similar to the one in Figure 1b, however, fits at the 3 indicated wavelength confirmed that the rise of the signal is instantaneous within the IRF of 25 fs. Thus, the fast component is absent if excitation occurs with lower photon energy. The transient absorption difference map that results from subtracting a measurement with 420 nm excitation from one with 430 nm excitation is shown in Figure 1c. It can clearly be seen that contributions to the signal have been redistributed from around 550 nm to around 570 nm upon increase of the excitation wavelength. At 570 nme it overlaps spectrally with the strong ground state bleach. This redistribution equilibrates after 200 fs. One way to explain the measurement is to assume a formation of a hot S2 state. Transient absorption of the hot S2 is red shifted and appears at the same energy as the S0-S1 transition and reduces the ground state bleach signal. Upon cooling the S2 state shifts to the blue and both transient absorption maps become identical, resulting in a loss of signal in the difference map. A simple simulation of a difference map composed of a hot state that is shifting towards a cold state at constant wavelength is shown in Figure S8. The simulation predicts a shift of the zero crossing point. However, the measurement is not conclusive. While the negative contribution clearly shifts to the blue, the positive contribution remains at a constant wavelength. A constant zero-crossing line is analogous to an isobestic point and would be indicative of a population redistribution between two states, i.e. from the state at 570 nm to the S2 state at 550 nm. However, the dynamic of the spectral shift is not known and does not have to be linear as it was assumed in our calculation. In addition, the map shown in Figure 1c is the difference of two normalized transient absorption difference spectra and can include subtraction artifacts. Thus, no conclusive assignment can be made based on these measurements.
Figure 1.
a) Ground state spectrum of Zn-TTP Soret band absorption and spectra of actinic pump pulses. b) TA map of Zn-TPP after excitation at 420 nm. c) TA difference map between (b) and a map recorded after 430 nm excitation on the red side of the Soret band (Figure S6). The dashed lines are guides to the eye. A vertical line would be indicative of repopulation between states, a sloped line would indicate a peak shift. d) Ground-state state resonant pump-DFWM spectrum.
As can be seen in Figure 1b, interpretation of the ultrafast excited-state dynamics of porphyrins are complicated by the manifold of molecular states with broad overlapping absorption spectra. A method that can identify vibrational relaxation of an individual vibrational mode that is coupled to the S0-S2 transition is necessary to unequivocally assign the initial relaxation to either vibrational cooling or to a higher lying state. Pump-DFWM allows measurement of changes in the vibrational spectrum on the sub-100 fs time scale and it is sensitive only to modes that couple to electronic excitation.
Pump-DFWM employs an initial actinic pump pulse preceding a four-wave mixing pulse sequence that measures Raman active modes in the time-domain. Modes that are coupled to the S2 state are selectively measured by: Firstly, subtracting a ground state measurement with blocked actinic pulse on a shot-to-shot basis. Secondly, by resonance enhancement via tuning the wavelength of the DFWM pulses in resonance with S2 excited-state absorption (Figure S2). And thirdly, by narrowing the waiting time window that is Fourier transformed into a spectrum to below 600 fs such that the vibrational spectrum is measured before S2-S1 internal conversion takes place. The oscillatory signal as a function of the waiting time t contains information on structural dynamics and dephasing times of the resonant PES. A scan range of 600 fs combined with 12 fs pulse length allows high frequency resolution of 3 cm−1 beyond 2000 cm−1. Experiments were performed with pulse energy below 50 nJ. The DFWM signal was recorded after a monochromator that was set at 510 nm with 3 nm FWHM. The setup was aligned to maximize the amplitude of the 1352 cm−1 mode at 1 ps actinic pump delay. The optical setup, the pulse sequence and the phase-matching condition are depicted in Figure S1.
The background free time-domain DFWM measurement eliminates influence from fluorescence that typically obfuscates Raman spectra of solution-phase Zn-TPP. A comparison between a ground state spectrum and a spectrum after excitation of the Soret band at 420 nm is shown in Figs. 1d and 2a. The excited-state spectrum reveals the presence of two major vibrational modes at 385 cm−1 (Zn-pyrrole stretch) and 1352 cm−1 (inner ring stretch)21,22 as well as an intense induced solvent mode at 910 cm−1.23 The ground state spectrum of Raman active modes agrees well with published results.24 Additional peaks at 180 cm−1, 650 cm−1, 1050 cm−1, and 1560 cm−1 are present in the ground state DFWM spectrum, but strongly suppressed in the excited-state spectrum. The presence of the non-resonant solvent peak in the pump-DFWM spectrum is unexpected. It is possible that the difference of absorbance at the DFWM wavelength before and after Soret excitation leads to oversubtraction, which would also explain the phase shift. However, careful rescaling with the amplitude of the non-oscillating signal that is proportional to the pulse intensity did not remove the solvent peak entirely and did not affect the phase shift. An alternative explanation is the change of the polarizability of the solvent shell due to the excitation of Zn-TPP. This interpretation would associate the phase-shift of π to the delayed response of the solvent shell (Figure 2b). However, an in-depth study of the solvent response is not the scope of this paper and none of the above explanations can be excluded.
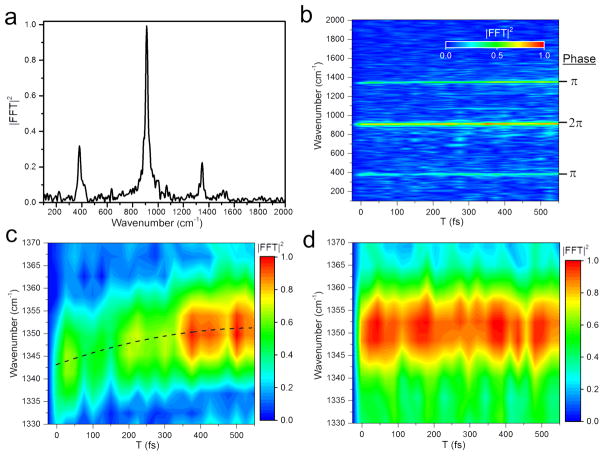
Figure 2.
a) excited-state resonant pump-DFWM spectra (T=100 fs). Dominant features are 385 cm−1 metalpyrrrole stretch, 1352 cm−1 inner ring stretch, and 910 cm−1 solvent mode. b), c), and d) Actinic pump delay dependent DFWM spectra and magnification of the 1350 cm−1 mode at c) 420 nm excitation and d) 430 nm excitation. The black dotted line is a fit with a 12 cm−1/85 fs time constant.
Figures 2b, c, and d show maps of the Fourier transformed waiting time t that corresponds to the DFWM spectrum plotted as a function of the actinic pump delay (T). The dominant mode around 1350 cm−1 in Figure 2b shows a time dependent shift towards higher vibrational frequencies while no shift is observed for the lower 385 cm−1 mode. A small shift of around 3 cm−1 is observed in the solvent mode that is just within the resolution of the instrument. However, the absence of the shift in the 385 cm−1 mode excludes a systematic error. Figure 2c shows a magnification of the inner ring stretching mode that shifts from 1340 cm−1 to 1352 cm−1 in about 85 fs. The frequency shift is accompanied by a rise in amplitude in about 250 fs. The 385 cm−1 Zn-TPP mode shows no clear rise (Figure S9), while the solvent mode shows a much smaller rise.
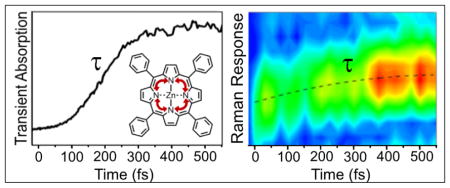
The continuous frequency shift towards higher vibrational energies can be explained by relaxation in an anharmonic S2 potential.25–27 As the vibrational energy spacing increases, the vibrational energy increases. The shift in frequency is best fit with a second order polynomial. This fit would indicate a third order perturbation to the harmonicity of the potential energy surface.28 Computational studies beyond the scope of this work could provide more detailed insight into the exact shape of the PES. The continuous rise of the signal amplitude can be explained by a shift of the transition into resonance for S2 excited-state absorption while the mode relaxes.7,11 It is consistent with the TA kinetics that show a rise in excited-state absorption on the same timescale.
The alternative explanation that involves intersystem crossing between a dark state and S2 would not result in a continuous shift in frequency. For this case two scenarios are possible. If both states couple to the same mode no change in frequency would be expected. In case both states couple to different modes, an abrupt change in frequency should be observed.25,29 Thus the continuous shift of the 1352 cm−1 mode over 12 cm−1 is strong evidence that the initial dynamics that has been observed in numerous TA measurements is associated with vibrational cooling in the S2 state. At lower pump energy the S2 state is excited in the vibrational ground state and no relaxation is expected in this case. Measurements with lower excitation energy at 430 nm show the expected absence of the vibrational shift as well as a constant signal (Figure 2d). While in itself this is not a proof for vibrational cooling, it supports the assignment. As mentioned above, a detailed analysis of the data would allow to extract the shape of the PES in direction of the 1352 cm−1 mode. Unfortunately, knowledge about the rate of IVR that is necessary to connect the peak shift kinetics to the PES profile is not available. However, an order of magnitude consistency check can be done using common anharmonic corrections. For the benzene ring stretching mode at 1494 cm−1 the correction is 10 cm−1, slightly below the 12 cm−1 correction we observed for the 1352 cm−1 inner ring stretching mode but in overall good agreement.30
We applied TA and pump-DFWM to elucidate the origin of the initial relaxation dynamics on the 100 fs time scale that has been reported for Zn-TPP two decades ago. Comparing TA maps for different excitation energies we were able to explain the observed rise time in the TA signals as a shift or redistribution of population from 550 nm to 570 nm. The TA maps did not allow a conclusive assignment of the dynamics to either IVR or IC from a higher lying dark state. Pump-DFWM with excitation on the blue side of the Soret band revealed that the inner ring stretching mode that couples strongly to the excitation shifts on the relevant time scale. This mode showed a continuous shift over 12 cm−1 and a continuous increase in peak amplitude. The continuous shift and rise can only be explained by IVR in the anharmonic S2 PES and is not compatible with IC. Pump-DFWM after excitation on the red side of the Soret band showed no signal rise or peak shifts as expected. Given the structural and electronic similarities between metal-porphyrins, these results can likely be extended to other compounds. Pump-DFWM has proven to be an appropriate tool to reveal electronic-vibrational coupling and relaxation pathways on the sub 100 fs time scale. Increased sensitivity and improved signal to noise ratio will allow internal vibrational redistribution to be followed in real time and will reveal the vibrational modes that are involved in chemical reactions.
Supplementary Material
Acknowledgments
We thank David McCamant for fruitful discussions. J.N.-P. acknowledges support from the NIH COBRE Program, NIH NIGMS P20GM104316.
Footnotes
The Supporting Information is available free of charge on the ACS Publications website at DOI:.
Sample preparation, sample characterization, pump-DFWM setup, wavelength dependent TA measurements, and additional pump-DFWM data.
References
- 1.Lu K, He C, Lin W. A Chlorin-Based Nanoscale MetalOrganic Framework for Photodynamic Therapy of Colon Cancers. Journal of the American Chemical Society. 2015;137:7600–7603. doi: 10.1021/jacs.5b04069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie Y, Tang Y, Wu W, Wang Y, Liu J, Li X, Tian H, Zhu WH. Porphyrin Cosensitization for a Photovoltaic Efficiency of 11. 5%: A Record for Non-Ruthenium Solar Cells Based on Iodine Electrolyte. Journal of the American Chemical Society. 2015;137:14055–14058. doi: 10.1021/jacs.5b09665. [DOI] [PubMed] [Google Scholar]
- 3.Omagari T, Suzuki A, Akita M, Yoshizawa M. Efficient Catalytic Epoxidation in Water by Axial N-Ligand-Free Mn-Porphyrins within a Micellar Capsule. Journal of the American Chemical Society. 2016;138:499–502. doi: 10.1021/jacs.5b11665. [DOI] [PubMed] [Google Scholar]
- 4.Williams DE, Rietman JA, Maier JM, Tan R, Greytak AB, Smith MD, Krause JA, Shustova NB. Energy Transfer on Demand: Photoswitch-Directed Behavior of Metal Porphyrin Frameworks. Journal of the American Chemical Society. 2014;136:11886–11889. doi: 10.1021/ja505589d. [DOI] [PubMed] [Google Scholar]
- 5.Son HJ, Jin S, Patwardhan S, Wezenberg SJ, Jeong NC, So M, Wilmer CE, Sarjeant AA, Schatz GC, Snurr RQ, et al. Light-Harvesting and Ultrafast Energy Migration in Porphyrin-Based MetalOrganic Frameworks. Journal of the American Chemical Society. 2013;135:862–869. doi: 10.1021/ja310596a. [DOI] [PubMed] [Google Scholar]
- 6.Kumble R, Palese S, Lin VSY, Therien MJ, Hochstrasser RM. Ultrafast Dynamics of Highly Conjugated Porphyrin Arrays. Journal of the American Chemical Society. 1998;120:11489–11498. [Google Scholar]
- 7.Kullmann M, Hipke A, Nuernberger P, Bruhn T, Gotz DCG, Sekita M, Guldi DM, Bringmann G, Brixner T. Ultrafast Exciton Dynamics After Soret- or Q-band Excitation of a Directly β, β′-linked Bisporphyrin. Phys Chem Chem Phys. 2012;14:8038–8050. doi: 10.1039/c2cp23608g. [DOI] [PubMed] [Google Scholar]
- 8.Perdomo-Ortiz A, Widom JR, Lott GA, Aspuru-Guzik A, Marcus AH. Conformation and Electronic Population Transfer in Membrane-Supported Self-Assembled Porphyrin Dimers by 2D Fluorescence Spectroscopy. The Journal of Physical Chemistry B. 2012;116:10757–10770. doi: 10.1021/jp305916x. [DOI] [PubMed] [Google Scholar]
- 9.Colvin MT, Smeigh AL, Giacobbe EM, Conron SMM, Ricks AB, Wasielewski MR. Ultrafast Intersystem Crossing and Spin Dynamics of Zinc meso-Tetraphenylporphyrin Covalently Bound to Stable Radicals. The Journal of Physical Chemistry A. 2011;115:7538–7549. doi: 10.1021/jp2021006. [DOI] [PubMed] [Google Scholar]
- 10.Yu HZ, Baskin JS, Zewail AH. Ultrafast Dynamics of Porphyrins in the Condensed Phase: II. Zinc Tetraphenylporphyrin. The Journal of Physical Chemistry A. 2002;106:9845–9854. [Google Scholar]
- 11.Enescu M, Steenkeste K, Tfibel F, Fontaine-Aupart MP. Femtosecond Relaxation Processes from Upper Excited States of Tetrakis(N-methyl-4-pyridyl)porphyrins Studied by Transient Absorption Spectroscopy. Phys Chem Chem Phys. 2002;4:6092–6099. [Google Scholar]
- 12.Gurzadyan GG, Tran-Thi TH, Gustavsson T. Time-resolved Fluorescence Spectroscopy of High-lying Electronic States of Zn-tetraphenylporphyrin. The Journal of Chemical Physics. 1998;108:385–388. [Google Scholar]
- 13.Liu X, Tripathy U, Bhosale SV, Langford SJ, Steer RP. Photophysics of Soret-Excited Tetrapyrroles in Solution. II. Effects of Perdeuteration, Substituent Nature and Position, and Macrocycle Structure and Conformation in Zinc(II) Porphyrins. The Journal of Physical Chemistry A. 2008;112:8986–8998. doi: 10.1021/jp804792x. [DOI] [PubMed] [Google Scholar]
- 14.Lukaszewicz A, Karolczak J, Kowalska D, Maciejewski A, Ziolek M, Steer RP. Photophysical processes in electronic states of zinc tetraphenyl porphyrin accessed on one- and two-photon excitation in the soret region. Chemical Physics. 2007;331:359– 372. [Google Scholar]
- 15.Tripathy U, Kowalska D, Liu X, Velate S, Steer RP. Photophysics of Soret-Excited Tetrapyrroles in Solution. I. Metalloporphyrins: MgTPP, ZnTPP, and CdTPP. The Journal of Physical Chemistry A. 2008;112:5824–5833. doi: 10.1021/jp801395h. [DOI] [PubMed] [Google Scholar]
- 16.Moore B, Autschbach J. Density Functional Study of Tetraphenylporphyrin Long-Range Exciton Coupling. ChemistryOpen. 2012;1:184–194. doi: 10.1002/open.201200020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khairutdinov RF, Serpone N. Photo-luminescence and Transient Spectroscopy of Free Base Porphyrin Aggregates. The Journal of Physical Chemistry B. 1999;103:761–769. [Google Scholar]
- 18.Li Ye LMX, Han Wei-Wei. Spectroscopic and Crystal Structural Analyses of Zinc (II) Tetraphenylporphyrin J-aggregates. Acta Physico-Chimica Sinica. 2009;25:2493. [Google Scholar]
- 19.Li Y, Steer RP. Kinetics of disaggregation of a non-covalent zinc tetraphenylporphyrin dimer in solution. Chemical Physics Letters. 2003;373:94– 99. [Google Scholar]
- 20.Karolczak J, Kowalska D, Lukaszewicz A, Maciejewski A, Steer RP. Photophysical Studies of Porphyrins and Metalloporphyrins: Accurate Measurements of Fluorescence Spectra and Fluorescence Quantum Yields for Soret Band Excitation of Zinc Tetraphenylporphyrin. The Journal of Physical Chemistry A. 2004;108:4570–4575. [Google Scholar]
- 21.Even U, Magen J, Jortner J, Friedman J, Levanon H. Isolated ultra-cold porphyrins in supersonic expansions. I. Free-base tetraphenylporphyrin and Zn-tetraphenylporphyrin. The Journal of Chemical Physics. 1982;77:4374–4383. [Google Scholar]
- 22.Spiro TG, Czernuszewicz RS, Li XY. Metalloporphyrin structure and dynamics from resonance raman spectroscopy. Coordination Chemistry Reviews. 1990;100:541– 571. [Google Scholar]
- 23.Hauer J, Buckup T, Motzkus M. Pump-Degenerate Four Wave Mixing as a Technique for Analyzing Structural and Electronic Evolution: Multidimensional Time-Resolved Dynamics near a Conical Intersection. The Journal of Physical Chemistry A. 2007;111:10517–10529. doi: 10.1021/jp073727j. [DOI] [PubMed] [Google Scholar]
- 24.Hard AP, Jayasooriya UA, Cammidge AN. Molecular sensors using a resonance Raman template. Analyst. 2003;128:70–74. doi: 10.1039/b209526b. [DOI] [PubMed] [Google Scholar]
- 25.Marek MS, Buckup T, Motzkus M. Direct Observation of a Dark State in Lycopene Using Pump-DFWM. The Journal of Physical Chemistry B. 2011;115:8328–8337. doi: 10.1021/jp202753j. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Yu W, Bragg AE. Structural Relaxation of Photoexcited Quaterthiophenes Probed with Vibrational Specificity. The Journal of Physical Chemistry Letters. 2015;6:3496–3502. doi: 10.1021/acs.jpclett.5b01472. [DOI] [PubMed] [Google Scholar]
- 27.Oliver TAA, Lewis NHC, Fleming GR. Correlating the Motion of Electrons and Nuclei with Two-Dimensional ElectronicVibrational Spectroscopy. Proceedings of the National Academy of Sciences. 2014;111:10061–10066. doi: 10.1073/pnas.1409207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bender CM, Wu TT. Anharmonic Oscillator. Phys Rev. 1969;184:1231–1260. [Google Scholar]
- 29.Brazard J, Bizimana LA, Gellen T, Carbery WP, Turner DB. Experimental Detection of Branching at a Conical Intersection in a Highly Fluorescent Molecule. The Journal of Physical Chemistry Letters. 2016;7:14–19. doi: 10.1021/acs.jpclett.5b02476. [DOI] [PubMed] [Google Scholar]
- 30.Goodman L, Ozkabak AG, Thakur SN. A Benchmark Vibrational Potential Surface: Ground-State Benzene. The Journal of Physical Chemistry. 1991;95:9044–9058. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.