Abstract
Despite tremendous advances in critical care, multiple-organ failure continues to be a significant problem. However, in recent years, far fewer patients with multiple-organ failure die early, but many experience ongoing immune dysregulation and are developing persistent inflammation, immunosuppression, and catabolism syndrome (PICS). Most PICS patients are discharged to nonhome destinations, fail to rehabilitate, and succumb to indolent death. From a nutrition perspective, patients with PICS experience persistent inflammation-induced cachexia despite evidenced-based recommended intensive care unit nutrition support. Recent basic and translational research indicates that prolonged expansion of myeloid-derived suppressor cells plays a central role in the pathogenesis of PICS. Myeloid-derived suppressor cells express arginase 1, which depletes arginine, causing immunosuppression and impaired wound healing. This is the rationale for arginine supplementation in PICS. Other nutrition support recommendations for PICS are based on inferences made from other patient populations who experience similar persistent inflammation-induced cachexia. These include patients with established cancers, major burns, and sarcopenia. These patients experience anabolic resistance, but studies show that this can be overcome by providing higher levels of protein and certain specific amino acids. Nutrition support guidelines recommend provision of >1.5 g/kg/d of protein and indicate that higher levels may be needed. Protein composition is also important. There is good evidence that leucine can promote anabolism in patients with cancer and sarcopenia. Finally, anabolic interventions—including intensive insulin, oxandrolone, propranolol, and resistance exercise—have proven to be effective in patients with major burns and are likely relevant in combating PICS cachexia.
Keywords: critical illness, nutritional support, enteral nutrition, parenteral nutrition, dietary proteins, multiple organ failure, inflammation, arginine, leucine
Multiple-organ failure (MOF) has plagued surgical intensive care units (ICUs) for >4 decades. Its epidemiology has evolved through a series of predominant clinical presentations (or phenotypes) that have faded in importance as a result of research and resultant advances in care that were directed at them.1 Irrespective of the phenotype, patients with MOF consume tremendous healthcare resources, and long-term mortality remains prohibitively high. The term persistent inflammation, immunosuppression, and catabolism syndrome (PICS) has been coined to describe the most recent observed phenotype of chronic MOF, which we believe represents the next challenge in surgical critical care. Patients with PICS experience prolonged low-grade inflammation and catabolism with resultant loss of lean body mass. Thus, nutrition support is assumed to be an important and potentially pivotal component of their care. Early enteral nutrition (EEN) has been shown to be beneficial in MOF primarily in preventing nosocomial infections.2-4 However, EEN fails to prevent ongoing catabolism. Traditionally, this was presumed to be due to difficulties in placing patients in early positive caloric and nitrogen balance with EEN. However, attempts to optimized EEN with feeding protocols or by using enteral protein supplements or concurrent parenteral nutrition (PN) have not prevented the progressive cachexia seen in these ICU survivors. To better understand the implications of nutrition support in PICS, this article discusses the PICS paradigm, PICS cachexia, nutrition support, and anabolic adjuncts.
PICS Paradigm
The PICS paradigm (see Figure 1) is based on recent clinical observations and research data.1,5 Following major insults (trauma, burns, pancreatitis, sepsis), there is simultaneous systemic proinflammation (called systemic inflammatory response syndrome) and anti-inflammation (called compensatory anti-inflammatory response syndrome). In some cases, systemic inflammatory response syndrome can become overwhelming, leading to an early MOF and fulminant death trajectory. Fortunately, modern ICU care is directed at early detection and prevention of this trajectory's fatal expression. If patients do not die of early MOF, there are 2 alternatives. Their aberrant immunology rapidly recovers (ie, achieves homeostasis), or its dysfunction persists and they enter chronic critical illness (CCI; defined as >14 days in the ICU with organ dysfunction). These patients with CCI experience ongoing immunosuppression (eg, lymphopenia) and inflammation (eg, neutrophilia) associated with a persistent acute phase response (eg, high C reactive protein and low prealbumin levels) with ongoing protein catabolism. Despite aggressive nutrition intervention, there is a remarkable loss of lean body mass associated with a proportional decrease in functional status and poor wound healing. Clinically, patients with PICS suffer from recurrent nosocomial infections and poor wound healing, and they often develop pressure ulcers. They are frequently discharged to long-term acute care facilities, where they experience sepsis recidivism requiring rehospitalization, failure to rehabilitate, and indolent death. While other investigators have described the growing epidemic of CCI under a variety of descriptive terms (including postintensive care syndrome) in a variety of patient populations,6,7 absent is any unifying mechanistic explanation. In recent laboratory work with chronic murine models of sepsis and trauma, Moldawer and colleagues identified the expansion of myeloid-derived suppressor cells (MDSCs) to explain the persistent immunosuppression, concurrent low-grade inflammation, and associated ongoing catabolism that are being observed in patients with PICS (similar to that seen in the chronic phase of neoplastic disease).8-10 In a recent focused translational study of surgical patients with severe sepsis, they confirmed the clinical relevance of these laboratory observations. They showed that MDSCs are persistently elevated out to 28 days after sepsis.11 Importantly, these MDCSs were shown to suppress T lymphocyte proliferation and decrease the release of Th1 and Th2 cytokines. Moreover, MDSC expansion correlated with the following adverse outcomes: (1) early MDSC expansion was associated with early mortality; (2) persistent expansion was associated with prolonged ICU stays; and (3) persistent expansion was a strong independent predictor of nosocomial infections and poor postdischarge disposition. This MDSC expansion is a well-conserved response to a variety of insults and is called emergency myelopoiesis.12 It is the bone marrow's attempt to preserve innate immunity, and to accomplish this, the bone marrow concurrently suppresses lymphopoiesis and erythropoiesis with resulting lymphopenia and anemia (commonly observed in patients with CCI). Hemopoietic stem cells are preferentially directed down the common myeloid progenitor cell line to produced MDSCs. These MDSCs are not allowed to mature into granulocytes, monocytes, and dendritic cells but are released early from the bone marrow. While a primary role of MDSCs is to fight infections, they are poor phagocytes and do not present antigens well. Their immunosuppressive activity is attributed to a number of mechanisms, including the upregulation of arginase 1; increased interleukin 10 production and cell surface expression of programmed death ligand 1; nitrosylation of major histocompatibility complex molecules, preventing their appropriate interaction with the T-cell receptors and coreceptors as well as promoting T-cell receptor dissociation; and promotion of regulatory T-cell expansion. While best known for their detrimental suppression of adaptive immunity in chronic cancer, MDSCs also produce inflammatory mediators (including nitric oxide, reactive oxygen species, tumor necrosis factor, etc) that cause persistent low-grade inflammation that characterizes both cancer and PICS cachexia. In addition to MDSCs, patients with sepsis and trauma suffer from significant tissue injury with release of damage-associated molecular patterns.13 While these endogenous alarmins are less well studied, they may also contribute to the persistent inflammation in PICS.
Figure 1.
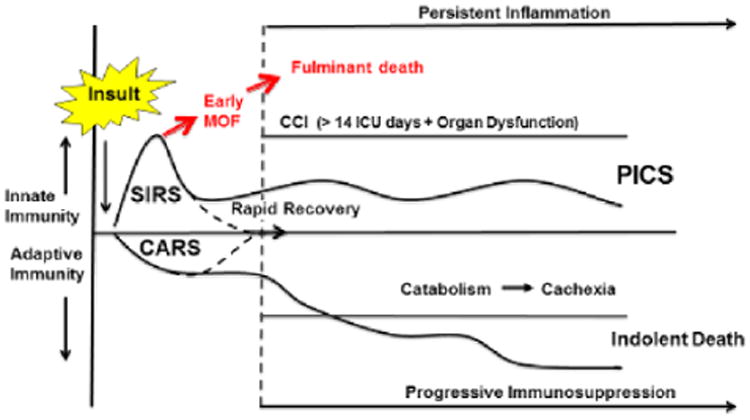
The PICS paradigm. Following a major inflammatory insult (trauma, sepsis, burns, acute pancreatitis, etc), there is simultaneous SIRS and CARS. The early MOF fulminant death trajectory is now rarely seen, due to the early recognition of shock and the rapid implementation of effective evidence-based interventions. Survivors may progress through 2 pathways: (1) patients readily return to immune homeostasis and achieve a rapid recovery; (2) patients smolder in the ICU with CCI, developing chronic inflammation, suppression of adaptive immunity, and ongoing protein catabolism with cachectic wasting and suffering from recurrent nosocomial infections. These patients often suffer from PICS, many of which fail to achieve functional independence, are discharged to long-term acute care facilities, have an extremely poor quality of life, and ultimately succumb to an indolent death. CARS, compensatory anti-inflammatory response syndrome; CCI, chronic critical illness; ICU, intensive care unit; MOF, multiple-organ failure; PICS, persistent inflammation, immunosuppression, and catabolism syndrome; SIRS, systemic inflammatory response syndrome.
PICS Cachexia
It has long been recognized that the loss of lean body mass in patients with prolonged ICU stays is dramatic. In a classic study, Graham Hill and colleagues performed serial body composition studies in critically injured patients over 25 days in the ICU.14 They demonstrated that despite placing patients in early positive caloric and nitrogen balance, there was an obligatory 16% loss of lean body mass, and the excessive administration of nutrition substrates was converted into fat. This tremendous loss lean body mass was recently confirmed by Putucheary et al, who performed muscle biopsies and serial ultrasound to assess the cross-sectional area of the rectus femoris over the first 10 days of ICU stay. They demonstrated a 20% decrease in cross-sectional area, and in the subset of patients with MOF, this decrease in cross-sectional area approached 30%.15 They assessed leg protein synthesis (measured by leucine incorporation)and breakdown (measured by femoral vein dilution of phenylalanine) on day 1 (with no nutrition support) and on day 7 (all fed enterally). Interestingly, on day 7, protein synthesis was variably increased, but breakdown was consistently higher in all patients with resultant negative protein balance. Muscle biopsies were also performed of day 7 to look at intracellular regulators of protein homeostasis, which revealed decreased anabolic and increased catabolic signaling. Surprisingly, hematoxylin and eosin staining of day 7 biopsies revealed necrosis and a cellular infiltrate in 40% of patients. Immunostaining indicated that these cells were neutrophils and macrophages, suggesting that innate inflammation-induced myonecrosis may play an important role in loss on muscle mass in CCI patients. There are multiple inciting factors that that contribute to PICS cachexia, and a full discussion is beyond this scope of this article. However, the underlying process is persistent or recurrent low-grade inflammation that drives catabolism and blocks anabolism.
Nutrition Support
Parenteral Nutrition
As mentioned earlier, in the mid-1970s, MOF emerged as a new, highly lethal ICU syndrome (mortality >80%), and with the increasing availability of PN, there was considerable interest in optimizing PN to match the metabolic demands of MOF patients to prevent their demise.2,3 In the early 1980s, “stress formula” PNs were developed and widely used with the belief that, by reestablishing more normal serum amino acid levels, catabolism could be attenuated. However, over the next decade, numerous prospective randomized trials (PRTs) failed to demonstrate improved mortality, and in several trials, PN actually caused harm by increasing infectious morbidity. At the same time, PRTs convincing demonstrated that EEN had improved outcomes when compared with early PN (mechanism described below). As a result, interest in using early PN in MOF patients faded. However, with ongoing improvement in ICU care and understanding of PN nutrient delivery, early MOF mortality decreased substantially, and there has been renewed interest in early PN to ensure that patients were placed in early positive caloric and nitrogen balance. Unfortunately, recent experience (including a large PRT) has again demonstrated that early PN in stressed ICU patients does not improve mortality and worsens infectious morbidity.4,16 The current American Society for Parenteral and Enteral Nutrition / Society of Critical Care Medicine recommendation to start PN in stressed ICU patients after 7 days appears to be warranted. The earlier use of PN is recommended only in those who arrive in the ICU severely malnourished or at high nutrition risk who are unable to be fed enterally.
Early Enteral Nutrition
Compared with early PN, EEN has long been recognized to be beneficial in high-risk surgical ICU patients.2-4 However, there has been considerable debate over its underlying mechanism. The preponderance of evidence suggests that this is due to the beneficial effect of EEN rather than a harmful effect of PN. MOF research in the 1990s focused on the defining the role of the gut in MOF and provided a plausible explanation for how EEN promotes vital gut functions (eg, mucosal perfusion, intestinal transit, mucosal permeability).17 Most important, the gut was recognized to be an important immunologic organ, and the severity of systemic immunosuppression can be lessened by feeding the gut. Enteral nutrition supports the function of the mucosal-associated lymphoid tissue that produces 70% of the body's secretory immunoglobulin A. Naive T and B lymphocytes target and enter the gut-associated lymphoid tissue, where they are sensitized and stimulated by antigens sampled from the gut lumen and thereby become more responsive to potential pathogens in the external environment. These stimulated lymphocytes then migrate via mesenteric lymph nodes and the thoracic duct and into the vascular tree for distribution to the extraintestinal sites of mucosal-associated lymphoid tissue. These provide protection with subsequent bacterial inoculation. This is key in preventing late infections, which serve to promote ongoing inflammation in the PICS paradigm. Very recent work has shown the benefit of EEN on stabilizing and possibly supporting the patient's microbiome.
High Protein
Traditionally, nutrition support of MOF patients emphasized early protein intake levels >1.2 g/kg/d.3,4 Recently, a PRT demonstrated worse outcomes with early supplemental PN, and the investigators proposed that early high protein nutrition support was harmful because of inhibition of autophagy.16 While this is an interesting hypothesis, it is unproven, and other recent data refute this concern.18-20 Additionally, this is not relevant to the later phase of anabolic nutrition for PICS. While there are no specific data concerning optimal protein levels in PICS, a recent review article by Delano and Moldawer demonstrates that patients with cancer cachexia experience remarkably similar alterations in metabolism as seen in PICS.9 Therefore, what works in cancer cachexia should be applicable to PICS. Recent studies have shown a linear relationship between dietary amino acid intake and net protein anabolism in patients with advanced cancer (including non–small cell lung cancer and pancreatic cancer) similar to that observed in healthy controls.21 These data support the recent guideline recommendation that cancer patients should consume at least 1.2–2.0 g/kg/d of protein.22 Aging sarcopenia is another example of chronic inflammation-induced cachexia, and here the evidence-based recommendations are to provide 1.5 g/kg/d of protein.23,24 Finally, burn patients suffer from persistent catabolism, and the classic study by Alexander et al demonstrated improved survival and less bacteremia in burned children who received early aggressive high-protein nutrition support.25 Recent recommendations from Herndon and Tompkins is to provide 2.0 g/kg/d based on the observation that amino acid oxidation in burn patients is twice that of normal healthy controls.26
Arginine
Immune-enhancing enteral diets fortified with arginine have convincingly been shown to be beneficial in surgical patients undergoing major operations and in trauma patients at high risk for MOF.27,28 However, the use of arginine in sepsis is controversial. Arginine is a semiessential amino acid that promotes lymphocyte and fibroblast proliferation and serves as an intra-cellular substrate for nitric oxide production in macrophages to improve bactericidal activity. It is a secretagogue that increases levels of growth hormone, prolactin, somatostatin, insulin, and glucagon. The sepsis controversy centers on the fact that arginine is a competitive substrate for arginase 1 and inducible nitric oxide synthase. During severe sepsis/septic shock, there is upregulation of inducible nitric oxide synthase, and available arginine is converted to citrulline and nitric oxide. Arginine levels are depleted, which suppresses lymphocyte proliferation (causing immunosuppression). The concern is that arginine supplementation in severe sepsis/septic shock would result in increased nitric oxide, causing pathologic vasodilation-amplifying shock and thus contributing to patient demise. This scenario is physiologically possible but clinically unproven and not relevant, because arginine is rarely provided in high doses during severe sepsis/septic shock resuscitation. However, Ochoa et al have convincingly demonstrated upregulation of arginase 1 in MDSCs after trauma, and others have shown that the same occurs in chronic sepsis.11,29-31 When arginase 1 is upregulated, arginine is shunted into the production of ornithine, which is converted to polyamines and prolines. These are used in wound healing. Arginine supplementation in this setting would increase arginine levels and restore lymphocyte proliferation, and converted arginine would increase polyamines and prolines, which would promote healing. Given that MDSC upregulation is a prominent feature in patients with PICS, arginine supplementation is rationale intervention, but study is needed.
Leucine
Interest in branched-chain amino acids (BCAAs; leucine, isoleucine, and valine) dates back to the 1980s, with the demonstration that these amino acids decreased muscle protein catabolism and stimulated protein synthesis.3 This provided the rationale for stress formula PN fortified with BCAAs. Frank Cerra championed this as a strategy to prevent “septic autocannibalism” that was occurring in patients with MOF despite standard PN.32 In a PRT, he demonstrated that the use of BCAA-fortified PN in surgical patients (1) increased plasma levels of BCAAs and arginine, (2) improved nitrogen balance (by promoting protein synthesis), (3) increased prealbumin levels, and (4) increased absolute lymphocyte counts. Unfortunately, this PRT and others failed to demonstrate improved mortality or other major clinical outcome parameters, and because BCAA PNs were expensive, interest in their use faded. However, with more recent understanding of the intracellular regulation of protein synthesis, there has been renewed interest in leucine because it has been shown to be a potent stimulator of protein synthesis via the mammalian target of rapamycin (mTOR) complex pathway.33 Consistent with the idea that leucine supplementation should stimulate protein synthesis, it has recently been shown to be effective in elderly subjects.34 Additionally, this has recently been shown to be true in patients with cancer.35 In acute sepsis, mTOR is down-regulated; thus, early leucine supplementation may be ineffective.36 However, it is unknown whether this persists into the chronic phase of PICS, and given the similarity of the metabolic alterations with aging sarcopenia and cancer cachexia, leucine supplementation in PICS is worthy of future study.
Anabolic Adjuncts
Herndon et al have shown that after major burns, patients experience persistent inflammation and catabolism similar to PICS. Over the last 15 years, they have performed a series of PRTs testing various anabolic strategies to combat the sustained muscle breakdown. Several of these anabolic interventions have proven to be effective and are likely relevant to PICS. These include intensive insulin therapy, oxandrolone, propranolol, and resistance exercise.37-40 In these well-performed PRTs, Herndon et al have shown that strict glucose control (80–160 mg/dL) significantly increased bone mineralization and muscle strength in children sustaining major burns. They have also demonstrated that oxandrolone substantially decreased resting energy expenditure, increased insulin-like growth factor 1 secretion during the first year after burn injury, and, in combination with exercise, increased lean body mass and muscle strength considerably. Finally, they showed a reduction in burn-induced proteolysis with an increase in muscle anabolism following propranolol administration. These are all easily employed in patients with PICS and are worthy of future study.
Summary
With improvements in ICU care over the past decade, there has been a disturbing increase in patients with CCI who survive the ICU but are discharged to long-term acute care facilities, where they suffer indolent deaths. A substantial portion of patients with CCI in surgical ICUs are developing a new predominant chronic phenotype of MOF called PICS. From a nutrition perspective, the most glaring problem in patients with PICS is persistent inflammation-induced cachexia that precludes effective rehabilitation. This is occurring despite traditional nutrition support (which has its merits during the acute phase of critical illness). Thus, there is a need to develop anabolic nutrition strategies as these patients enter the recovery phase of CCI. While there are limited nutrition support data available from this newly described PICS population, there are observations made in other patient populations (including burns, cancer, and sarcopenia) that are likely relevant to PICS. Based on these observation, high-protein diets supplemented with leucine (to promote anabolism) and arginine (to overcome MDSC expansion) appear to be logical recommendations. Anabolic adjuncts warrant further study.
Discussion
Steve McClave
Glucose control and insulin therapy keep coming up in a number of our talks, and yet, our intensivists are saying that we need moderate blood glucose control. When you look at what is happening in the ICU, you find out that the blood glucose levels run in the 200s often. Tight glucose control has been shown to increase rates of hypoglycemia and mortality. What needs to be done for glucose control in the ICU setting?
Fred Moore
The NICE-SUGAR study demonstrated that if you get too aggressive with insulin therapy, targeting 80–110, you hurt patients with hypoglycemia. As a result, most people have backed off and said we are not going to 80–110, and instead the goal should be between 120–150. One point to keep in mind is that insulin is an anabolic hormone, and some of the early studies by Dr Allison showed dramatic anabolic responses by adding insulin to the TPN [total parenteral nutrition] solutions. As I have said, when everybody gets nervous in our ICU to start an insulin drip, I feel that it could be good thing because of the anabolic effect.
Robert Martindale
That was a great summary of PICS, and I've been following this issue for a long time. One of the interesting points from your talk was the risk of arginine in sepsis. Do you use it early? When do you supplement with arginine, and do you think that the timing is critical for survival?
Fred Moore
I use immune-enhancing diets in all of our high-risk patients right out of the box.
Robert Martindale
You are not worried about using arginine in patients with sepsis?
Fred Moore
No, we are not worried about the risk of arginine use in sepsis. As we move through the clinical course using immune-enhancing diets after 10 days, we switch over to a less expensive high-protein formula and then start giving additional arginine supplementation.
Stuart Phillips
One question I have is whether some of the sarcomere disruption that you described is the result of a rapid turnover of proteins. In addition to early provision of protein, what are your thoughts about the use of neuromuscular electrical stimulation? This can be done fairly easily in the critical care setting, as it does not require physical therapy and has been shown to reduce decline in muscle cross-sectional area in ICU patients.
Fred Moore
I agree with the use of nerve stimulation in these patients. There are 2 different nerve stimulations that we routinely use. One is phrenic nerve stimulation for the diaphragm, and the second is stimulation of the thigh muscles. The data on the efficacy of nerve stimulation are mixed, but the problem with these patients is that you can't get them to voluntarily exercise. Part of our program initially was to enroll patients into a leg press protocol, and we had problems getting them to volunteer to do that. We are now involved in a prospective randomized study using a fairly aggressive nerve stimulator of the leg muscles.
Jayshil Patel
We used to think of sepsis as a predominantly systemic inflammatory response syndrome, where you highlighted that it's probably a combination of both SIRS and the compensatory anti-inflammatory response syndrome. Do you find a difference in the medical ICU and trauma patients and the development of PICS? In the medical ICU, we don't see as much damage associated with the molecular proteins compared to trauma patients. These trauma patients may have a tremendous amount of physical damage and perhaps a greater shift of Th1 to Th2 response, leading to more of a CARS response.
Fred Moore
I have never thought of myself as an expert in medical ICU issues, but this is likely PICS. Surgical and trauma ICU patients suffer from recurrent inflammatory insults. Trauma patients are going to get an initial damage control laparotomy, often followed by subsequent operations. These operations are often followed by nosocomial infections. These repeated inflammatory insults can occur for weeks. I actually think mechanical ventilation is a proinflammatory disease, and I think that if you were to study your medical ICU patients, you would find that they are persistently inflamed if they are chronically ventilated. In addition, medical ICU patients may have nosocomial infections like the surgical patients. This is my opinion, but I don't pretend to be an expert about medical ICU patients.
Craig McClain
Do you perform late muscle biopsies in addition to the early ones you showed in your talk? If you did perform both early and late biopsies, did the histology improve? I agree with both your and Stuart Phillips's comments about the use of nerve stimulation in these patients.
Fred Moore
We are doing muscle biopsies in these patients and are working with the Aging Institute at the University of Florida. They routinely perform muscle biopsies in their elderly sarcopenic patients. They have numerous interventions that they perform in these patients, but the key is exercise. If they can get these sarcopenic patients exercising, they can have a positive impact on outcomes. We have demonstrated, during prolonged operations, when you stimulate the diaphragm, you can decrease the loss of its twitch. This idea of stimulating the diaphragm in patients who get stuck on the ventilator because of diaphragm dysfunction could revolutionize the way we take care of ICU patients.
Beth Taylor
You talked about giving a lot of arginine, and usually I would do arginine for 7–10 days and switch over to the standard formula, but I don't continue to supplement. You're saying you can continue the arginine supplemental packets? How much arginine do you give and for how long?
Fred Moore
We continue arginine supplementation and use a commercial product that provides about 10 g of arginine per packet, and we give them 3 packets per day for a total of 30 g.
Juan Ochoa
What we are really trying to determine is whether the patient has an arginine deficiency state or not. In addition we need to determine if this arginine deficiency state is caused by MDSCs versus an accumulation of other problems in the liberation of arginase through other mechanisms. It is really important to try to differentiate a true arginine deficiency as a maladaptive state from a physiologic regulation of arginine by MDSCs. What we see, really, that is easily defined in the surgical patient is that first they come with an arginine deficiency state because of the tumors that we operate upon. If you get a renal cell carcinoma or head and neck cancer, almost 100% of those patients are already arginine deficient when they reach the operating room, which can be exacerbated by surgery. What we do not know is whether in sepsis the elevation of MDSCs is adaptive or maladaptive, which it may be either. If you have a toxic shock syndrome, you actually want to have T-cell regulation, and a regulatory mechanism is that of MDSCs depleting arginine to block protein synthesis and T lymphocytes. In maladaptive conditions and infections such as staphylococcal, pseudomonas, and malaria infections, arginine deficiency is a real issue. In these types of infections, there should be clinical benefit with arginine supplementation.We need causality constructs to help determine whether replacement is going to be a cost benefit before we say that all patients should be supplemented with arginine. I agree: in PICS patients, arginine may be beneficial in the long term. We need to remember that, in your animal models and the models that Link did, by blocking arginase there was high mortality.
Fred Moore
Of course, the MDSC response is normal. The body's doing it for a reason, and it's trying to promote its innate immune response. The body wants to preserve the innate immune system and will sacrifice lymphocytes to do it. What we're saying is that people weren't supposed to survive these massive inflammatory insults. When they do, you have this persistent expansion of the MDSCs, and you're seeing the detrimental effect of MDSCs. I don't think the answer is to deplete MDSCs; rather, I think modulating them somehow is the key.
Robert Martindale
Do you think we can cause earlier resolution or earlier change back to normal physiology? Do you think the macrophages and their M1 and M2 class changes may be involved in this? This seems like a big thing now with the macrophage research; with the goal of shifting from an M1 to an M2, we may be able to increase resolution physiology.
Fred Moore
One way I look at this question is that there's ebb and there's the flow, and so I've been spending my entire career trying to manipulate the flow phase of metabolism. I have come to the conclusion that we have not had much impact on the flow phase, and we have to focus on getting the patient into the recovery phase. One of the problems is that the recovery phase may not start immediately. There are 2 different phenotypes of MDSCs—the monocytic and the granulocytic—and in normal situations, there is a higher number of monocytic versus granulocytic cells. In our septic patients, this ratio changes, which is important because the granulocytic MDSCs are more immunosuppressive. Granulocytic MDSCs do not mature into monocytes and macrophages; rather, they mature into neutrophils. Because of this, they're not going to present antigens and do things that macrophages and monocytes would do.
Craig McClain
I have a practical question on exercise and mobilization. We've known for 10 years that it is important to have patients in the ICU exercise and have early mobilization. During this Protein Summit, we have all stressed the importance of exercise and mobilization. We've been nodding our heads like “Oh, we do that at our institution.” I had this conversation with our director of surgical nutrition at home and asked if we are doing this, for which he replied that we are not. He acknowledges that it is important, but because of numerous reasons, we are not able to implement exercise protocols. Is it a cultural leadership issue from the physician perspective that we know it's important but just aren't there yet to implement it? Or is it more of staffing issue, such as a lack of personnel, like physical therapists?
Fred Moore
I think this is more of a resource issue. We have an early mobility program, and on weekends somehow it doesn't happen, due to the lack of physical therapy coverage. Physical therapists are expensive, and they're in short supply. You can talk to the administrators until you're blue in the face; they don't buy it. I think the nurses in our units have bought into the principle that if you lie in bed for 2 weeks, it is likely not good for you. Then the issue becomes, is it dangerous to get these people up? The initial resistance by the bedside nurse is that it may be dangerous to get these bed-bound patients up, and if something happens during mobilization, who gets blamed?
Acknowledgments
Supported by P50 GM-111152 (F. A. Moore) awarded by the National Institute of General Medical Sciences. Financial support for the publication of the supplement in which this article appears was provided by Nestlé HealthCare Nutrition, Inc.
Footnotes
Conflicts of interest: None declared.
Statement of Authorship: All authors drafted and critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
References
- 1.Gentile LF, Efron PA, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FA, Moore EE. The evolving rationale for early enteral nutrition based on paradigms of multiple organ failure: a personal journey. Nutr Clin Pract. 2009;24:297–304. doi: 10.1177/0884533609336604. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal MD, Vanzant EL, Martindale RG, Moore FA. Evolving paradigms in the nutritional support of critically ill surgical patients. Curr Probl Surg. 2015;52:147–182. doi: 10.1067/j.cpsurg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 4.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) JPEN J Parenter Enteral Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 5.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76:21–29. doi: 10.1097/TA.0b013e3182ab1ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich O, Reid MB, Van den Berghe G, et al. The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev. 2015;95:1025–1029. doi: 10.1152/physrev.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott D, Davidson JE, Harvey MA, et al. Exploring the scope of post-intensive care syndrome therapy and care: engagement of non-critical care providers and survivors in a second stakeholders meeting. Crit Care Med. 2014;42:2518–2526. doi: 10.1097/CCM.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 8.Cuenca AG, Delano MJ, Kelly-Scumpia KM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17:281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract. 2006;21:68–81. doi: 10.1177/011542650602100168. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371:380–383. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 11.Mathias B, Delmas AL, Ozrazgat-Baslanti T, et al. Human myeloid-derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock [published online May 9, 2016] Ann Surg. doi: 10.1097/SLA.0000000000001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamanouchi S, Kudo D, Yamada M, Miyagawa N, Furukawa H, Kushimoto S. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care. 2013;28:1027–1031. doi: 10.1016/j.jcrc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Monk DN, Plank LD, Franch-Arcas G, Finn PJ, Streat SJ, Hill GL. Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann Surg. 1996;223:395–405. doi: 10.1097/00000658-199604000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 16.Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 17.Hassoun HH, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Postinjury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Weijs PJ, Looijaard WG, Beishuizen A, Girbes ARJ, Oudemans-van Straaten HM. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care. 2014;18:701. doi: 10.1186/s13054-014-0701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weijs PJ, Sauerwein HP, Kondrup J. Protein recommendations in the ICU: G protein/kg bodyweight—which body weight for underweight and obese patients? Clin Nutr. 2012;31:774–775. doi: 10.1016/j.clnu.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Allingstrup MJ, Esmailzadeh N, Wilkens Knudsen A, et al. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin Nutr. 2012;31:462–468. doi: 10.1016/j.clnu.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Engelen MP, van der Meij BS, Deutz NE. Protein anabolic resistance in cancer: does it really exist? Curr Opin Clin Nutr Metab Care. 2016;19:39–47. doi: 10.1097/MCO.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arends J, Bodoky G, Bozzetti F, et al. ESPEN guidelines on enteral nutrition: nonsurgical oncology. Clin Nutr. 2006;25:245–259. doi: 10.1016/j.clnu.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr. 2008;27:675–684. doi: 10.1016/j.clnu.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Morley JE, Argiles JM, Evans WJ, et al. Society for Sarcopenia, Cachexia, and Wasting Disease. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;1:391–396. doi: 10.1016/j.jamda.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander JW, MacMillan BG, Stinett JD, et al. Beneficial effects of aggressive protein feeding in severely burned children. Ann Surg. 1980;192:505–507. doi: 10.1097/00000658-198010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 27.Drover JW, Dhaliwal R, Weitzel L, Wischmeyer PE, Ochoa JB, Heyland DK. Perioperative use of arginine-supplemented diets: a systematic review of the evidence. J Am Coll Surg. 2011;212:385–399. doi: 10.1016/j.jamcollsurg.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Moore FA. Effects of immune enhancing diets on infectious morbidity and multiple organ failure. JPEN J Parenter Enteral Nutr. 2001;25:S36–S42. doi: 10.1177/014860710102500209. [DOI] [PubMed] [Google Scholar]
- 29.Zhu X, Pribis JP, Rodriguez PC, et al. The central role of arginine catabo-lism in T-cell dysfunction and increased susceptibility to infection after physical injury. Ann Surg. 2014;259:171–178. doi: 10.1097/SLA.0b013e31828611f8. [DOI] [PubMed] [Google Scholar]
- 30.Darcy CJ, Minigo G, Piera KA, et al. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit Care. 2014;118:R163. doi: 10.1186/cc14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gey A, Tadie JM, Caumont-Prim A, et al. Granulocytic myeloid-derived suppressor cells inversely correlate with plasma arginine and overall survival in critically ill patients. Clin Exp Immunol. 2015;180:280–288. doi: 10.1111/cei.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerra FB, Siegel JH, Coleman B, Border JR, McMenamy RR. Septic autocannibalism: a failure of exogenous nutritional support. Ann Surg. 1980;192:570–580. doi: 10.1097/00000658-198010000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cynober L, De Bandt JP, Moinard C. Leucine and citrulline: two major regulators of protein turnover. World Rev Nutr Diet. 2013;105:97–105. doi: 10.1159/000341278. [DOI] [PubMed] [Google Scholar]
- 34.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 35.Deutz NE, Safar A, Schutzler S, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30:759–768. doi: 10.1016/j.clnu.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laufenberg LJ, Pruznak AM, Navaratnarajah M, Lang CH. Sepsis-induced changes in amino acid transporters and leucine signaling via mTOR in skeletal muscle. Amino Acids. 2014;6:2787–2798. doi: 10.1007/s00726-014-1836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;17:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;17:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porro LJ, Herndon DN, Rodriguez NA, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg. 2012;14:489–502. doi: 10.1016/j.jamcollsurg.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]


