ABSTRACT
Neural stem cells (NSCs) are a multipotent, self-renewing source of undifferentiated cells in the periventricular region of the mammalian central nervous system (CNS). Since their original discovery 25 years ago, much has been learned about their development, persistence, localization, properties and potential. Herein we discuss the current state of knowledge pertaining to neural stem cells with a focus on the lineage relationship between two NSC populations along the neuraxis and their regionally distinct niches in the CNS.
KEYWORDS: adult stem cells, central canal, development, forebrain, injury, neural stem cells, spinal cord, stem cell lineage, stem cell niche, subventricular zone
In the 1960s, James Till and Ernest McCulloch performed groundbreaking research that proved the existence of stem cells.1 Known as the “fathers of stem cell research,” their discovery marked the beginning of adult stem cell research. In the last 25 years of neuroscience research, one of the landmark events was the discovery that neural stem cells (NSCs) existed in the central nervous system.2 This finding defaced the dogma that the adult central nervous system (CNS) was unable to replace lost neurons and glia. Since their original discovery, tremendous strides in the characterization and utilization of NSCs have been achieved.2 Herein we will discuss the most current understanding of the neural stem cell lineage through development and into adulthood in the periventricular region of the CNS.
Adult stem cells
Most mammalian adult tissues contain resident stem cells. An adult stem cell is defined by 2 cardinal properties.2-4 First, is the ability to self-renew and generate copies of itself. This process allows for the maintenance of the stem cell pool (through asymmetric division) or expansion of the size of the stem cell pool (through symmetric division).5 Stem cell self-renewal depends on the proliferative state of the cell, ranging from a quiescent state to an actively proliferative state. Stem cell quiescence is often viewed as a dormant state with minimal basal activity and is reversible. More proliferative stem cells are found in tissues where cell proliferation compensates for tissue loss throughout the life of the organism. The second property is multipotency whereby a stem cell has the ability to give rise to progeny that generate the different cell types found in the tissue of origin. Resident stem cells throughout the body give rise to cells specific for their tissue of origin. Stem cell progeny, the direct descendants of stem cell proliferation, are considered to have restricted lineage potential and limited self-renewal capacity. Similar to stem cells, progenitor cells have the ability to generate tissue specific cell types by undergoing asymmetric and/or symmetric division – with limited longevity. The kinetic similarities between stem and progenitor cells poses a challenge when studying cell lineage through development and into adulthood.6 Further, significant overlap exists in terms of protein expression between stem cells and their progeny making isolation of these distinct populations challenging. Classic in vitro colony forming assays have allowed for the isolation of clonally derived stem and progenitor populations and permit proliferation and differentiation to be assessed.2,7,8 In vivo, common immunohistochemistry paradigms or fluorescent protein expression in genetically modified rodents has been widely adopted for the isolation and characterization of neural stem and progenitor cells.2,9-11 In the past decades, isolation of distinct populations has been done using cell sorting technologies coupled with marker expression.12 Most recent advances in single cell analysis (e.g. single cell RNA-seq13 or retroviral barcoding14) has enabled the sensitivity required to examine distinct cellular phenotypes within heterogeneous populations.
Origin of neural stem cells
During development, a blastocyst forms which contains an inner cell mass comprised of pluripotent embryonic stem cells (ESCs) and an outer layer of trophoblast cells.15 ESCs give rise to all cells that comprise the embryo whereas trophoblast cells are restricted to generating extraembryonic tissue cells including the placenta and yolk sac. ESCs can be isolated from developing blastocysts in the mouse at embryonic day 3.5 (E3.5).16 They express various pluripotency markers such as Oct3/4, Sox2, Rex-1 and TERT and can be maintained in culture as pluripotent cells in the presence of leukemia inhibitory factor (LIF). By E6.5, the developing embryo comprises 3 germ layers (endoderm, mesoderm and ectoderm) and at E7.5, neural induction begins where neural cells are specified from the ectoderm through a default mechanism regulated by inhibitory signals from molecules such as the bone morphogenetic protein (BMP) and transforming growth factor (TGFβ).17 Neurulation is the process of neural tube formation, which will ultimately give rise to the brain and spinal cord.18 The neural tube comprises a relatively homogenous population of neurepithelial cells that will proliferate and expand through symmetric division. By E8.5–10 the patterning of the developing neural tube to form initial specification of the forebrain, midbrain, hindbrain and spinal cord occurs, guided by morphogens such as BMP, fibroblast growth factor (FGF), sonic hedgehog (Shh) and retinoic acid.19 During this process, neuroepithelial cells can divide asymmetrically to generate a radial glial cell, which has higher levels of notch signaling, and a sibling cell, with lower notch signaling, that adopts a neuronal fate.20
Radial glia are the precursor cells responsible for the formation of the mammalian cerebral cortex, a process that has been extensively studied by many groups.21,22 In the developing embryo, radial glial cells comprise the NSC population that divide symmetrically to increase the size of the NSC pool as well as give rise to progenitors that will migrate away from the periventricular germinal zone where the NSCs reside. The NSC progeny migrate into the developing parenchyma where they form the structures of developing CNS. Toward the end of development, radial glia leaves the ventricular niche and migrates into the parenchyma where they give rise to astrocytes. Lineage tracking experiments using viral constructs and electroporation at embryonic time points to label precursors and radial glia in the developing brain support the hypothesis that a subset of radial glial cells persist in the periventricular region into adulthood as adult NSCs.21,23
Neural stem cells can also be characterized during development through protein expression and cytokine responsiveness. There are 2 distinct NSC populations that can be found in the developing CNS. As early as E5.5 (before neural tube formation), a LIF responsive NSC population expressing low levels of the pluripotency marker Oct4, can be isolated using the clonal colony forming assay (neurosphere assay) from the brain germinal zone lining the ventricular space. Known as the primitive NSCs (pNSCs), these cells give rise to all of the cells of the neural lineage and further, can incorporate into developing blastocysts following morula aggregation, suggesting that they may be capable of generating non-neural tissue.24,25 This pNSC population peaks during early embryonic development and early postnatally before declining drastically while persisting into adulthood.10,25 By E7.5–8.5, a second population of fibroblast growth factor 2 (FGF2) dependent NSCs can be isolated from the developing CNS and by E12.5, epidermal growth factor (EGF) dependent NSCs are observed.26 By E16.5, these FGF and EGF responsive NSC populations, known as the definitive NSCs (dNSCs) express the intermediate filament protein, glial fibrillary acidic protein (GFAP), a mature astrocytic marker.26,27 This marker persists into adulthood in dNSCs that co-express EGF and FGF2 receptors (Fig. 1). Throughout development, the NSC populations are found in the periventricular germinal zone of the CNS.
Figure 1.
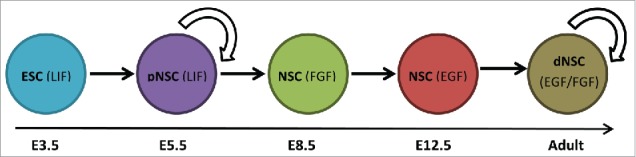
Neural stem cell lineage. The neural stem cell (NSC) lineage from development to adulthood in the mouse includes the primitive NSC (pNSC) derived from pluripotent embryonic stem cells (ESC) during early development. Definitive NSCs (dNSC) are derived from pNSCs during development as early at embryonic day (E) 8.5. The individual NSC populations are responsive to leukemia inhibitory factor (LIF) (pNSC) and both epidermal growth factor (EGF) and fibroblast growth factor (FGF) (dNSCs) in adulthood. Arrow indicates self-renewing in vivo.
Neural stem cells in the adult brain
In the adult brain, the NSC niche comprises the 2–4 cell layer thick subependymal zone (SEZ) (also referred to as the subventricular zone).28,29 The SEZ is immediately adjacent to the single cell layer of ciliated ependymal cells that lines the fluid filled lateral ventricles in the forebrain. Debate between whether ependymal or subependymal cells are the source of dNSCs has persisted for several years but to date, no convincing studies have demonstrated that the ependymal cells are able to give rise to multipotent, self-renewing, clonally derived colonies of cells. The dNSC (EGF and FGF2 responsive) found in the SEZ expresses GFAP and is located within a pinwheel-like structure within this region that is made up of ependymal cells lining the walls of the lateral ventricles.30 Mirazedeh et al., (2008) demonstrated that dNSCs have their cell bodies within the SEZ and extend one apical process through the ependymal layer to contact the CSF through the center of the pinwheel structure.30 The basal end feet of the dNSC extend to the blood vessels found along the border of the SEZ and the striatum.
The SEZ is the largest neurogenic region in the adult mammalian CNS, generating functional olfactory bulb interneurons throughout the lifetime of the rodent. Notably, the subgranular zone of the dentate gyrus of the hippocampus also contributes to ongoing neurogenesis throughout life, albeit at a lower level and with more limited cell migration (a distance of a few cell bodies in the dentate gyrus versus a few millimeters in the SEZ). It is important to note that although this is true in rodents, in humans the SEZ (initially a larger pool) becomes exhausted and does not persist in adulthood – whereas the subgranular zone does and contributes to ongoing adult neurogenesis.31 Neurogenesis in the SEZ results from the asymmetric division of the definitive NSC to give rise to neurogenic progeny (neuroblasts) that proliferate and migrate along the well-defined rostral migratory stream (RMS) to the olfactory bulb where they differentiate into mature interneurons.32-35 Since then, multiple groups have shown heterogeneity within the definitive NSC population. Codega et al., (2014) reported 2 distinct states of GFAP/CD133 expressing NSCs within the SEZ: a mitotically active, EGF receptor expressing dNSC and a subpopulation of mitotically quiescent dNSCs, that could be activated to proliferate upon upregulation of EGF receptor and nestin expression.36 The concept of quiescent vs. activated NSCs was also reported by Mich et al., (2014).12 They reported a population of pre-GEPCOT cells that expressed high levels of Glast (a mature astrocyte marker) and low levels of EGF receptor and semaphorin receptor Plexin B2. Downstream GEPCOT cells, on the other hand, express lower levels of Glast and high levels of both EGF receptor and Plexin B2. Notably, in all cases the dNSC subtypes can give rise to clonally-derived colonies of neural stem and progenitor cells (i.e. neurospheres) in vitro. While the exact relationship between these different subpopulations of dNSCs is not known, and their lineage relationship is not fully established, it is clear that the SEZ contains subpopulations of quiescent and mitotically active dNSCs in the adult brain.
More recently, a novel NSC was found to exist in the SEZ of the adult brain. Sachewsky et al., (2014) reported the isolation and characterization of an Oct4 expressing, GFAP negative population of adult NSCs, similar to the pNSC population found in development as early as E5.5.10,25 The neurospheres derived from Oct4 expressing pNSCs (isolated from Oct4-GFP mice) were isolated in the presence of LIF and were smaller in diameter than the dNSC derived neurospheres. Unlike the dNSCs, which primarily give rise to astrocytes when differentiated in vitro, the differentiation profile of primitive neurospheres from adult brains revealed equal proportions of astrocytes, oligodendrocytes and neurons. The pNSCs were also different from the dNSCs in that they were 100-fold less in number in the adult brain. Sachewsky et al., (2014) also revealed the lineage relationship between the primitive and definitive NSCs whereby the Oct 4 expressing pNSC is found upstream of the GFAP expressing definitive NSC. The authors used in vitro passaging, in vivo ablation studies, and cell transplantation studies to show that a pNSC could give rise to a dNSC; however, the reverse was not seen. The lineage relationship between the different populations of NSCs and their potential roles in vivo following injury or disease, poses interesting questions for future studies.
Neural stem cells in the spinal cord: Are they really different?
The caudal neural tube gives rise to the spinal cord. During development, similar to the forebrain, neuroepithelial cells first divide symmetrically to expand the size of the neural stem cell pool and this is followed by asymmetric divisions to generate progenitor cells that will generate post-mitotic motor, sensory and interneurons between E10–14.37 Gliogenesis begins after neurogenesis and continues postnatally. Similar to the brain, the germinal zone is found in the periventricular region of the central canal and this shrinks down in the adult spinal cord to be only a few cell layers thick.37,38 It is proposed that spinal cord NSCs reside in this periventricular region, with many reports that the ependymal cells are in fact the NSCs. Different from the brain is (1) the ciliated ependymal cells lining the central canal appear to have a low level of mitotic activity under baseline conditions and (2) the spinal cord is a non-neurogenic region.37-39
In 1996, Reynold and Weiss first isolated EGF and FGF2 dependent NSCs from the spinal cord using the in vitro neurosphere assay.40 Since then, several groups have shown that the neurosphere initiating cells reside in the periventricular region lining the central canal and express the ependymal markers FoxJ1 and S100β.41 Distinct from the post-mitotic ependymal cells in the forebrain, proliferative spinal cord ependymal cells express nestin, GFAP, brain lipid-binding protein (BLBP) and CD15.38,41-43 Interestingly, electron microscopy analysis of the GFAP expressing spinal cord NSCs showed them residing adjacent to the ependymal cells with processes extending between ependymal cells to access the ventricular lumen, similar to what is observed in the SEZ of the forebrain.44 Along the same lines, Xu et al., recently reported that the GFAP expressing, EGF and FGF responsive dNSC was also found in the adult spinal cord.11 What had not been shown previously was that the adult spinal cord also harbored a rare population of Oct4 expressing pNSCs, identical to what was seen in the adult SEZ. The spinal cord pNSCs could be isolated through development (starting at E10.5 in the tail bud) and were maintained into adulthood. The group provided support for a similar lineage relationship between the primitive and definitive NSC populations by demonstrating that pNSCs could give rise to dNSCs but the reverse was not true. Hence it appears that similar populations of NSCs are found along the entire neuraxis of the developing and adult CNS.
Factors affecting neural stem cells
There are many types of molecular signals that directly or indirectly influence NSC proliferation, survival, migration and/or differentiation both in vivo and in vitro, including growth factors, cytokines, neurotrophic factors, neurotransmitters and morphogens. These have been extensively reviewed elsewhere.22,45 Most of the factors examined looked at their influence on dNSCs (EGF and FGF2 responsive). Leeder et al., (2016) are among the first to study the behavior of pNSCs in response to exogenous factors.46 Basing their study on receptor expression on pNSCs derived from ESCs, they demonstrated that inhibitors of C-kit and ErbB2 selectively increased the size of pNSC pool (more neurospheres following in vitro exposure and in vivo exposure to specific receptor blockers) with no concomitant effect on the dNSC pool. The ability to selectively manipulate the primitive and definitive NSC pools leads to more questions regarding their potential roles in homeostasis and in response to injury.
One of the more interesting observations regarding factors that regulate NSC behavior was the recent work that illuminated the role of mature myelin on NSC activation. Xu et al., (2016) found that myelin basic protein (MBP), a mature constituent of mature myelin, is able to regulate the proliferation of both primitive and definitive NSCs.11 MBP is normally found in the cytosolic leaflets of oligodendrocyte foot processes that wrap around and myelinate neuronal axons.47 NSC cultures containing extracellular endogenous spinal cord derived MBP or pure exogenous MBP however, revealed a dose dependent inhibition of proliferation of spinal cord NSCs (both primitive and definitive NSCs). Since MBP is not normally found within the extracellular microenvironment of uninjured tissue, but is found following injury (or in primary spinal cord NSC cultures) when mature oligodendrocytes are damaged/degenerating, this finding highlights the significance of environmental influences on NSC based strategies to promote tissue repair. Indeed, MBP in the damaged spinal cord can negatively impact both endogenous and exogenous NSCs to impair their proliferative capacity and subsequent expansion. Interestingly, other myelin associated protein receptors (such as NogoA48) known to be expressed on neurospheres and their effects on NSC survival and proliferation will be important to consider when developing therapeutic interventions for CNS repair.
Niche-dependent regulation of neural stem cells
Stem cells reside in anatomically specific microenvironments within their tissue of origin termed the niche. This niche is composed of specialized extracellular matrix (i.e., basal lamina), heterologous cell types and various signaling inputs (autocrine, paracrine, humoral, and/or neural) that all work together to regulate the activity of their resident stem cell population as required in that tissue.49 As mentioned previously, NSCs can be found in 3 well-defined niches in the adult CNS: the SEZ of the lateral ventricles, the subgranular zone of the dentate gyrus in the hippocampal formation, and the periventricular region of the central canal in the spinal cord.50 What is interesting is that, although all these NSCs arose from the same neuroepithelial cells lining the original neural tube in CNS development, these regionally distinct populations behave differently based on the NSC niche they reside in. For example, NSCs in the brain SEZ and subgranular zone are neurogenic and they actively proliferate and generate progeny that migrate to the olfactory bulb via the RMS or to the granule cell layer in the hippocampus, respectively.51-54 On the other hand, NSCs in the spinal cord are aneurogenic and are characterized by their relative quiescence in adulthood.40 One hypothesis from these observations is that the observed differences in NSC behavior may be due intrinsic differences in the regionally distinct stem cells themselves or alternatively, the differences reflect differential regulation of a common NSC by niche specific factors. Support for latter comes from inter-regional transplant studies in which NSCs removed from their native niche can be seen to adopt proliferation, migration, and differentiation characteristics of the host neural niche that they are transplanted to.55,56 Furthermore, it has also been shown that temporally distinct NSCs will respond to the niche in which they are transplanted. For example, NSCs from the aged brain that normally exhibit reduced proliferative and migratory potential can be rescued when transplanted to a younger stem cell niche in a similar region of the brain.57 Results from these transplant studies strongly support the idea that temporal and spatial differences in stem cell niche play a pivotal role in regulation of stem cell kinetics between these different NSC populations. Translationally this is very appealing, as drugs and/or other small molecules could theoretically be used to modulate the stem cell niche and optimize regenerative therapies using endogenous and/or exogenous stem cell sources.
Injury induced activation of adult neural stem cells
The discovery of distinct populations of NSCs in the adult brain (primitive and definitive NSCs) can potentially provide targets for therapeutic interventions to promote neural repair. It is well established that dNSCs are activated following injury alone, demonstrating increased proliferation (both symmetric and asymmetric division), survival and migration.45,58 While the factors that modulate the particular behaviors are not fully delineated, they include neurotrophic growth factors, cytokines, inflammatory signals such as chemokines and reactive oxygen species, neurotransmitters and cell-cell interactions. Sachewsky et al., (2014) demonstrated that stroke injury activated both primitive and definitive NSCs with distinct time courses post-stroke.10 The pNSCs underwent symmetric division to significantly increase the size of the pNSC pool by 4 days post-stroke. The dNSC pool was similarly increased in size however this was not seen until 7 days post stroke. This sequential activation of primitive and definitive pools supports the hypothesis that the pNSCs may be proliferating to generate the dNSCs (which appear at a later time in development) which can then go on to replace lost cells. The idea that pNSCs are a “reserve” pool of stem cells is an intriguing one and similar to what is see in the haematopoietic system where the true stem cell is thought to be exceedingly rare and brought into cycle following injury or disease.
In cases of SCI injury, several factors contribute to the observed pathology including breaking the blood-spinal cord barrier, necrosis, demyelination, axonal degeneration and an inflammatory response followed by apoptosis and the formation of the glial scar. In the injured mouse spinal cord, periventricular and ependymal cells will proliferate. Xu et al., (2016) and others have shown that injuries sparing the NSC niche lead to definitive NSC migration toward the injury site, as determined by neurosphere formation from cells derived directly from the lesion site; and pNSC proliferation rostral to the injury site.11,41,59 Interestingly, the migration of NSCs to the site of injury is also seen in the brain. Faiz et al., (2015) reported dNSC migration to the site of a stroke lesion within a few days post injury and most intriguingly, the NSCs generated reactive astrocytes which contributed to scar formation in the injured cortex.60 A sound knowledge of the factors that regulate NSC proliferation migration and differentiation is an important step toward the goal of neural repair.
Conclusion
Herein, we have highlighted that similar populations of NSCs persist along the entire neuraxis, despite regionally and temporally distinct NSC behaviors. This finding reveals the importance of understanding the factors that regulate their behavior to effectively develop regenerative strategies that will undoubtedly be different depending on the region of the CNS lost to injury or disease.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. 1961. Radiat Res 2012; 178:AV3-7; PMID:22870977; http://dx.doi.org/ 10.1667/RRAV01.1 [DOI] [PubMed] [Google Scholar]
- [2].Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992; 255:1707-10; PMID:1553558; http://dx.doi.org/ 10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- [3].Siminovitch L, Mcculloch EA, Till JE. The distribution of colony-forming cells among spleen colonies. J Cell Physiol 1963; 62:327-36; http://dx.doi.org/ 10.1002/jcp.1030620313 [DOI] [PubMed] [Google Scholar]
- [4].Till JE, McCulloch EA. Hemopoietic stem cell differentiation. Biochim Biophys Acta 1980; 605:431-59; PMID:7006701 [DOI] [PubMed] [Google Scholar]
- [5].Mione MC, Cavanagh JF, Harris B, Parnavelas JG. Cell fate specification and symmetrical/asymmetrical divisions in the developing cerebral cortex. J Neurosci Off J Soc Neurosci 1997; 17:2018-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci 2003; 26:125-31; PMID:12591214; http://dx.doi.org/ 10.1016/S0166-2236(03)00031-6 [DOI] [PubMed] [Google Scholar]
- [7].Coles-Takabe BL, Brain I, Purpura KA, Karpowicz P, Zandstra PW, Morshead CM, van der Kooy D. Don't look: growing clonal versus nonclonal neural stem cell colonies. Stem Cells Dayt Ohio 2008; 26:2938-44; http://dx.doi.org/ 10.1634/stemcells.2008-0558 [DOI] [PubMed] [Google Scholar]
- [8].Guo W, Patzlaff NE, Jobe EM, Zhao X. Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat Protoc 2012; 7:2005-12; PMID:23080272; http://dx.doi.org/ 10.1038/nprot.2012.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Leong C, Zhai D, Kim B, Yun SW, Chang YT. Neural stem cell isolation from the whole mouse brain using the novel FABP7-binding fluorescent dye, CDr3. Stem Cell Res 2013; 11:1314-22; PMID:24090932; http://dx.doi.org/ 10.1016/j.scr.2013.09.002 [DOI] [PubMed] [Google Scholar]
- [10].Sachewsky N, Leeder R, Xu W, Rose KL, Yu F, van der Kooy D, Morshead CM. Primitive neural stem cells in the adult mammalian brain give rise to GFAP-expressing neural stem cells. Stem Cell Rep 2014; 2:810-24; http://dx.doi.org/ 10.1016/j.stemcr.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu W, Sachewsky N, Azimi A, Hung M, Gappasov A, Morshead CM. Myelin basic protein regulates primitive and definitive neural stem cell proliferation from the adult spinal cord. Stem Cells Dayt Ohio 2016; 35(2):485-96; http://dx.doi.org/ 10.1002/stem.2488 [DOI] [PubMed] [Google Scholar]
- [12].Mich JK, Signer RA, Nakada D, Pineda A, Burgess RJ, Vue TY, Johnson JE, Morrison SJ. Prospective identification of functionally distinct stem cells and neurosphere-initiating cells in adult mouse forebrain. Elife 2014; 3:e02669; PMID:24843006; http://dx.doi.org/ 10.7554/eLife.02669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A. Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell 2015; 17:329-40; PMID:26235341; http://dx.doi.org/ 10.1016/j.stem.2015.07.002 [DOI] [PubMed] [Google Scholar]
- [14].Mayer C, Jaglin XH, Cobbs LV, Bandler RC, Streicher C, Cepko CL, Hippenmeyer S, Fishell G. Clonally related forebrain interneurons disperse broadly across both functional areas and structural boundaries. Neuron 2015; 87:989-98; PMID:26299473; http://dx.doi.org/ 10.1016/j.neuron.2015.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000; 24:372-6; PMID:10742100; http://dx.doi.org/ 10.1038/74199 [DOI] [PubMed] [Google Scholar]
- [16].Rossant J. Stem cells from the Mammalian blastocyst. Stem Cells Dayt Ohio 2001; 19:477-82; http://dx.doi.org/ 10.1634/stemcells.19-6-477 [DOI] [PubMed] [Google Scholar]
- [17].Panchision DM, Pickel JM, Studer L, Lee SH, Turner PA, Hazel TG, McKay RD. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev 2001; 15:2094-110; PMID:11511541; http://dx.doi.org/ 10.1101/gad.894701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weinstein DC, Hemmati-Brivanlou A. Neural induction. Annu Rev Cell Dev Biol 1999; 15:411-33; PMID:10611968; http://dx.doi.org/ 10.1146/annurev.cellbio.15.1.411 [DOI] [PubMed] [Google Scholar]
- [19].Zappaterra MW, Lehtinen MK. The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell Mol Life Sci 2012; 69:2863-78; PMID:22415326; http://dx.doi.org/ 10.1007/s00018-012-0957-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stolfi A, Wagner E, Taliaferro JM, Chou S, Levine M. Neural tube patterning by Ephrin, FGF and Notch signaling relays. Dev Camb Engl 2011; 138:5429-39 [DOI] [PubMed] [Google Scholar]
- [21].Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A 2004; 101:17528-32; PMID:15574494; http://dx.doi.org/ 10.1073/pnas.0407893101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tong CK, Alvarez-Buylla A. SnapShot: adult neurogenesis in the V-SVZ. Neuron 2014; 81:220.e1; http://dx.doi.org/ 10.1016/j.neuron.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gallagher D, Norman AA, Woodard CL, Yang G, Gauthier-Fisher A, Fujitani M, Vessey JP, Cancino GI, Sachewsky N, Woltjen K, et al.. Transient maternal IL-6 mediates long-lasting changes in neural stem cell pools by deregulating an endogenous self-renewal pathway. Cell Stem Cell 2013; 13:564-76; PMID:24209760; http://dx.doi.org/ 10.1016/j.stem.2013.10.002 [DOI] [PubMed] [Google Scholar]
- [24].Hitoshi S, Seaberg RM, Koscik C, Alexson T, Kusunoki S, Kanazawa I, Tsuji S, van der Kooy D. Primitive neural stem cells from the mammalian epiblast differentiate to definitive neural stem cells under the control of Notch signaling. Genes Dev 2004; 18:1806-11; PMID:15289455; http://dx.doi.org/ 10.1101/gad.1208404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 2001; 30:65-78; PMID:11343645; http://dx.doi.org/ 10.1016/S0896-6273(01)00263-X [DOI] [PubMed] [Google Scholar]
- [26].Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol 1999; 208:166-88; PMID:10075850; http://dx.doi.org/ 10.1006/dbio.1998.9192 [DOI] [PubMed] [Google Scholar]
- [27].Karpowicz P, Inoue T, Runciman S, Deveale B, Seaberg R, Gertsenstein M, Byers L, Yamanaka Y, Tondat S, Slevin J, et al.. Adhesion is prerequisite, but alone insufficient, to elicit stem cell pluripotency. J Neurosci Off J Soc Neurosci 2007; 27:5437-47; http://dx.doi.org/ 10.1523/JNEUROSCI.0300-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 1994; 13:1071-82; PMID:7946346; http://dx.doi.org/ 10.1016/0896-6273(94)90046-9 [DOI] [PubMed] [Google Scholar]
- [29].Morshead CM, van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci Off J Soc Neurosci 1992; 12:249-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 2008; 3:265-78; PMID:18786414; http://dx.doi.org/ 10.1016/j.stem.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Altman J. Are new neurons formed in the brains of adult mammals? Science 1962; 135:1127-8; PMID:13860748; http://dx.doi.org/ 10.1126/science.135.3509.1127 [DOI] [PubMed] [Google Scholar]
- [32].Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science 1994; 264:1145-8; PMID:8178174; http://dx.doi.org/ 10.1126/science.8178174 [DOI] [PubMed] [Google Scholar]
- [33].Morshead CM, Craig CG, van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Dev Camb Engl 1998; 125:2251-61 [DOI] [PubMed] [Google Scholar]
- [34].Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999; 97:703-16; PMID:10380923; http://dx.doi.org/ 10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- [35].Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci Off J Soc Neurosci 1997; 17:5046-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Codega P, Silva-Vargas V, Paul A, Maldonado-Soto AR, Deleo AM, Pastrana E, Doetsch F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 2014; 82:545-59; PMID:24811379; http://dx.doi.org/ 10.1016/j.neuron.2014.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fu H, Qi Y, Tan M, Cai J, Hu X, Liu Z, Jensen J, Qiu M. Molecular mapping of the origin of postnatal spinal cord ependymal cells: evidence that adult ependymal cells are derived from Nkx6.1+ ventral neural progenitor cells. J Comp Neurol 2003; 456:237-44; PMID:12528188; http://dx.doi.org/ 10.1002/cne.10481 [DOI] [PubMed] [Google Scholar]
- [38].Sabourin JC, Ackema KB, Ohayon D, Guichet PO, Perrin FE, Garces A, Ripoll C, Charité J, Simonneau L, Kettenmann H, et al.. A mesenchymal-like ZEB1(+) niche harbors dorsal radial glial fibrillary acidic protein-positive stem cells in the spinal cord. Stem Cells Dayt Ohio 2009; 27:2722-33; http://dx.doi.org/ 10.1002/stem.226 [DOI] [PubMed] [Google Scholar]
- [39].Smart IH. Proliferative characteristics of the ependymal layer during the early development of the spinal cord in the mouse. J Anat 1972; 111:365-80; PMID:4560930 [PMC free article] [PubMed] [Google Scholar]
- [40].Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci Off J Soc Neurosci 1996; 16:7599-609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Meletis K, Barnabé-Heider F, Carlén M, Evergren E, Tomilin N, Shupliakov O, Frisén J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol 2008; 6:e182; PMID:18651793; http://dx.doi.org/ 10.1371/journal.pbio.0060182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hamilton LK, Truong MK, Bednarczyk MR, Aumont A, Fernandes KJL. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience 2009; 164:1044-56; PMID:19747531; http://dx.doi.org/ 10.1016/j.neuroscience.2009.09.006 [DOI] [PubMed] [Google Scholar]
- [43].Bodega G, Suárez I, Rubio M, Fernández B. Ependyma: phylogenetic evolution of glial fibrillary acidic protein (GFAP) and vimentin expression in vertebrate spinal cord. Histochemistry 1994; 102:113-22; PMID:7822213; http://dx.doi.org/ 10.1007/BF00269015 [DOI] [PubMed] [Google Scholar]
- [44].Fiorelli R, Cebrian-Silla A, Garcia-Verdugo JM, Raineteau O. The adult spinal cord harbors a population of GFAP-positive progenitors with limited self-renewal potential. Glia 2013; 61:2100-13; PMID:24123239; http://dx.doi.org/ 10.1002/glia.22579 [DOI] [PubMed] [Google Scholar]
- [45].Bernstock J, Verheyen J, Huang B, Hallenbeck J, Pluchino S.. Typical and atypical stem cell niches of the adult nervous system in health and inflammatory brain and spinal cord diseases. Adult Stem Cell Niches 2014; 8:211-88. [Google Scholar]
- [46].Reeve RL, Yammine SZ, DeVeale B, van der Kooy D. Targeted activation of primitive neural stem cells in the mouse brain. Eur J Neurosci 2016; 43:1474-85; PMID:26946195; http://dx.doi.org/ 10.1111/ejn.13228 [DOI] [PubMed] [Google Scholar]
- [47].Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci 2006; 63:1945-61; PMID:16794783; http://dx.doi.org/ 10.1007/s00018-006-6094-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hou T, Shi Y, Cheng S, Yang X, Li L, Xiao C. Nogo-A expresses on neural stem cell surface. Int J Neurosci 2010; 120:201-5; PMID:20374087; http://dx.doi.org/ 10.3109/00207450903506502 [DOI] [PubMed] [Google Scholar]
- [49].Scadden DT. The stem-cell niche as an entity of action. Nature 2006; 441:1075-9; PMID:16810242; http://dx.doi.org/ 10.1038/nature04957 [DOI] [PubMed] [Google Scholar]
- [50].Picard-Riera N, Nait-Oumesmar B, Baron-Van Evercooren A. Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J Neurosci Res 2004; 76:223-31; PMID:15048920; http://dx.doi.org/ 10.1002/jnr.20040 [DOI] [PubMed] [Google Scholar]
- [51].Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 1993; 11:173-89; PMID:8338665; http://dx.doi.org/ 10.1016/0896-6273(93)90281-U [DOI] [PubMed] [Google Scholar]
- [52].Lois C, García-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science 1996; 271:978-81; PMID:8584933; http://dx.doi.org/ 10.1126/science.271.5251.978 [DOI] [PubMed] [Google Scholar]
- [53].Bayer SA. Changes in the total number of dentate granule cells in juvenile and adult rats: a correlated volumetric and 3H-thymidine autoradiographic study. Exp Brain Res 1982; 46:315-23; PMID:7095040; http://dx.doi.org/ 10.1007/BF00238626 [DOI] [PubMed] [Google Scholar]
- [54].Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 1965; 124:319-35; PMID:5861717; http://dx.doi.org/ 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- [55].Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci Off J Soc Neurosci 2000; 20:8727-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol 2001; 167:27-39; PMID:11161590; http://dx.doi.org/ 10.1006/exnr.2000.7539 [DOI] [PubMed] [Google Scholar]
- [57].Piccin D, Tufford A, Morshead CM. Neural stem and progenitor cells in the aged subependyma are activated by the young niche. Neurobiol Aging 2014; 35:1669-79; PMID:24559648; http://dx.doi.org/ 10.1016/j.neurobiolaging.2014.01.026 [DOI] [PubMed] [Google Scholar]
- [58].Grégoire CA, Goldenstein BL, Floriddia EM, Barnabé-Heider F, Fernandes KJ. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia 2015; 63:1469-82; PMID:25921491; http://dx.doi.org/ 10.1002/glia.22851 [DOI] [PubMed] [Google Scholar]
- [59].Barnabé-Heider F, Göritz C, Sabelström H, Takebayashi H, Pfrieger FW, Meletis K, Frisén J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010; 7:470-82; PMID:20887953; http://dx.doi.org/ 10.1016/j.stem.2010.07.014 [DOI] [PubMed] [Google Scholar]
- [60].Faiz M, Sachewsky N, Gascón S, Bang KW, Morshead CM, Nagy A. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell 2015; 17:624-34; PMID:26456685; http://dx.doi.org/ 10.1016/j.stem.2015.08.002 [DOI] [PubMed] [Google Scholar]


