Summary
According to the N-end rule, the N-terminal residue of a protein determines its stability. In bacteria, the adaptor ClpS mediates proteolysis by delivering substrates bearing specific N-terminal residues to the protease ClpAP. We now report that the Salmonella adaptor ClpS binds to the N-terminus of the regulatory protein PhoP, resulting in PhoP degradation by ClpAP. We establish that the PhoP-activated protein MgtC protects PhoP from degradation by outcompeting ClpS for binding to PhoP. MgtC appears to act exclusively on PhoP as it did not alter the stability of a different ClpS-dependent ClpAP substrate. Removal of five N-terminal residues rendered PhoP stability independent of both the clpS and mgtC genes. By preserving PhoP protein levels, MgtC enables normal temporal transcription of PhoP-activated genes. The identified mechanism provides a simple means to spare specific substrates from an adaptor-dependent protease.
Keywords: PhoP, Protease adaptor ClpS, Protease ClpAP, Protease substrate specificity, Protein stability
Introduction
Cells modify the levels of specific proteins via the combined effects of protein synthesis and degradation. The fastest way to modify the levels of a given protein is via proteolysis, which governs multiple cellular functions (Ehrmann and Clausen, 2004; Gur et al., 2011). Proteases utilize a variety of strategies to target specific proteins for degradation (Sauer and Baker, 2011). For example, certain proteases require adaptor proteins for recognition and delivery of specific substrates (Kirstein et al., 2009). Thus, conditions that alter the production and/or availability of an adaptor impact the degradation of the corresponding substrates (Battesti and Gottesman, 2013; Kirstein et al., 2009). Because some protease adaptors recognize multiple targets (Kirstein et al., 2009), it is presently unclear how cells achieve differential stability among the substrates of a given adaptor-dependent protease.
The AAA+ family protease machines contribute to protein quality control by promoting proteolysis of a variety of proteins in all domains of life (Bonnet et al., 2013; Buczek et al., 2016; Mogk et al., 2007; Song et al., 2015; Williams et al., 2014). In bacteria, the AAA+ protease ClpAP is composed of the serine protease ClpP and the regulatory ATPase ClpA (Thompson and Maurizi, 1994). ClpA uses the adaptor ClpS to target substrates of the N-end rule degradation pathway (Dougan et al., 2010; Kirstein et al., 2009; Mogk et al., 2007) (Figure 1). ClpS recognizes the N-terminus of substrates and delivers them to ClpAP for proteolysis (Kirstein et al., 2009; Mogk et al., 2007; Schuenemann et al., 2009). Designated N-degrons, these N-terminal residues are key determinants in substrate recognition (Kirstein et al., 2009; Mogk et al., 2007; Schuenemann et al., 2009).
Figure 1. Differential stability of protease substrate by sequestration from protease adaptor.
(A) Substrate specificity modulation mechanism via competition between an adaptor protein and a specific adaptor competitor. The ClpS adaptor recognizes the substrates Oat and PhoP, and delivers them to the ClpAP protease, which degrades them. The MgtC protein competes with ClpS for PhoP, thereby protecting PhoP, but not Oat, from ClpS-dependent ClpAP-mediated proteolysis. Western blot and SDS-PAGE analysis for time course in vitro degradation of PhoP (B) or heat aggregated Mdh (C). PhoP (0.2 μM) and Mdh (0.5 μM) were mixed with ClpAP in the absence or presence of ClpS (0.5 μM), and/or 3 μl in vitro of synthesized MgtC. Reactions contained 2 μl in vitro of synthesized ClpP and ClpA. All reactions were carried out at 30°C for the indicated times in the presence of an ATP regeneration system and started by the addition of substrates. After incubation, protein amounts were determined by anti-PhoP, anti-MgtC, and anti-Mdh antibodies and Coomassie-stained band following separation by 4–12% SDS-PAGE gel. Data are representative of three independent experiments, which gave similar results. See also Figures S1 and S3.
PhoP is a DNA binding regulatory protein that governs Mg2+ homeostasis and virulence in several Gram-negative species (Ernst et al., 2001; Grabenstein et al., 2004; Groisman, 2001). The levels of active (i.e., phosphorylated) PhoP protein (PhoP-P) are determined by PhoQ, a sensor of extracytoplasmic Mg2+ (Garcia Vescovi et al., 1996) and antimicrobial peptides (Bader et al., 2005), and of cytoplasmic pH (Choi and Groisman, 2016; Prost et al., 2007). In Salmonella enterica serovar Typhimurium, the PhoP/PhoQ system is required for survival in macrophages (Alpuche Aranda et al., 1992; Fields et al., 1989; Groisman et al., 1989; Miller et al., 1989) and growth in low Mg2+ (Garcia Vescovi et al., 1996). These abilities are mediated, in part, by the PhoP-activated mgtC gene (Blanc-Potard and Groisman, 1997; Lee et al., 2013), which specifies an inhibitor of Salmonella’s own F1Fo ATPase (Blanc-Potard and Groisman, 1997; Lee et al., 2013), the machine responsible for making most of the ATP in the cell (Senior, 1990). PhoP controls expression of ~9% of the Salmonella genes (Colgan et al., 2016). Transcription of PhoP-activated horizontally acquired genes requires larger amounts of PhoP-P than that of PhoP-activated ancestral genes (Park and Groisman, 2013; Zwir et al., 2012).
We now report a regulatory mechanism that confers differential stability to the substrates of an adaptor-mediated protease (Figure 1A). We identify PhoP as a ClpS- and ClpAP-dependent substrate, and determined N-terminal residues required for PhoP proteolysis. We determine that the PhoP-activated MgtC protein protects PhoP from degradation by ClpSAP, and that, unexpectedly, MgtC protects PhoP independently of its ability to reduce ATP levels. Moreover, we establish that MgtC-dependent protection of PhoP is essential for transcription of a subset of the PhoP regulon. The identified mechanism achieves differential stability of a specific protease substrate without compromising degradation of other adaptor-dependent substrates.
Results and Discussion
ClpAP promotes PhoP degradation in a ClpS-dependent manner
The protease ClpP can pair with two different regulatory ATPases: ClpA and ClpX (Gottesman, 1996; Ortega et al., 2004). We hypothesized that ClpP degrades the PhoP protein because PhoP’s N-terminus has putative recognition motifs for ClpA (Ninnis et al., 2009), ClpX (Flynn et al., 2003), and the adaptor ClpS (Ninnis et al., 2009) (Figure S1A). For instance, the N-terminal residues of PhoP matched part of the ClpX recognition motif present in the ClpX substrate DadA (Flynn et al., 2003) (Figure S1A, blue bar). Likewise, the N-terminal residues of PhoP include those normally recognized by the adaptor ClpS (Dougan et al., 2010; Humbard et al., 2013) (Figure S1A, red bar). In addition, PhoP has a motif that resembles the ClpA substrate Dps (Figure S1A, green bar) (Ninnis et al., 2009). Due to the overlapping specificities of ClpX, ClpA and the adaptor ClpS (Figure S1A), certain proteins, such as Dps, are degraded by both ClpXP and ClpSAP (Farrell et al., 2005; Stephani et al., 2003).
To determine whether ClpA, ClpX and/or ClpS promote PhoP degradation in vivo, we examined PhoP protein levels in mutants lacking the clpA, clpX or clpS genes. PhoP levels were higher in clpA and clpS mutants than in wild-type Salmonella or the clpX mutant (Figure 2A). By contrast, the levels of the control protein AtpB were similar in all four strains (Figure 2A). Wild-type levels of PhoP were restored to the clpS mutant by a clpS-expressing plasmid but not by the vector control (Figure 2B), indicating that the higher PhoP levels exhibited by the clpS mutant are due to absence of clpS (as opposed to polarity on the downstream clpA gene).
Figure 2. The PhoP-activated MgtC protein protects PhoP from ClpS-mediated proteolysis.
(A) Western blot analysis of crude extracts prepared from wild-type (14028s), clpA (JY650), clpS (JY651) and clpX (JY649) Salmonella grown under low Mg2+ conditions. Samples were analysed using anti-PhoP and anti-AtpB antibodies. The data are representative of three independent experiments, which gave similar results. (B) Western blot analysis of crude extracts prepared from wild-type (14028s), clpS (JY651) and clpS Salmonella with no plasmid, with the plasmid vector (vector, pUHE-21-2-lacIq) or with a clpS-expressing plasmid (pclpS; pUHE-clpS). clpS transcription from pClpS was induced with IPTG (100 μM). Samples were analysed using anti-PhoP and anti-OmpA antibodies. Samples were loaded on 4–12% NuPAGE gels with normalization via cell abundance with optical density. Data are representative of three independent experiments, which gave similar results. (C) The PhoP half-life was determined in wild-type (14028s), clpA (JY650), clpS (JY651) and clpX (JY649) Salmonella. Protein synthesis was inhibited with chloramphenicol (1 mg/ml). Samples were removed at the indicated time points and analysed by Western blotting with anti-PhoP antibodies. PhoP half-lives (t1/2) were calculated by regression analysis of the exponential decay of PhoP. 5-fold less protein was loaded for the samples from the clpS and clpA strains than for the samples from the wild-type and clpX strains. (D) Western blot analysis of crude extracts prepared from wild-type (14028s), mgtC (EL4), clpS (JY651), clpS mgtC (JY655), clpX (JY649), clpX mgtC (JY652), clpA (JY650) and clpA mgtC (JY653) Salmonella. Samples were analysed using anti-PhoP and anti-AtpB antibodies. Data are representative of three independent experiments, which gave similar results. (E) PhoP stability was determined in wild-type (14028s) and mgtC (EL4) Salmonella. (F) PhoP stability was determined in clpS (JY651) and clpS mgtC (JY655) Salmonella. Samples were analysed using anti-PhoP and anti-AtpB antibodies. 5-fold more protein was loaded for the mgtC samples than for the wild-type and mgtC/clpS samples (E–F). Bacteria were grown in N-minimal media pH 7.7 containing 15 μM MgCl2 for 6 h. See also Figures S1 and S2.
Degradation of certain ClpS-dependent substrates requires the amino acyl transferase (Aat) protein, which attaches a leucine or phenylalanine residue to the N-terminal basic amino acids lysine or arginine, thereby allowing recognition by ClpS (Humbard et al., 2013). However, Aat is not necessary for ClpS-dependent degradation of PhoP because PhoP levels were not altered upon deletion of the aat gene (Figure S1B). The ClpA and ClpS proteins control PhoP stability because PhoP’s half-life was much longer in clpA and clpS mutants than in wild-type and clpX Salmonella (Figure 2C).
The results described above suggested that PhoP is a ClpS-dependent but Aat-independent substrate of ClpAP. This was surprising because the deduced amino acid sequence of the annotated phoP gene begins with MMR, and neither Met nor Arg are normally recognized by ClpS in vitro (Varshavsky, 2011). This is to say, the primary destabilizing residues in N-degrons, which are recognized by ClpS and degraded by ClpAP without modification, are Leu, Tyr, Trp and Phe at the very N-terminus (Varshavsky, 2011). Arg and Lys (and occasionally Met) are secondary destabilizing residues that are turned into functional degrons by the Aat-mediated covalently attachment of an Leu or an Phe to their N-terminus (Ninnis et al., 2009).
To determine the N-terminal residue of the PhoP protein, we performed Edman degradation of the PhoP protein purified from a clpS Salmonella strain to avoid PhoP degradation. The amino acid sequence was MRVLVV, which exhibits a perfect match to the deduced amino acid sequence of the phoP gene except for the first annotated start codon. (Note that the additional AUG codon located immediately upstream of the bona fide start codon is not conserved in other enteric phoP genes; and that Edman degradation was performed on a purified PhoP sample showing no additional bands (Figure S1C). These data indicate that the N-terminus of PhoP did not arise from peptidase cleavage. Moreover, they suggest that the in vivo specificity of the bacterial adaptor ClpS may be broader than previously assumed.
The adaptor ClpS interacts with PhoP
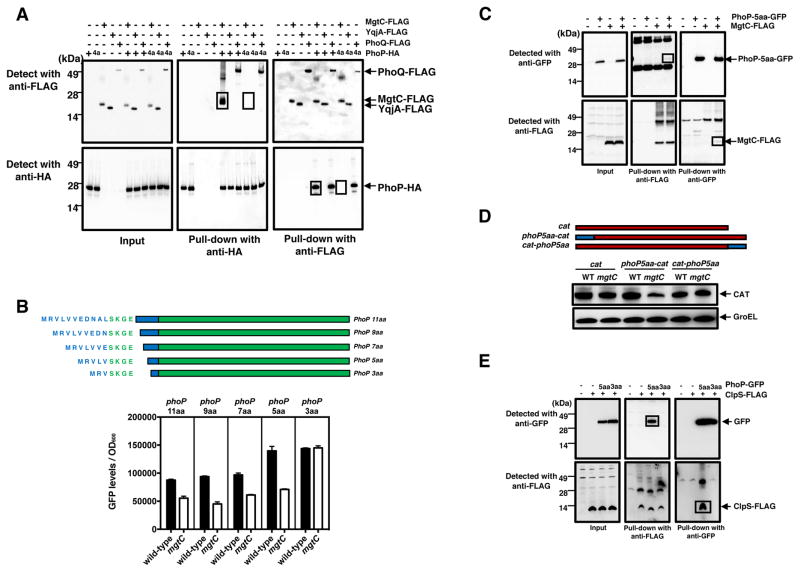
Three independent experiments support the notion that ClpS binds to the PhoP protein. First, a bacterial two-hybrid system assay showed that PhoP interacts with ClpS as it does with the positive control protein PhoQ (Figure S2A). Second, a pull-down assay using in vitro synthesized PhoP-HA and ClpS-FLAG, and anti-HA and anti-FLAG antibodies demonstrated that PhoP binds to ClpS (Figure 3A). Binding is specific because PhoP also bound to the MgtC protein (Figure 3A), which was used as a positive control (Lee et al., 2013), but not to AtpB (Figure 3A), an MgtC-interacting partner (Lee et al., 2013) used as a negative control. And third, in vivo immunoprecipitaction experiments with wild-type Salmonella as well as strains expressing PhoP-HA and/or ClpS-FLAG proteins from their normal promoters and chromosomal locations demonstrated that anti-HA antibodies pull down ClpS-FLAG, and that anti-FLAG antibodies pull down PhoP-HA in a strain specifying both PhoP-HA and ClpS-FLAG (Figure 3B). These pull downs were specific because they were not observed in wild-type Salmonella (with no epitope-tagged proteins) or in strains specifying only one of the epitope-tagged proteins (Figure 3B).
Figure 3. The PhoP and ClpS proteins interact in vitro and in vivo.
(A) Interaction between the MgtC-FLAG, ClpS-FLAG and AtpB-FLAG proteins synthesized using an in vitro transcription/translation system. Proteins were incubated with purified PhoP-HA protein at room temperature for 2 h, and then incubated with anti-FLAG and anti-HA magnetic beads for an additional 2 h. Immunoprecipitated samples were analysed using anti-FLAG and anti-HA antibodies. The data are representative of two independent experiments, which gave similar results. Boxes highlight relevant bands. (B) A pull-down assay was performed in wild-type (14028s), phoP-HA (EG13918), clpS-FLAG (JY674) and phoP-HA/clpS-FLAG (JY676) Salmonella following growth in N-minimal media pH 7.7 containing 15 μM MgCl2 for 6 h. Lysed cells were incubated with anti-FLAG and anti-HA beads overnight. Samples were loaded onto 4–12% NuPAGE gels and analysed using anti-HA and anti-FLAG antibodies. The data are representative of two independent experiments, which gave similar results. Boxes highlight relevant bands. See also Figure S2.
The MgtC protein protects PhoP from degradation
We hypothesized that the PhoP-activated mgtC gene hinders PhoP proteolysis because the MgtC protein decreases ATP levels (Lee et al., 2013), and ClpAP is an ATP-dependent protease (Weber-Ban et al., 1999). Moreover, an increase in cytoplasmic ATP levels stimulates proteolysis by ClpXP (Peterson et al., 2012). As hypothesized, PhoP protein levels were lower in the mgtC mutant than in wild-type Salmonella (Figure 2D and Figure S3A). The defect of the mgtC mutant was corrected by a plasmid expressing the mgtC gene from a heterologous promoter but not by the vector control (Figure S3A).
Inactivation of the clpS or clpA genes also corrected PhoP levels in the mgtC mutant (Figure 2D), but inactivation of clpX did not (Figure 2D). In agreement with these results, the decreased half-life of the PhoP protein exhibited by the mgtC mutant (Figure 2E) was overcome by inactivation of the clpS gene (Figure 2F).
Growth in low Mg2+ promotes phosphorylation of the PhoP protein at Asp52 (Shin and Groisman, 2005). To test whether PhoP phosphorylation impacts PhoP degradation by ClpSAP and protection from degradation by MgtC, we examined the levels of the PhoP D52V variant, which cannot be phosphorylated (Shin and Groisman, 2005). PhoP D52V levels were lower in the mgtC mutant than in wild-type Salmonella (Figure S2B), and higher in clpA and clpS mutants than in wild-type Salmonella (Figure S2B), mimicking the behaviour of the phosphorylatable PhoP protein. Taken together, the results presented in this section established that MgtC protects PhoP from ClpSAP-mediated degradation regardless of PhoP’s phosphorylation status.
MgtC protects PhoP directly and independently of its effect on ATP levels
Surprisingly, the mgtC mutant displayed lower PhoP protein amounts than the wild-type strain even when the ATP levels of the mgtC mutant were corrected to those of the wild-type strain (Figures S2C and S2D) using a plasmid that expresses the soluble subunit of the F1Fo ATPase (Koebmann et al., 2002). This is to say, MgtC enhances PhoP stability independently of its ability to decrease ATP levels.
Because anti-FLAG antibodies pulled down PhoP in a strain expressing an MgtC-FLAG protein (Lee et al., 2013), we wondered whether MgtC interacts with PhoP directly. Pull-down assays with in vitro synthesized proteins demonstrated that the MgtC-FLAG protein binds to C-terminally HA-tagged PhoP (Figure 4A). Control experiments revealed that the PhoP-HA protein also binds to the positive control PhoQ-FLAG protein (Figure 4A), but not to the negative control YqjA-FLAG protein (Figure 4A). This result raised the possibility of MgtC protecting PhoP from degradation by hindering ClpS access to PhoP.
Figure 4. The five N-terminal amino acids of PhoP are required for protection by MgtC.
(A) In vitro protein-protein interactions examined by co-immunoprecipitation using MgtC-FLAG, PhoQ-FLAG, YqjA-FLAG, PhoP-HA, and PhoP-HA derivative lacking amino acids 2 to 4 (4a). All proteins were synthesized using an in vitro transcription/translation system incubated at room temperature for 2 h, and then incubated with anti-FLAG or anti-HA magnetic beads for an additional 2 h. Immunoprecipitated samples with FLAG and HA magnetic beads were analysed by Western blotting using anti-HA or anti-FLAG antibodies. Boxes highlight relevant bands. (B) Fluorescence of wild-type (14028s) and mgtC (EL4) Salmonella harboring plasmids expressing PhoP-GFP chimeric proteins (pUHE-phoP-11aa-gfp, pUHE-phoP-9aa-gfp, pUHE-phoP-7aa-gfp, pUHE-phoP-5aa-gfp, and pUHE-phoP-3aa-gfp). Top, schematic of chimeric proteins with GFP indicated in green and PhoP in blue. Bottom, Fluorescence normalized by optical density. Shown are the mean and SD from four independent experiments, which gave similar results. (C) Pull-down assay of wild-type (14028s), phoP-five amino acids-GFP (KW64), mgtC-FLAG (EG16539) and phoP-five amino acids-GFP mgtC-FLAG (JY610) Salmonella. Samples were analysed with anti-GFP and anti-FLAG antibodies. Boxes highlight relevant bands. Data are representative of two independent experiments, which gave similar results. (D) Western blot analysis of wild-type (14028s) and mgtC (EL4) Salmonella carrying the CAT-expressing plasmids (pUHE-cat, pUHE-phoP-5aa-cat and pUHE-cat-phoP-5aa) using anti-CAT and anti-GroEL antibodies. Top, schematic of CAT and chimeric proteins with CAT indicated in red and PhoP in blue. Data are representative of three independent experiments, which gave similar results. (E) Pull-down assay using wild-type (14028s) and clpS-FLAG (JY674) Salmonella, and derivatives of the latter strain harboring the GFP-expressing plasmids pUHE-phoP-5aa-gfp or pUHE-phoP-3aa-gfp. Samples were analysed using anti-GFP and anti-FLAG antibodies. Boxes highlight relevant bands. Data are representative of two independent experiments, which gave similar results. Bacteria were grown in N-minimal media pH 7.7 containing 15 μM MgCl2 and 0.1 mM IPTG for plasmids for 6 h. All pull-down assay samples were incubated with magnetic beads at 4°C overnight. See also Figure S4.
In vitro reconstitution of MgtC protection of PhoP from ClpSAP-mediated proteolysis
To determine whether the MgtC protein directly protects PhoP from degradation, we performed an in vitro degradation assay with purified PhoP protein (corresponding to the sample used in the Edman degradation assay), purified ClpS protein, and in vitro synthesized MgtC, ClpA and ClpP proteins. ClpAP degraded PhoP in a ClpS-dependent manner (Figure 1B), and MgtC protected PhoP from degradation (Figure 1B). By contrast, heat aggregated Mdh protein (Dougan et al., 2002), a previously described ClpS client, was degraded by ClpSAP both in the absence and presence of MgtC (Figure 1C). These results established that MgtC specifically protects PhoP from ClpSAP-dependent degradation independently of other cellular components.
The five N-terminal amino acids from PhoP are necessary and sufficient for MgtC to protect PhoP from degradation by ClpSAP
ClpS recognizes client proteins harboring Leu, Tyr, Trp or Phe at the very N-terminus (Varshavsky, 2011). Then, what does ClpS recognize in PhoP given that PhoP’s N-terminal Met is not removed? Because PhoP harbors a Leu residue at position 4, we wondered whether the five N-terminal amino acids from PhoP were sufficient for recognition by MgtC and protection from ClpSA-mediated proteolysis. We investigated this possibility in a pull down assay: in the presence of the MgtC-FLAG protein, anti-FLAG antibodies pulled down C-terminally HA-tagged full-length PhoP but not a derivative lacking amino acids 2 to 4 (Figure 4A). This PhoP derivative still interacted with PhoP’s cognate sensor protein PhoQ like the full-length PhoP protein (Figure 4A). In agreement with these results, the PhoP derivative lacking amino acids 2 to 4 interacted with PhoQ but not with ClpS and MgtC in the bacterial two-hybrid system assay (Figure S2B–D). These results argue that one or more of the five N-terminal amino acids of PhoP are necessary for interaction with the MgtC protein.
The five N-terminal amino acids of PhoP are sufficient to confer mgtC-dependent stability to heterologous proteins because: the fluorescence levels of Salmonella strains harbouring plasmids expressing chimeric proteins containing 5, 7, 9 or 11 N-terminal amino acids from PhoP fused to a full-length GFP were lower in an mgtC mutant than in the wild-type strain (Figure 4B). By contrast, there were no differences in fluorescence between the two strains when harbouring a plasmid expressing a chimeric protein consisting of the three N-terminal amino acids from PhoP fused to GFP (Figure 4B). The fluorescence data reflect the stability of the chimeric proteins because the PhoP-GFP chimera with the five N-terminal amino acids from PhoP was more rapidly degraded in the mgtC mutant than in the wild-type strain (Figure S4A), whereas the PhoP-GFP chimera harbouring only three N-terminal amino acids from PhoP was similarly degraded in wild-type and mgtC Salmonella (Figure S4A). In agreement with these results, the PhoP-GFP chimera with the five N-terminal amino acids from PhoP was pull-downed by the MgtC-FLAG protein (Figure 4C).
The difference in fluorescence between wild-type and mgtC Salmonella harbouring the PhoP-GFP chimera with the five N-terminal amino acids from PhoP is due to ClpS-dependent degradation because fluorescence levels were higher in the clpS mutant than in the wild-type strain (Figure S4B), which were, in turn, higher than in the mgtC mutant (Figure S4B). By contrast, there were no differences in fluorescence among the three strains harbouring a plasmid expressing the PhoP-GFP chimera with the three N-terminal amino acids from PhoP (Figure S4B). The differences in fluorescence appears to reflect binding of ClpS to the PhoP-GFP chimeras because ClpS bound to the PhoP-GFP chimera with the five N-terminal amino acids from PhoP (Figure 4E) but not to the chimera harbouring only three N-terminal amino acids from PhoP (Figure 4E). Thus, the five N-terminal amino acids from PhoP are sufficient for binding to ClpS and MgtC, and for MgtC to protect PhoP.
The degron originating from the N terminus of PhoP confers instability when present at the N-terminus of a heterologous protein. This is because the mgtC mutant had lower levels of a chimeric protein consisting of the five N-terminal amino acids from PhoP fused to chloramphenicol acetyl transferase (CAT) than the wild-type strain (Figure 4D), but similar levels of a chimera with the five N-terminal amino acids from PhoP placed at the C terminus of CAT (Figure 4D). In sum, the five N-terminal PhoP residues are necessary and sufficient for MgtC protection from ClpS-dependent proteolysis.
MgtC and ClpS compete for binding to PhoP
That the same five N-terminal amino acids of PhoP are sufficient for interactions with MgtC and ClpS suggested that these two proteins compete with one another for binding to PhoP. A pull-down assay with in vitro synthesized PhoP, MgtC and ClpS proteins revealed that MgtC has a higher affinity for PhoP compared with ClpS (Figure S5). When MgtC protein levels were gradually increased, the interaction between ClpS and PhoP decreased (Figure 5A). Increasing ClpS to high levels decreased binding between MgtC and PhoP (Figure 5B). To determine the binding specificity of MgtC and ClpS for PhoP, we calculated the half inhibitory concentration (IC50) values of MgtC-FLAG (Figure 5C, red line) and Clps-FLAG (Figure 5C, blue line) for PhoP-HA using increasing amounts of competitor ClpS-FLAG and MgtC-FLAG, respectively. The IC50 values of MgtC-FLAG and Clps-FLAG for PhoP-HA are 14.6 μM and 8.4 μM, respectively (Figure 5C), suggesting that MgtC outcompetes ClpS for binding to PhoP.
Figure 5. MgtC outcompetes ClpS for interaction with PhoP.
(A–B) In vitro competition by a pull-down assay using PhoP-HA, ClpS-FLAG, and MgtC-FLAG proteins. (A) Interaction between PhoP-HA and ClpS-FLAG proteins under increasing MgtC-FLAG amounts. (B) Interaction between PhoP-HA and MgtC-FLAG proteins under increasing ClpS-FLAG amounts. (C) Dissociation curves of MgtC-FLAG (red line) and ClpS-FLAG (blue line) from PhoP-HA (from A and B; see Experimental Procedures). The IC50 corresponds to the concentration at which half of the proteins are dissociated from PhoP-HA. Shown are the mean and SD from three independent experiments. (D) In vivo competition for binding to PhoP-HA in wild-type (14028s), phoP-HA clpA::cm (JY590), mgtC-FLAG (EG16539), phoP-HA clpA::cm mgtC-FLAG (JY591), and phoP-HA clpA::cm mgtC-FLAG Salmonella harbouring the clpS-expressing plasmid (pUHE-clpS). Bacteria were grown in N-minimal media pH 7.7 containing 15 μM MgCl2 and IPTG (0.05, 0.2, and 0.5 mM) for 6 h. Data are representative of two independent experiments, which gave similar results. (E) Levels of the MgtC, ClpS and PhoP proteins determined by Western blot analysis of crude extracts from phoP-HA/clpS-FLAG (JY676) Salmonella following growth under non-inducing (10 mM MgCl2, time zero) and inducing (15 μM MgCl2) conditions for 2.5, 3.5, 4.5, 5.5, 6.5, 8.5 or 24 h. Numbers of protein molecules were calculated using purified proteins (see Experimental Procedures). Shown are the mean and SD from three independent experiments. See also Figures S5 and S6.
To test whether MgtC vies with ClpS for binding to PhoP in vivo, we conducted pull-down assays using the strain specifying chromosomally encoded PhoP-HA and MgtC-FLAG proteins, and harbouring a plasmid with the clpS gene under the control of a derivative of the lac promoter. As clpS expression increased, the amount of MgtC-FLAG protein recovered following pull down with anti-HA antibodies decreased, indicating diminished interaction between MgtC and PhoP (Figure 5D). Taken together with the in vitro assays, these data imply that, when MgtC levels reach a certain threshold, PhoP is sequestered away from ClpS (Figure 1A).
MgtC determines PhoP’s half-life in vivo
The ability of MgtC and ClpS to compete for PhoP in vivo is determined both by the affinities of these proteins for PhoP, and by the number of molecules of each of these proteins, which may vary during growth. Thus, we examined the amounts of MgtC, ClpS and PhoP proteins in a strain expressing the PhoP-HA and ClpS-FLAG proteins from their normal chromosomal locations at different times after Salmonella was switched from non-inducing to inducing conditions for the PhoP and MgtC proteins. Purified PhoP, MgtC and ClpS proteins (Figure S6A) were used as standards to determine protein amounts (Figure S6B), and following immunoblotting (Figure S6C), to calculate the number of molecules (Figure 5E) and molar concentrations (Figure S6D) of the three proteins.
PhoP levels increased 30 fold over the first 2.5 h in low Mg2+ conditions (Figure 5E), reflecting that PhoP positively regulates its own transcription (Shin et al., 2006; Soncini et al., 1995). PhoP levels peaked at 6.5 h before slightly decreasing by 24 h (Figure 5E). The MgtC protein was not detected during the first 2.5 h due to transcription termination in the mgtC leader region promoted by cytoplasmic signals (Cromie et al., 2006; Lee and Groisman, 2012a; Lee et al., 2014). MgtC amounts increased dramatically starting at 3.5 h, matched those of ClpS by 4.5 h, peaked at 6.5 h and decreased by 24 h (Figure 5E), reflecting degradation of the mgtC mRNA (Lee and Groisman, 2012b) and MgtC protein (Alix and Blanc-Potard, 2008). By contrast, ClpS was present at constant high levels during the first 3.5 h, decreased six-fold between 4.5 h and 5.5 h (Figure 5E), and remained at the same level until 24 h (Figure 5E). The levels of the AtpB protein, which was used as a control, remained constant over the course of the experiment (Figure S6C). Thus, MgtC amounts matched those of ClpS starting at 4.5 h, exceeded them by 5.5 h and were lower than those of ClpS by 24 h (Figure 5E).
Taken together with the data presented in the previous section, these results argue that preferential PhoP binding to ClpS over the first 3.5 h favours PhoP degradation, and that the increased MgtC levels starting at 4.5 h protects PhoP. This protection results in a four-fold increase in PhoP’s half-life between 2 and 5 h (Figure 6A).
Figure 6. MgtC increases the levels of active PhoP protein.
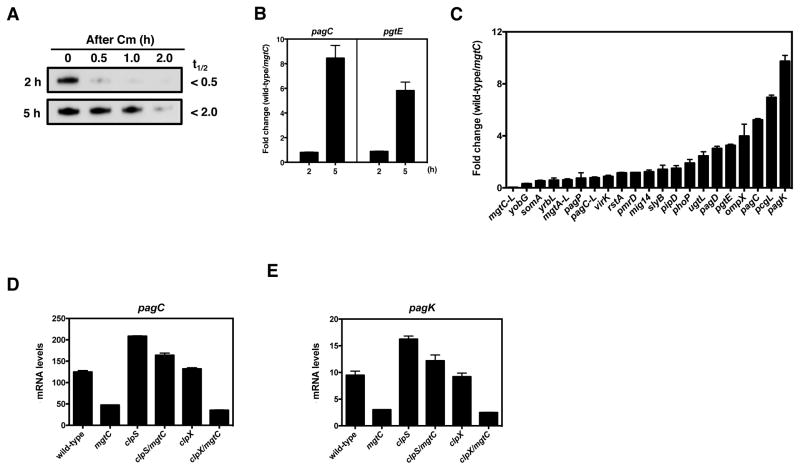
(A) Half-life of PhoP-HA in phoP-HA (EG13918) Salmonella grown in N-minimal media with 15 μM MgCl2 for 2 and 5 h. Protein synthesis was inhibited by chloramphenicol (1 mg/ml). Samples were removed at the indicated times and analysed by Western blotting with anti-HA antibodies. Three fold more protein was loaded in the 2 h samples than in the 5 h samples. Half-lives (t1/2) were calculated by regression analysis of the exponential decay of PhoP. (B) Fold change in the mRNA levels of the PhoP-activated pagC and pgtE genes produced by wild-type (14028s) and mgtC (EL4) Salmonella. Fold change was calculated by dividing the mRNA levels produced by wild-type Salmonella by those produced by the mgtC mutant. Bacteria were grown in N-minimal media pH 7.7 containing 10 μM MgCl2 for 2 and 5 h. (C) Fold change in the mRNA levels of 21 PhoP-activated genes produced by wild-type (14028s) and mgtC (EL4) Salmonella. Fold change was calculated by dividing the mRNA levels produced by wild-type Salmonella by those produced by the mgtC mutant. mRNA levels of target genes were normalized to those of the ompA gene (15054/15055). (D–E) mRNA levels of the pagC and pagK genes produced by wild-type (14028s), mgtC (EL4), clpS (JY651), clpS mgtC (JY655), clpX (JY649) and clpX mgtC (JY652) Salmonella following growth in N-minimal medium, pH 7.7, with 10 μM MgCl2 at 37°C for 5 h. mRNA levels were normalized to those of the ompA gene. Primers used in qRT-PCR are presented in Table S3. For all qRT-PCR analysis, shown are the mean and SD from three independent experiments. See also Figure S7.
MgtC is required for normal transcription of PhoP-activated genes
To explore the consequences of MgtC protecting PhoP from proteolysis, we examined the mRNA levels of the PhoP-activated pagC and pgtE genes in isogenic wild-type and mgtC strains at 5 h post induction, a time when MgtC stabilizes PhoP levels. mRNA levels were 5–8 times higher in the wild-type strain than in the mgtC mutant (Figure 6B). (This difference reflects a larger increase in the pagC and pgtE transcripts in the wild-type strain than in the mgtC mutant (Figure S7A and S7B). By contrast, the mRNA levels of the pagC and pgtE genes were similar in wild-type and mgtC Salmonella at 2 h post induction (Figures 6B and S7A–B), which makes sense given that the MgtC protein levels are exceedingly low at this time (Figure 5E).
Investigation of 21 genes directly activated by PhoP revealed differences in their dependence on mgtC for full expression (Figure 6C), which we ascribe to the distinct architectures of the corresponding PhoP-activated promoters (Zwir et al., 2012). Horizontally-acquired genes, which require larger amounts of active PhoP protein for transcription (Zwir et al., 2012), were most affected by inactivation of the mgtC gene (Figure 6C).
If the defective expression of PhoP-activated genes exhibited by the mgtC mutant results from lower PhoP levels, then, preventing PhoP degradation should restore wild-type expression to the mgtC mutant. As predicted, wild-type mRNA levels of the pagC (Figure 6D) and pagK (Figure 6E) genes returned to the mgtC mutant upon inactivation of the clpS gene. By contrast, inactivation of the clpX gene failed to rescue the mgtC mutant (Figures 6D and 6E). Thus, normal transcription of PhoP-regulated genes requires PhoP levels attained by the protective action of MgtC.
Removal of N-terminal amino acids renders PhoP stability mgtC- and clpS-independent
We reasoned that the stability of a PhoP variant lacking N-terminal residues should be oblivious to the presence/absence of the protease adaptor ClpS and its competitor protein MgtC because the five N-terminal amino acids of PhoP are sufficient for interaction with the MgtC and ClpS proteins (Figure 4C and E), and for MgtC-dependent protection of ClpS-dependent proteolysis (Figure 4B and D). As proposed, the PhoP-HA derivative lacking amino acids at position 2 to 4 was present at the same levels in isogenic wild-type, mgtC, clpS and mgtC clpS strains (Figure 7A, right). By contrast, the clpS and mgtC clpS mutants contained larger amounts of full-length PhoP-HA protein than the wild-type strain (Figure 7A, left) whereas the mgtC mutant had lower amounts (Figure 7A). (To avoid potential confounding effects resulting from PhoP positively regulating its own transcription (Shin et al., 2006; Soncini et al., 1995), we used plasmid-liked genes specifying wild-type and variant phoP-HA transcribed from a heterologous promoter.)
Figure 7. Removal or substitution of N-terminal residues renders PhoP stability independent of MgtC and ClpS, and MgtC does not regulate proteolysis of the ClpS client Oat.
(A) Protein levels of PhoP-HA in wild-type (14028s), mgtC (EL4), clpS (JY651), mgtC clpS (JY655), phoP (MS7953s) and phoP mgtC (EG16743) Salmonella harbouring a plasmid expressing phoP-HA (pphoP-HA; pUHE-phoP-HA), or a derivative lacking amino acids 2–5 (pphoP-4aa-HA; pUHE-phoP-5aa-HA). Bacteria were grown in N-minimal media pH 7.7 containing 15 μM MgCl2 and 100 μM IPTG for 6 h. Samples were loaded on 4–12% NuPAGE gels with normalization by cell abundance with optical density, and analysed using anti-HA, and anti-OmpA antibodies. Data are representative of two independent experiments, which gave similar results. (B) Protein levels of PhoP-HA in wild-type (14028s), mgtC (EL4) and clpS (JY651) Salmonella harboring a plasmid expressing HA-tagged PhoP (pphoP-HA; pUHE-phoP-HA), or a variant with the Leu4Ala substitution (pphoP-L4A-HA; pUHE-phoP-L4A-HA). Bacteria were grown as indicated in (A) with 300 μM IPTG. Samples were loaded on 4–12% NuPAGE gels with normalization by cell abundance with optical density, and analysed using anti-HA, and anti-OmpA antibodies. Data are representative of two independent experiments, which gave similar results. (C) Protein levels of the Oat protein, in oat-FLAG (JY655), oat-FLAG clpS (JY657) and oat-FLAG clpS mgtC (JY697) Salmonella harbouring no plasmid, the plasmid vector (pUHE-21-2-lacIq), or a clpS-expressing plasmid (pclpS; pUHE-clpS). Bacteria were growth as indicated in (A). Samples were loaded on 4–12% NuPAGE gels with normalization by cell abundance, and analysed using anti-FLAG, anti-PhoP and anti-OmpA antibodies. Data are representative of two independent experiments, which gave similar results. See also Figure S1.
Further support to the notion that amino acids 2–4 of PhoP are critical for MgtC-dependent protection of ClpS-dependent proteolysis was obtained in strains deleted for the phoP gene so that the only source of PhoP protein was the plasmid-encoded phoP gene transcribed from a heterologous promoter. This is to say, the PhoP-HA derivative lacking amino acids 2–4 was present at the same levels in phoP and mgtC phoP strains (Figure 7A) whereas the levels of the full-length PhoP-HA protein were lower in the mgtC phoP mutant than in the phoP strain (Figure 7A). Therefore, amino acids 2 to 4 of PhoP are necessary for the MgtC and ClpS proteins to regulate PhoP stability.
As stated above, ClpS recognizes the primary destabilizing residues Leu, Tyr, Trp and Phe at the very N-terminus of N-end rule substrates (Varshavsky, 2011). The first such residue in PhoP is a Leu residue at position 4. To test whether this residue is important for PhoP stability, we examined the behaviour of the PhoP Leu4Ala variant in isogenic wild-type, mgtC and clpS strains. The stability of the variant was similar in the three strains (Figure 7B), which is in contrast to the behaviour of the wild-type PhoP. This result implicates Leu4 in PhoP degradation by ClpSAP (Figure 7B).
The stability of the ClpS-dependent substrate Oat is unaffected by mgtC inactivation
The data presented above indicates that MgtC protects PhoP by outcompeting ClpS for binding to PhoP. This scenario predicts that degradation of other ClpS-dependent substrates should remain unimpeded in an mgtC mutant. As predicted, the mgtC mutant retained wild-type levels of the ClpS-dependent ClpAP substrate putrescine aminotransferase Oat (Ninnis et al., 2009) despite having reduced PhoP amounts (Figure 7C). (To avoid potential effects of mgtC on clpS transcription, this experiment was carried out in a strain deleted for the chromosomal clpS gene and expressing the wild-type clpS gene from a heterologous promoter.) This result suggests that MgtC acts on particular ClpS clients.
Concluding remarks
We have now uncovered a mechanism that confers differential stability to a specific substrate of a protease adaptor that has multiple substrates. We established that the adaptor ClpS delivers the regulatory protein PhoP to the ClpAP protease (Figure 2A–C), promoting its degradation (Figure 2A–C), and that degradation is prevented (Figure 2E and F) when the MgtC protein binds to the PhoP protein (Figure 3). Thus, when MgtC outcompetes ClpS (Figure 5), PhoP levels increase, resulting in transcription of its target genes (Figure 6).
Because mgtC is a PhoP-activated gene (Lejona et al., 2003; Soncini et al., 1996), the MgtC protein acts as a positive regulator of its own transcriptional activator. The positive feedback resulting from MgtC-mediated PhoP stabilization differs from previously described positive feedback mechanisms whereby PhoP enhances transcription from the phoP promoter (Soncini et al., 1995) or where the PhoP-activated MgtA protein enhances PhoQ-dependent phosphorylation of the PhoP protein (Park and Groisman, 2013). (See (Groisman, 2016) for a review on feedback on the PhoP protein.) As transcription elongation into the mgtC coding region is controlled by a long leader region that responds to cytosolic signals (Cromie et al., 2006; Lee and Groisman, 2012a; Lee et al., 2014), MgtC-dependent genes (Figure 6C) are transcribed only when the cytosolic conditions acting on the mgtC leader mRNA are met. Therefore, MgtC enables differential temporal expression of PhoP-activated genes.
The mechanism we uncovered spares specific substrates from proteolysis without compromising the protein quality control function of proteases (Figure 1A). Moreover, it differs from mechanisms involving competition between adaptors altering proteolysis of different substrates (Joshi et al., 2015), anti-adaptor proteins preventing substrate binding to an adaptor (Battesti and Gottesman, 2013), altering expression of proteases (Bellier and Mazodier, 2004; Guyet et al., 2013), or ATP levels (Peterson et al., 2012) in that the competition mechanism here described affects one substrate whereas the others impact multiple substrates (except for adaptors that recognize a single client).
The identified mechanism enables cells to regulate the stability of specific protease substrates in response to signals controlling products that hinder adaptor access to a degron. In all organisms, regulated degradation of specific proteins by the recognition of a degron governs a broad range of physiological functions (Varshavsky, 2011). Given that the N-end rule pathway is widely conserved in eukaryotes (Choi et al., 2010; Varshavsky, 2011) and prokaryotes (Erbse et al., 2006; Ninnis et al., 2009), the ClpS adaptor is essential for degradation of several N-end rule substrates in bacteria (Humbard et al., 2013), and the widespread phylogenetic distribution of ClpS-mediated proteolysis (Kirstein et al., 2009), we anticipate that analogous mechanisms may provide differential stability to other substrates dependent on ClpS and potentially other adaptors.
Finally, PhoP is degraded in a ClpS-dependent manner despite lacking a Leu, Tyr, Trp or Phe as the N terminus (Figure S1A), and independently of the amino acyl transferase Aat (Figure S1B). By contrast, the PhoP L4A variant was not degraded (Figure 7B). In agreement with our findings, ClpS promotes degradation of a ClpAP substrate in Pseudomonas aeruginosa that lacks classical destabilizing residues at the N-terminus (Zhao et al., 2016). Furthermore, a 25 amino acid region of ClpS exhibits similarity with a region of the eukaryotic ubiquitin ligase Ubr1 involved in recognition of N-terminal bulky hydrophobic amino acids (Varshavsky, 2011). Interestingly, the Saccharomyces cerevisiae Ubr1 protein recognizes an internal degron in its target CUP9 (Du et al., 2002; Hwang et al., 2009; Xia et al., 2008). Thus, like Ubr1, ClpS may recognize internal degrons in some of its clients.
STAR Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-FLAG | Sigma-Aldrich | F7425 |
| Rabbit polyclonal anti-HA | Sigma-Aldrich | H6908 |
| Mouse monoclonal anti-GFP | Thermo Scientific | MA5-15256 |
| Rabbit polyclonal anti-CAT | Sigma-Aldrich | C9336 |
| Mouse monoclonal anti-AtpB | Abcam | ab110280 |
| Rabbit polyclonal anti-OmpA | LSbio | LS-C369146 |
| Mouse monoclonal anti-GroEL | Abcam | ab82592 |
| Rabbit polyclonal anti-PhoP | This study | N/A |
| Rabbit polyclonal anti-MgtC | This study | N/A |
| Mouse monoclonal anti-FLAG | Sigma-Aldrich | F1804 |
| Mouse monoclonal anti-HA | Sigma-Aldrich | H3663 |
| Anti-rabbit IgG HRP-linked antibody | GE healthcare | NA934 |
| Anti-mouse IgG HRP-linked antibody | GE healthcare | NA931 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Ampicillin | Sigma-Aldrich | A0166 |
| Kanamycin | Sigma-Aldrich | K1377 |
| Tetracycline | Sigma-Aldrich | T7660 |
| Chloramphenicol | Sigma-Aldrich | C0378 |
| L-α-phosphatidylcholine | Sigma-Aldrich | P5638 |
| FLAG peptide | Sigma-Aldrich | F3290 |
| HA peptide | Sigma-Aldrich | I2149 |
| Supersignal West Femto Chemiluminescent Substrate | Thermo Scientific | 34096 |
| B-PER reagent | Thermo Scientific | 78248 |
| Anti-FLAG magnetic bead | Sigma-Aldrich | M8823 |
| Anti-HA magnetic bead | Thermo Scientific | 88836 |
| Anti-GFP magnetic bead | MBL international corporation | D153-10 |
| Deposited Data | ||
| Raw image data | This study; Mendeley Data | doi:10.17632/4t4svy52fb.1 |
| Experimental Models: Organisms/strains | ||
| Salmonella enterica serovar Typhimurium 14028s | (Fields et al., 1989) | N/A |
| A detailed strain list can be found in Table S1. | this study | N/A |
| Recombinant DNA | ||
| A detailed plasmid list can be found in Table S1. | this study | N/A |
| Software and Algorithms | ||
| ImageJ | NIH | ver. 1.49u |
| GraphPad Prism | http://www.graphpad.com | version 6.05 MACOS |
Contact for Reagents and Resource Sharing
Requests for reagents will be fulfilled by the corresponding author Eduardo A. Groisman (eduardo.groisman@yale.edu).
Experimental Model and Subject Details
Bacterial Strains, Plasmids and Growth Conditions
Bacterial strains and plasmids used in this study are presented in Table S1. All S. enterica serovar Typhimurium strains are derived from strain 14028s (Fields et al., 1986) and were constructed by phage P22-mediated transductions as described (Davis et al., 1980). DNA oligonucleotides used in this study are presented in Table S2. Bacteria were grown at 37°C in Luria-Bertani broth (LB) and N-minimal media (pH 7.7) (Snavely et al., 1991) supplemented with 0.1% casamino acids, 38 mM glycerol, and the indicated concentrations of MgCl2. Escherichia coli DH5α was used as the host for preparation of plasmid DNA. Ampicillin was used at 50 μg/ml, kanamycin at 50 μg/ml, tetracycline at 12.5 μg/ml and chloramphenicol at 25 μg/ml except for the protein stability assays when it was used at 1 mg/ml.
Construction of Chromosomal Mutants and Plasmids
Chromosomal mutants were constructed with the one-step disruption method (Datsenko and Wanner, 2000) with minor modifications. To construct clpX (JY649), clpA (JY650), and clpS (JY651) mutant strains, a cat cassette was introduced in the clpX, clpA, and clpS genes as follows: a cat gene fragment was amplified from plasmid pKD3 using primer pairs 6644/6645, 15943/15944 and 15925/15926, then introduced into wild-type Salmonella 14028s and mgtC mutant (EL4) harboring plasmid pKD46. The resulting strains were kept at 30°C and transformed with pCP20 to remove the cat cassette. Strain JY619 was made by transducing the phoP::Tn10 (Fields et al., 1989) into strain JY651 using a P22 lysate grown in strains MS7953s.
To construct aat mutant strains (JY634, JY616, and JY617), a cat cassette was introduced in the aat gene as follows: a cat gene fragment was amplified from pKD3 using primers 15945/15946, then introduced into wild-type, clpA and clpS Salmonella harboring plasmid pKD46. The resulting strain was kept at 30°C and transformed with pCP20 to remove the cat cassette.
To construct strains specifying a C-terminally FLAG-tagged ClpS protein (JY674 and JY676), a cat cassette was introduced at the 3′ end of clpS: a cat gene fragment was amplified from pKD3 using primers 15969/15970 for clpS-FLAG, then introduced into wild-type Salmonella 14028s harboring plasmid pKD46. The resulting strain was kept at 30°C and transformed with pCP20 to remove the cat cassette. Strain JY676 was made by transducing the phoP-HA::cm (Shin and Groisman, 2005) into strain JY674 using a P22 lysate. The resulting strain was kept at 30°C and transformed with pCP20 to remove the cat cassette.
To construct strains specifying a C-terminally FLAG-tagged Oat protein (JY655, JY657and JY697), a cat cassette was introduced at the 3′ end of oat: a cat gene fragment was amplified from pKD3 using primers 15954/15955 for oat-FLAG, then introduced into wild-type Salmonella 14028s and clpS (JY651) harboring plasmid pKD46. The resulting strain was kept at 30°C and transformed with pCP20 to remove cat cassette. Strain JY697 was made by transducing the mgtC::km (Lee et al., 2013) into strain JY657 using a P22 lysate. The resulting strain was kept at 30°C and transformed with pCP20 to remove the km cassette.
Strain JY626 was made by transducing the mgtC-FLAG::cm insertion into strain EG13918 using a P22 lysate generated in strain EG13248. The resulting strain was transformed with pCP20 to remove the cat cassette. Strain EG16743 was made by transducing the phoP::Tn10 insertion into strain EL4 using a P22 lysate generated in strain MS7953s.
Strain EG15598 was transformed with pCP20 to remove the cat cassette from EG15599 (Shin and Groisman, 2005). Strain JY622, JY623 and JY624 were made by transducing the mgtC::km, clpA::cm and clpS::cm insertion into strain EG15598 using a P22 lysate generated in strain EL1, JY199 and JY570, respectively.
Strains JY590 and JY591 were made by transducing the clpA::cm insertion into strains JY626 and EG13918 using a P22 lysate grown in strain JY199. The phoP-6aa-gfp strain was constructed as described (Howe et al., 2010). The phoP-6 amino acids-gfp fragment was amplified by PCR using primers 14302/14303 and 14304/14305. Then, a second amplification round was performed with primers 14304/14305. The resulting fragment was introduced into wild-type Salmonella. Strain JY610 was made by transducing the mgtC-FLAG::cm insertion into strain KW64 using a P22 lysate generated in strain EG13248. The resulting strain was transformed with pCP20 to remove the cat cassette.
Plasmids expressing ClpS and ClpS-FLAG were constructed as follows: the clpS and clpS-FLAG genes were amplified using primer pairs 15947/15948 and 15947/15968, respectively. The PCR products were digested with BamHI and HindIII and then introduced between the BamHI and HindIII sites of pUHE21-2lacIq (Soncini et al., 1996). Plasmids expressing GFP derivatives were constructed as follows: the gfp derivatives’ genes were amplified using 15919-15923 as forward primer and 15924 as reverse primer. The PCR products were digested with BamHI and HindIII, and then were introduced between the BamHI and HindIII sites of pUHE21-2lacIq. Plasmids expressing CAT derivatives were constructed as follows: the cat derivatives’ genes were amplified with primer pair 15904/15907 using pKD3 as template. Amplified cat derivatives genes had an amino acid substitution at the ninth residue from threonine into leucine. Then, PCR products were introduced between the BamHI and HindIII sites of pUHE21-2lacIq (Soncini et al., 1996). Plasmids expressing PhoP derivatives were constructed as follows: the phoP, phoP-5aa, and phoP-L4A derivatives genes were amplified with primer pair 16017/16019, 16018/16019, and 16114/16115, respectively, using EG13918 strain as template. Then, PCR products were introduced between the BamHI and HindIII sites of pUHE21-2lacIq (Soncini et al., 1996).
Western Blot Assay
Cells were grown in N-minimal medium containing 15 μM MgCl2. Crude extracts were prepared in B-PER reagent (Pierce) with 100 μg/mL lysozyme and EDTA-free protease inhibitor (Roche). Samples were loaded on 4–12% NuPAGE gels (Life Technologies) and transferred to nitrocellulose membrane using the iBot machine (Life Technologies). Membranes were blocked with 3% skim milk solution at room temperature for 1 h. Then, samples were analysed using anti-HA, anti-FLAG, anti-PhoP, anti-MgtC, anti-GFP, anti-CAT, anti-GroEL or anti-AtpB antibodies. Rabbit anti-HA, anti-FLAG, anti-MgtC and anti-PhoP antibodies were used at 1:2,000 dilution. Mouse anti-HA, anti-FLAG, anti-GFP and anti-CAT antibodies were used at 1:2,000 dilution. Mouse anti-GroEL and anti-AtpB were used as control at 1:5,000 dilution. Secondary horseradish peroxidase-conjugated anti-rabbit or anti-mouse antiserum (GE healthcare) was used at 1:5,000 dilution. The blots were developed with the Amersham ECL Western Blotting Detection Reagents (GE Healthcare) or SuperSignal West Femto Chemiluminescent system (Pierce).
Edman degradation analysis for determination of PhoP N-terminal sequence
For purification of the PhoP protein, phoP clpS (JY651) Salmonella harboring plasmids pT7-7-PhoP-His6 was used to inoculate N-minimal medium at pH 7.7 with 10 mM MgCl2 media. Overnight cultures were inoculated into N-minimal medium at pH 7.7 with 10 μM MgCl2, and expression of the PhoP-His6 protein was achieved by addition of 0.1 mM IPTG at 37°C for 6 h. Cells were pelleted, resuspended in 1 × TBS buffer with 150 Yg/ml lysozyme, and subjected to sonication. Cell debris was removed by centrifugation (12,000 g, 10 min), and the supernatant was applied to 1 ml of Ni-NTA agarose (Qiagen) per the manufacturer’s protocol. Protein was recovered by elution buffer (1 ×TBS buffer and 250 mM imidazole), exchanged with 1 ×TBS buffer (Sigma), followed by TBS buffer containing 20 % glycerol, and concentrated using an Amicon Ultra-3 (MW 3,000; Millipore) filter. The purified PhoP protein was separated on 4–12% SDS-polyacrylamide gel, then transferred on PVDF membrane (Invitrogen). The PVDF membrane stained in 0.1% Coomassie brilliant blue R-250 (Invitrogen). PhoP band was excised from the PVDF membrane, then the sample was subjected to 6 cycles of automated Edman degradation using an Applied Biosystems 494 Procise Protein sequencing system. The latter analysis was carried out by the Protein Facility of the Iowa State University Office of Biotechnology.
In Vivo Protein Degradation Assay
To measure PhoP stability, cells were grown in 20 ml N-minimal medium containing 15 μM MgCl2 for 5.5 h. Cells were treated with chloramphenicol (1 mg/ml), and 700 μL samples were removed at the indicated time points and harvested at 4°C. Pelleted cells were kept on dry ice for 30 min. Then, all samples were resuspended in B-PER reagent (Pierce) with 100 μg/mL lysozyme and EDTA-free protease inhibitor (Roche). After addition of the same volume of SDS sample buffer, samples were separated on 4–12% SDS-polyacrylamide gel and analyzed by Western blotting. For quantitative analysis of the blots, we used the ImageJ (NIH, ver. 1.49u) program. PhoP half-lives (t1/2) were calculated by regression analysis of the exponential decay of PhoP. To measure stability of the PhoP-GFP proteins, cells were grown in 1 ml N-minimal medium containing 15 μM MgCl2 and 100 μM IPTG for 5.5 h, and then treated with chloramphenicol (1 mg/ml). 100 μl of the treated samples were transferred to a microplate reader (Victor 3; Bio-rad) to measure fluorescence levels. Fluorescence levels were normalized by cell amounts (OD600).
In vitro substrate degradation and protection assays
In vitro substrate degradation assays were performed as described earlier with some modifications (Dougan et al., 2002). The assay performed in a solution containing 50 mM Tris-HCl (pH 7.5), 150 mM KCl, 20 mM MgCl2 and 2mM DTT. Purified proteins were used as 0.5 μM ClpS, 0.2 μM PhoP and 0.5 μM Mdh (Roche). In vitro synthesized ClpA, ClpP and MgtC-FLAG proteins were produced using the cell-free PURExpress in vitro protein synthesis system (NEB) at 37°C for 3 h. Then, in vitro synthesized proteins were diluted 10 fold in 1×TBS (Tris-buffered saline) buffer containing 10 % glycerol. 5 μl In vitro synthesized ClpA, ClpP and MgtC-FLAG were used for each reaction. Samples were removed from the reactions at the indicated time points and stopped by the addition of sample buffer. After separation by SDS–PAGE, proteins were detected by Western blotting using anti-PhoP, anti-Mdh or anti-MgtC antibodies and Coomassie blue staining.
Purification of the MgtC, ClpS and PhoP proteins for determination of the in vivo levels
For purification of the MgtC-FLAG and ClpS-FLAG proteins, overnight cultures of wild-type Salmonella harboring plasmids pUHE-MgtC-FLAG or pUHE-ClpS-FLAG were used to inoculate N-minimal medium at pH 7.7 with 10 mM MgCl2 media. Cells were grown at 37°C to logarithmic phase (OD600 ≈ 0.3) and the expression of proteins was induced by addition of 0.5 mM of IPTG at 37°C for an additional 4 h. Cells were collected, washed once with 10 mM Tris-HCl (pH 8.0) and resuspended in a solution containing 20 mM Tris-HCl (pH 8.0), 20% sucrose, 5 mM M EDTA, 20 mM MgCl2 (final concentration) and 150 μg/ml lysozyme. After 30-min incubation at 4°C, cells were centrifuged (18,000×g, 4°C, 20 min). Cells were resuspended with 10 mM Tris-HCl (pH 8.0) and 10 mM MgCl2, subjected to sonication, and membranes were collected by centrifugation (25,000×g, 4°C, 1 h). Isolated membranes were solubilized in 1 × binding buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, and 0.1 % n-dodecyl β-D-maltoside [Sigma]) on ice for 1 h. Solubilized proteins were recovered by centrifugation (25,000×g, 4°C, 1 h) and applied to FLAG® M Purification Kit (Sigma) per the manufacturer’s protocol. Finally, the eluate was exchanged with TKM buffer, followed by TKM buffer containing 50 % glycerol, and concentrated using an Amicon Ultra-3 (MW 3,000; Millipore) filter.
For purification of the PhoP-HA protein, wild-type Salmonella harboring plasmids pUHE-PhoP-HA was used to inoculate N-minimal medium at pH 7.7 with 10 mM MgCl2 media. Overnight cultures were inoculated into fresh medium, and expression of the PhoP-HA protein was achieved by addition of 1 mM IPTG at 30°C for 5 h. Cells were pelleted, resuspended in 1 × TBS buffer with 150 μg/ml lysozyme, and subjected to sonication. Cell debris was removed by centrifugation (12,000×g, 10 min), and the supernatant was applied to anti-HA agarose Kit (Thermoscientific) per the manufacturer’s protocol. Protein was recovered by elution buffer (1 ×TBS buffer and 1mg/mL HA peptide), exchanged with 1 ×TBS buffer (Sigma), followed by TBS buffer containing 20 % glycerol, and concentrated using an Amicon Ultra-3 (MW 3,000; Millipore) filter.
Bacterial Two-Hybrid Analysis to Examine Protein-Protein Interactions
We used the BACTH system with the constructs described as follows (Battesti and Bouveret, 2012). The clpS, phoQ, mgtC, pmrB, cigR and four amino acids deleted phoP genes were PCR amplified, and the PCR fragments were cloned between the XbaI and KpnI sites of the pUT18 vector to produce the corresponding fusion proteins. Removing the XbaI and KpnI sites modified the pKT25 vector. Recombinant plasmids carrying the clpS, phoQ, mgtC, pmrB, cigR and four amino acids deleted phoP genes were co-transformed into E. coli strain BTH101. Transformants were plated on LB agar plates containing ampicillin (100 μg/ml) and kanamycin (50 μg/ml) and incubated at 30°C for 24 h. To examine the interaction between the hybrid proteins, bacteria were grown at 30°C overnight as recommended in the BACHT protocol in LB Amp Kan liquid medium supplemented with 0.5 mM IPTG. All samples were spotted onto LB agar plates supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), X-Gal (40 μg/ml), and IPTG (0.5 mM).
Pull-down Assay with Proteins Synthesized in vitro
Pull-down assay was performed with proteins produced using an in vitro transcription/translation system as previously described with some modifications (Lee et al., 2013). Proteins were produced using the cell-free PURExpress in vitro protein synthesis system (NEB) in the presence of 0.12 mg/ml proteoliposomes at 37°C for 3 h. Proteoliposomes were prepared using soybean L-α-phosphatidylcholine (Sigma) in buffer (20 mM Tricine, 20 mM succinic acid, 80 mM NaCl and 0.6 mM KOH, adjusted to pH8.0) to a concentration of 32 mg/ml as described (Kuruma et al., 2012). We prepared the DNA templates according to the manufacturer’s instructions. To synthesize mgtC-FLAG, phoP-HA, atpB-FLAG, clpS-FLAG, phoQ-FLAG, yqjA-FLAG, and five amino acids deleted phoP-HA, we used primers 14298/14299 for mgtC-FLAG, 14296/14297 for phoP-HA, 15208/15209 for atpB-FLAG, 15208/15209 for yqjA-FLAG, 14068/14069 for phoQ-FLAG, 15949/15950 for clpS-FLAG, and 14059/14060 for five amino acids deleted phoP-HA. At the end of the reaction, samples were diluted with 20 volumes in TBS (Tris-buffered saline) buffer. Diluted reactions were mixed in 500 μL TBS and incubated at room temperature for 2 h. Then, samples were pulled down with either anti-HA or anti-FLAG antibodies at room temperature for 2 h. Pulled down samples were analysed by Western blotting using anti-HA or anti-FLAG antibodies.
In vivo pull-down Assay
Interaction between the MgtC-FLAG, PhoP-HA and PhoP-6aa-GFP proteins was investigated using a strain expressing the FLAG-tagged mgtC gene, HA-tagged phoP gene and GFP-tagged phoP five amino acid gene from their normal chromosomal locations. Cells were grown overnight in N-minimal media containing 10 mM Mg2+. 1 ml of the overnight culture was washed in the N-minimal media without Mg2+ and resuspended in 1 ml of the same media. 1/50 dilution of bacteria was inoculated in 15 ml of N-minimal media containing 15 μM Mg2+ and grown for 6 h. To express the clpS gene, different concentrations of IPTG were added to strains harbouring plasmid pClpS. Crude extracts were prepared as described above and incubated with anti-HA magnetic beads (Pierce) or anti-FLAG magnetic beads (Sigma) overnight at 4°C. After washing the beads, binding proteins were eluted in 100 μL SDS sample buffer without reducing agents and separated on 4–12% SDS-polyacrylamide gel and analysed by Western blotting using anti-FLAG or anti-HA antibodies described above.
Interaction between ClpS-FLAG and PhoP-4aa-GFP or PhoP-6aa-GFP was investigated using a strain expressing a FLAG-tagged ClpS protein, (JY674) and GFP-tagged with five or three amino acids from PhoP. Bacteria were grown overnight in N-minimal media containing 10 mM Mg2+. One ml of the overnight culture was washed in N-minimal media without Mg2+ and resuspended in 1 ml of the same media. 1/50 dilution of bacteria was used to inoculate 20 ml of N-minimal media containing 15 μM Mg2+ and grown for 6 h. To express the PhoP-GFP proteins, 0.1 mM IPTG was added to strains harbouring the corresponding plasmids. The lysed samples were incubated with anti-GFP magnetic beads (MBL International) or anti-FLAG magnetic beads (Sigma) overnight at 4°C. After washing the beads, binding proteins were eluted in 100 μL SDS sample buffer and separated on 4–12% SDS-polyacrylamide gel and analyzed by Western blotting using anti-FLAG or anti-GFP antibodies described above.
In vitro and In vivo Competition Assays
MgtC-FLAG and ClpS-FLAG proteins were synthesized by the PURExpress in vitro synthesis system (Promega) per the manufacturer’s protocol. To measure the concentrations of MgtC-FLAG, ClpS-FLAG, and purified PhoP-HA, known amounts of FLAG peptide (Sigma) and HA peptide (Sigma) were run on the same gel and used as standards. Standard curves calculated from FLAG and HA peptides in the same blots used for calculation of molecular concentrations and binding affinities of the MgtC-FLAG, ClpS-FLAG, and PhoP-HA proteins. All proteins were incubated in 500 μL TBS and incubated at room temperature for 2 h. Then, samples were pulled down with either anti-HA or anti-FLAG antibodies at room temperature for 2 h. Proteins were then electrotransferred onto nitrocellulose membrane (iBlot; Life Technologies) following the manufacturer’s protocol, detected with immunoblotting using monoclonal antibodies against FLAG or HA, and the secondary antibody, horseradish peroxidase-conjugated anti-mouse IgG fragment (GE). All proteins were visualized by the Supersignal West Femto Chemiluminescent Substrate (Thermo Scientific) and LAS-4000 (FujiFilm). The densities of protein bands were determined by quantification using the ImageJ (NIH, ver. 1.49u) program. The amounts of MgtC-FLAG, ClpS-FLAG and PhoP-HA proteins were then calculated from the standard curve derived from a serial dilution of the FLAG and HA peptides standards run on the same blot (see Fig. S4A). For in vivo competition assay, bacteria were grown in N-minimal media pH 7.7 containing 15 μM MgCl2 and IPTG (0.05, 0.2, and 0.5 mM) for 6 h. Samples were analysed using anti-FLAG and anti-HA antibodies by immunoblotting.
Determination of the in vivo levels of the MgtC, ClpS and PhoP proteins
An overnight culture of Salmonella (JY676) was grown in N-minimal medium at pH 7.7 with 10 mM MgCl2, washed twice with N-minimal medium containing no Mg2+ and used to inoculate 50 mL N-minimal medium containing 15 μM MgCl2 at a 1:50 dilution. 0.5 ml of each culture was taken at the indicated time points, except for 2.5 h samples (when 2.5 ml were taken), and 100 μl B-PER solution was added. Known amounts of purified MgtC-FLAG, ClpS-FLAG, and PhoP-HA proteins were run on the same gel and used as standards. Protein purification was carried out as described in the Experimental Procedures. Purified proteins were then electrotransferred onto nitrocellulose membrane (iBlot; Life Technologies) following the manufacturer’s protocol, detected with immunoblotting using the antiserum against MgtC, FLAG or HA and the secondary antibody, horseradish peroxidase-conjugated anti-rabbit IgG fragment (GE). Levels of the MgtC, ClpS and PhoP proteins determined by Western blot analysis in phoP-HA/clpS-FLAG (JY676) Salmonella following growth under non-inducing (10 mM MgCl2, time zero) and inducing (15 μM MgCl2) conditions for 2.5, 3.5, 4.5, 5.5, 6.5, 8.5 or 24 h. All proteins were visualized by the Supersignal West Femto Chemiluminescent Substrate (Thermo Scientific) and LAS-4000 (FujiFilm). The densities of protein bands were determined by quantification using the ImageJ (NIH, ver. 1.49u) program. The amounts of MgtC, ClpS and PhoP proteins were then calculated from the standard curve derived from a serial dilution of purified MgtC-FLAG, ClpS-FLAG and PhoP-HA protein standards run on the same blot (see Figure S6).
Quantitative RT-PCR in Low Mg2+
To measure the relative mRNA levels in low Mg2+, cells were grown in N-minimal medium containing with 10 μM MgCl2 at 37°C for 5 h. Total RNA was purified by using RNeasy Kit (Qiagen) with on-column DNase treatment, and cDNA was synthesized by using VILO Super Mix (Life Technologies). Quantification of transcripts was carried out by qRT- PCR using SYBR Green PCR Master Mix (Applied Biosystems) in an QuantStudio™ 6 Flex Real-Time PCR System (Applied Biosystems). The relative amount of mRNA was determined by using a standard curve obtained from PCR with serially diluted genomic DNA, and results were normalized to the levels of ompA. The mRNA levels of the PhoP-activated genes were measured by using the indicated primer sets (Table S3). Data shown are an average from at least three independent experiments.
Measurement of ATP Levels
ATP levels were determined as previously described (Pontes et al., 2015) with with a few modifications. ATP levels were determined as previously described (Pontes et al., 2015) with a few modifications. Intracellular ATP levels were measured using a microplate reader (Tecan, Infinite M1000 PRO). Bacteria were grown overnight in N-minimal media containing 10 mM Mg2+. One ml of the overnight culture was washed three times in the N-minimal medium without Mg2+ and resuspended in 1 ml of the same media. Diluted (1/50) bacteria were inoculated into 1 ml of N-minimal media containing 10 μM Mg2+ and grown for 6 h. Cells were normalized by OD600 and heated at 70°C for 10 min for inactivation. Intracellular ATP was measured using the BacTiter-Glo Microbial Cell Viability Assay Kit (Promega) according to the manufacturer’s instructions.
Data and Software Availability
The unprocessed image files used to prepare the figures in this manuscript have been deposited to Mendeley Data and are available at doi:10.17632/4t4svy52fb.1.
Supplementary Material
Acknowledgments
We thank the reviewers for their valuable comments. This research was supported by NIH grant AI49561 to EAG.
Footnotes
Author Contributions
Conceptualization, J.Y. and E.A.G.; Designed research, J.Y. and E.A.G.; Performed research, J.Y., K.J.W. and E.A.G.; Analysed data, J.Y., K.J.W. and E.A.G.; Wrote the paper, J.Y. and E.A.G.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alix E, Blanc-Potard AB. Peptide-assisted degradation of the Salmonella MgtC virulence factor. EMBO J. 2008;27:546–557. doi: 10.1038/sj.emboj.7601983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpuche Aranda CM, Swanson JA, Loomis WP, Miller SI. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Battesti AL, Bouveret E. The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli. Methods. 2012;58:325–334. doi: 10.1016/j.ymeth.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Battesti A, Gottesman S. Roles of adaptor proteins in regulation of bacterial proteolysis. Current Opinion in Microbiology. 2013;16:140–147. doi: 10.1016/j.mib.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellier A, Mazodier P. ClgR, a novel regulator of clp and lon expression in Streptomyces. J Bacteriol. 2004;186:3238–3248. doi: 10.1128/JB.186.10.3238-3248.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc-Potard AB, Groisman EA. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 1997;16:5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet M, Stegmann M, Maglica Ž, Stiegeler E, Weber-Ban E, Hennecke H, Mesa S. FixK2, a key regulator in Bradyrhizobium japonicum, is a substrate for the protease ClpAP in vitro. FEBS Lett. 2013;587:88–93. doi: 10.1016/j.febslet.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Buczek MS, Cardenas Arevalo AL, Janakiraman A. ClpXP and ClpAP control the E. coli division protein ZapC by proteolysis. Microbiology. 2016;162:909–920. doi: 10.1099/mic.0.000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamnongpol S, Groisman EA. Acetyl phosphate-dependent activation of a mutant PhoP response regulator that functions independently of its cognate sensor kinase. J Mol Biol. 2000;300:291–305. doi: 10.1006/jmbi.2000.3848. [DOI] [PubMed] [Google Scholar]
- Choi J, Groisman EA. Acidic pH sensing in the bacterial cytoplasm is required for Salmonella virulence. Mol Microbiol. 2016;101:1024–1038. doi: 10.1111/mmi.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Jeong BC, Joo YJ, Lee MR, Kim J, Eck MJ, Song HK. Structural basis for the recognition of N-end rule substrates by the UBR box of ubiquitin ligases. Nat Struct Mol Biol. 2010;17:1175–1181. doi: 10.1038/nsmb.1907. [DOI] [PubMed] [Google Scholar]
- Colgan AM, Kröger C, Diard M, Hardt WD, Puente JL, Sivasankaran SK, Hokamp K, Hinton JCD. The Impact of 18 Ancestral and Horizontally-Acquired Regulatory Proteins upon the Transcriptome and sRNA Landscape of Salmonella enterica serovar Typhimurium. PLoS Genet. 2016;12:e1006258. doi: 10.1371/journal.pgen.1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA Sensor for Intracellular Mg2+ Cell. 2006;125:71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RW, 1941, Botstein D, Roth JR., 1939 . Advanced bacterial genetics. Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- Dougan DA, Truscott KN, Zeth K. The bacterial N-end rule pathway: expect the unexpected. Mol Microbiol. 2010;76:545–558. doi: 10.1111/j.1365-2958.2010.07120.x. [DOI] [PubMed] [Google Scholar]
- Dougan DA, Reid BG, Horwich AL, Bukau B. ClpS, a substrate modulator of the ClpAP machine. Mol Cell. 2002;9:673–683. doi: 10.1016/s1097-2765(02)00485-9. [DOI] [PubMed] [Google Scholar]
- Du F, Navarro-Garcia F, Xia Z, Tasaki T, Varshavsky A. Pairs of dipeptides synergistically activate the binding of substrate by ubiquitin ligase through dissociation of its autoinhibitory domain. Proc Natl Acad Sci USA. 2002;99:14110–14115. doi: 10.1073/pnas.172527399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbse A, Schmidt R, Bornemann T, Schneider-Mergener J, Mogk A, Zahn R, Dougan DA, Bukau B. ClpS is an essential component of the N-end rule pathway in Escherichia coli. Nature. 2006;439:753–756. doi: 10.1038/nature04412. [DOI] [PubMed] [Google Scholar]
- Ernst RK, Guina T, Miller SI. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes and Infection. 2001;3:1327–1334. doi: 10.1016/s1286-4579(01)01494-0. [DOI] [PubMed] [Google Scholar]
- Farrell CM, Grossman AD, Sauer RT. Cytoplasmic degradation of ssrA-tagged proteins. Mol Microbiol. 2005;57:1750–1761. doi: 10.1111/j.1365-2958.2005.04798.x. [DOI] [PubMed] [Google Scholar]
- Fields PI, Groisman EA, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, Neher SB, Kim YI, Sauer RT, Baker TA. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol cell. 2003;11:671–683. doi: 10.1016/s1097-2765(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Garcia Vescovi E, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- Grabenstein JP, Marceau M, Pujol C, Simonet M, Bliska JB. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infection and Immunity. 2004;72:4973–4984. doi: 10.1128/IAI.72.9.4973-4984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA, Chiao E, Lipps CJ. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci USA. 1989;86:7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA. Feedback Control of Two-Component Regulatory Systems. Annu Rev Microbiol. 2016;70:103–124. doi: 10.1146/annurev-micro-102215-095331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyet A, Gominet M, Benaroudj N, Mazodier P. Regulation of the clpP1clpP2 operon by the pleiotropic regulator AdpA in Streptomyces lividans. Arch Microbiol. 2013;195:831–841. doi: 10.1007/s00203-013-0918-2. [DOI] [PubMed] [Google Scholar]
- Howe K, Karsi A, Germon P, Wills RW, Lawrence ML, Bailey RH. Development of stable reporter system cloning luxCDABE genes into chromosome of Salmonella enterica serotypes using Tn7 transposon. BMC Microbiol. 2010;10:197. doi: 10.1186/1471-2180-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbard MA, Surkov S, De Donatis GM, Jenkins LM, Maurizi MR. The N-degradome of Escherichia coli: limited proteolysis in vivo generates a large pool of proteins bearing N-degrons. J Biol Chem. 2013;288:28913–28924. doi: 10.1074/jbc.M113.492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CS, Shemorry A, Varshavsky A. Two proteolytic pathways regulate DNA repair by cotargeting the Mgt1 alkylguanine transferase. Proc Natl Acad Sci USA. 2009;106:2142–2147. doi: 10.1073/pnas.0812316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi KK, Bergé M, Radhakrishnan SK, Viollier PH, Chien P. An Adaptor Hierarchy Regulates Proteolysis during a Bacterial Cell Cycle. Cell. 2015;163:419–431. doi: 10.1016/j.cell.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein J, Molière N, Dougan DA, Turgay K. Adapting the machine: adaptor proteins for Hsp100/Clp and AAA+ proteases. Nature Review Micobiol. 2009;7:589–599. doi: 10.1038/nrmicro2185. [DOI] [PubMed] [Google Scholar]
- Koebmann BJ, Westerhoff HV, Snoep JL, Nilsson D, Jensen PR. The glycolytic flux in Escherichia coli is controlled by the demand for ATP. J Bacteriol. 2002;184:3909–3916. doi: 10.1128/JB.184.14.3909-3916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruma Y, Suzuki T, Ono S, Yoshida M, Ueda T. Functional analysis of membranous Fo-a subunit of F1Fo-ATP synthase by in vitro protein synthesis. Biochem J. 2012;442:631–638. doi: 10.1042/BJ20111284. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Groisman EA. Control of a Salmonella virulence locus by an ATP-sensing leader messenger RNA. Nature. 2012a;486:271–275. doi: 10.1038/nature11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Groisman EA. Tandem attenuators control expression of the Salmonella mgtCBRvirulence operon. Mol Microbiol. 2012b;86:212–224. doi: 10.1111/j.1365-2958.2012.08188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Choi J, Groisman EA. Control of a Salmonella virulence operon by proline-charged tRNA(Pro) Proc Natl Acad Sci USA. 2014;111:3140–3145. doi: 10.1073/pnas.1316209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Pontes MH, Groisman EA. A Bacterial Virulence Protein Promotes Pathogenicity by Inhibiting the Bacterium’s Own F1Fo ATP Synthase. Cell. 2013;154:146–156. doi: 10.1016/j.cell.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejona S, Aguirre A, Cabeza ML, García Véscovi E, Soncini FC. Molecular characterization of the Mg2+-responsive PhoP-PhoQ regulon in Salmonella enterica. J Bacteriol. 2003;185:6287–6294. doi: 10.1128/JB.185.21.6287-6294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SI, Kukral AM, Mekalanos JJ. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Schmidt R, Bukau B. The N-end rule pathway for regulated proteolysis: prokaryotic and eukaryotic strategies. Trends Cell Biol. 2007;17:165–172. doi: 10.1016/j.tcb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Ninnis RL, Spall SK, Talbo GH, Truscott KN, Dougan DA. Modification of PATase by L/F-transferase generates a ClpS-dependent N-end rule substrate in Escherichia coli. EMBO J. 2009;28:1732–1744. doi: 10.1038/emboj.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega J, Lee HS, Maurizi MR, Steven AC. ClpA and ClpX ATPases bind simultaneously to opposite ends of ClpP peptidase to form active hybrid complexes. J Struct Biol. 2004;146:217–226. doi: 10.1016/j.jsb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Park SY, Groisman EA. Signal-specific temporal response by the Salmonella PhoP/PhoQ regulatory system. Mol Microbiol. 2013;91:135–144. doi: 10.1111/mmi.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CN, Levchenko I, Rabinowitz JD, Baker TA, Silhavy TJ. RpoS proteolysis is controlled directly by ATP levels in Escherichia coli. Genes & Development. 2012;26:548–553. doi: 10.1101/gad.183517.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes MH, Lee EJ, Choi J, Groisman EA. Salmonella promotes virulence by repressing cellulose production. Proc Natl Acad Sci USA. 2015;112:5183–5188. doi: 10.1073/pnas.1500989112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, Miller SI. Activation of the Bacterial Sensor Kinase PhoQ by Acidic pH. Molecular Cell. 2007;26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Sauer RT, Baker TA. AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem. 2011;80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- Schuenemann VJ, Kralik SM, Albrecht R, Spall SK, Truscott KN, Dougan DA, Zeth K. Structural basis of N-end rule substrate recognition in Escherichia coli by the ClpAP adaptor protein ClpS. EMBO Rep. 2009;10:508–514. doi: 10.1038/embor.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior AE. The proton-translocating ATPase of Escherichia coli. Annu Rev Biophys Biophys Chem. 1990;19:7–41. doi: 10.1146/annurev.bb.19.060190.000255. [DOI] [PubMed] [Google Scholar]
- Shin D, Groisman EA. Signal-dependent binding of the response regulators PhoP and PmrA to their target promoters in vivo. Journal of Biological Chemistry. 2005;280:4089–4094. doi: 10.1074/jbc.M412741200. [DOI] [PubMed] [Google Scholar]
- Shin D, Lee EJ, Huang H, Groisman EA. A positive feedback loop promotes transcription surge that jump-starts Salmonella virulence circuit. Science. 2006;314:1607–1609. doi: 10.1126/science.1134930. [DOI] [PubMed] [Google Scholar]
- Snavely MD, Miller CG, Maguire ME. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. Journal of Biological Chemistry. 1991;266:815–823. [PubMed] [Google Scholar]
- Soncini FC, Garcia Vescovi E, Solomon F, Groisman EA. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J Bacteriol. 1996;178:5092–5099. doi: 10.1128/jb.178.17.5092-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soncini FC, Vescovi EG, Groisman EA. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J Bacteriol. 1995;177:4364–4371. doi: 10.1128/jb.177.15.4364-4371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Sundqvist G, Malm E, de Bruijn I, Kumar A, van de Mortel J, Bulone V, Raaijmakers JM. Lipopeptide biosynthesis in Pseudomonas fluorescens is regulated by the protease complex ClpAP. BMC Microbiol. 2015;15:29. doi: 10.1186/s12866-015-0367-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephani K, Weichart D, Hengge R. Dynamic control of Dps protein levels by ClpXP and ClpAP proteases in Escherichia coli. Mol Microbiol. 2003;49:1605–1614. doi: 10.1046/j.1365-2958.2003.03644.x. [DOI] [PubMed] [Google Scholar]
- Thompson MW, Maurizi MR. Activity and specificity of Escherichia coli ClpAP protease in cleaving model peptide substrates. Journal of Biological Chemistry. 1994;269:18201–18208. [PubMed] [Google Scholar]
- Varshavsky A. The N-end rule pathway and regulation by proteolysis. Protein Science. 2011;20:1298–1345. doi: 10.1002/pro.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Ban EU, Reid BG, Miranker AD, Horwich AL. Global unfolding of a substrate protein by the Hsp100 chaperone ClpA. Nature. 1999;401:90–93. doi: 10.1038/43481. [DOI] [PubMed] [Google Scholar]
- Williams B, Bhat N, Chien P, Shapiro L. ClpXP and ClpAP proteolytic activity on divisome substrates is differentially regulated following the Caulobacter asymmetric cell division. Mol Microbiol. 2014;93:853–866. doi: 10.1111/mmi.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Webster A, Du F, Piatkov K, Ghislain M, Varshavsky A. Substrate-binding sites of UBR1, the ubiquitin ligase of the N-end rule pathway. Journal of Biological Chemistry. 2008;283:24011–24028. doi: 10.1074/jbc.M802583200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Yu X, Zhu M, Kang H, Ma J, Wu M, Gan J, Deng X, Liang H. Structural and Molecular Mechanism of CdpR Involved in Quorum-Sensing and Bacterial Virulence in Pseudomonas aeruginosa. PLoS Biol. 2016;14:e1002449. doi: 10.1371/journal.pbio.1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwir I, Latifi T, Perez JC, Huang H, Groisman EA. The promoter architectural landscape of the Salmonella PhoP regulon. Mol Microbiol. 2012;84:463–485. doi: 10.1111/j.1365-2958.2012.08036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.