Abstract
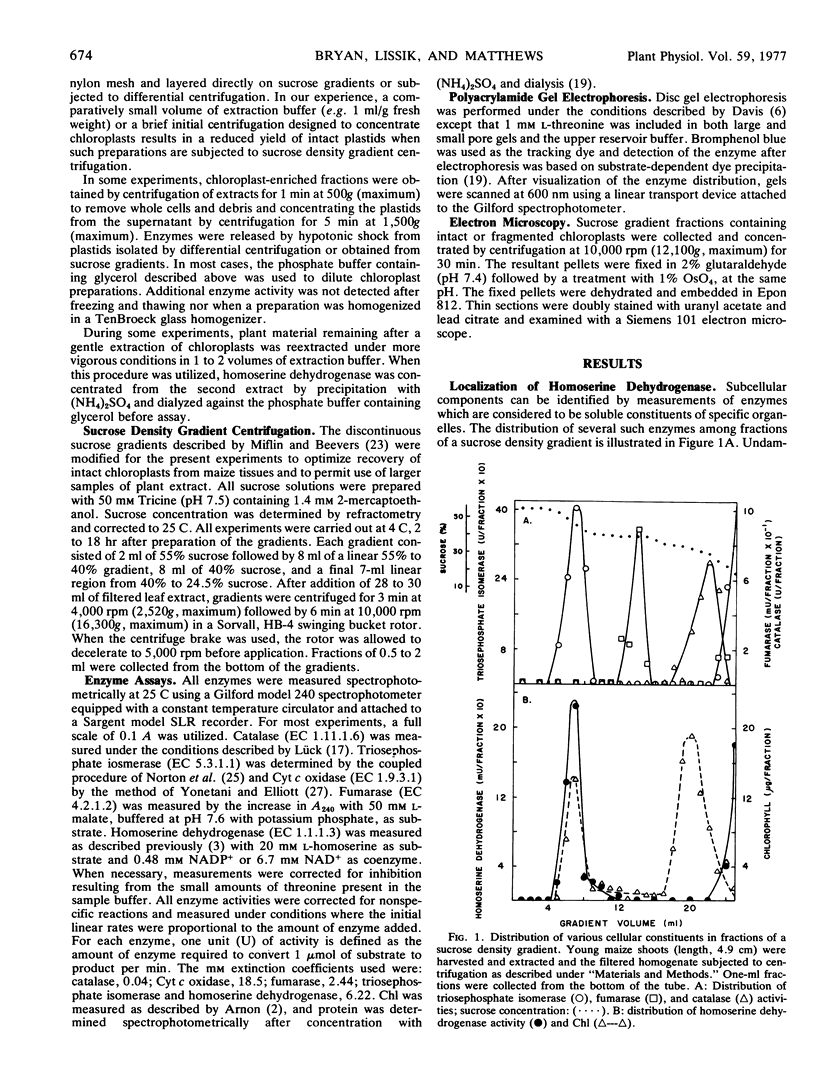
Extracts of leaf tissue of Zea mays L. seedlings were fractionated on nonlinear sucrose gradients to separate subcellular organelles. Homoserine dehydrogenase (EC 1.1.1.3) was identified in those fractions containing intact chloroplasts, as judged by the presence of chlorophyll and triosephosphate isomerase activity. Neither enzyme activity was detected in fractions containing ruptured chloroplasts, mitochondria, or microbodies. Quantitative measurements of enzyme activity and chlorophyll, and electron microscopic analysis of plastid preparations support the conclusion that maize mesophyll chloroplasts contain a significant fraction of the total cellular content of homoserine dehydrogenase.
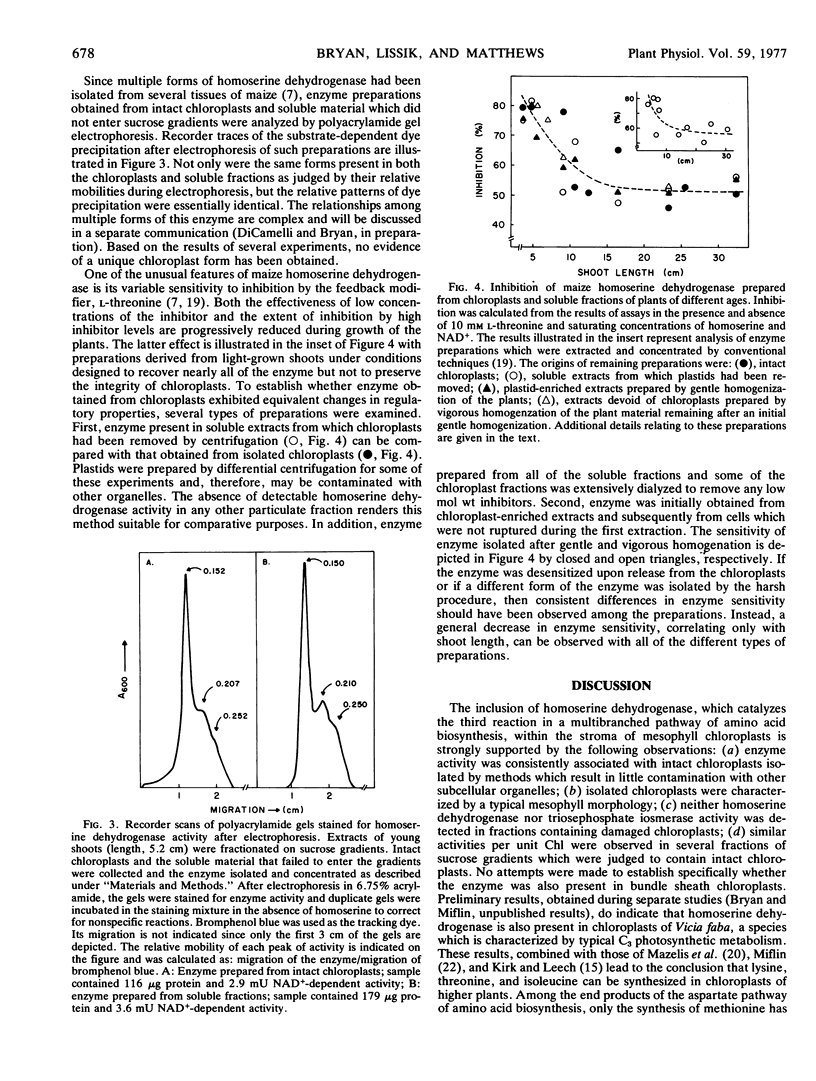
A survey of representative kinetic, regulatory, and physical properties did not reveal any significant differences between enzyme released from isolated, undamaged chloroplasts and that obtained from soluble cellular fractions.
Examination of enzyme prepared from chloroplasts of different age seedlings indicated that the sensitivity of homoserine dehydrogenase to inhibition by the feedback modifier l-threonine was progressively diminished during growth of the plants. This systematic change in regulatory properties of the enzyme occurred to the same extent for the enzymes obtained from chloroplasts and soluble fractions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J. K. Studies on the catalytic and regulatory properties of homoserine dehydrogenase of Zea mays roots. Biochim Biophys Acta. 1969 Feb 11;171(2):205–216. doi: 10.1016/0005-2744(69)90154-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dicamelli C. A., Bryan J. K. Changes in Enzyme Regulation during Growth of Maize: II. Relationships among Multiple Molecular Forms of Homoserine Dehydrogenase. Plant Physiol. 1975 Jun;55(6):999–1005. doi: 10.1104/pp.55.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm H. E., Margulies M. M. In vitro protein synthesis by plastids of Phaseolus vulgaris. IV. Amino acid incorporation by etioplasts and effect of illumination of leaves on incorporation by plastids. Plant Physiol. 1970 Apr;45(4):435–442. doi: 10.1104/pp.45.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk P. R., Leech R. M. Amino Acid Biosynthesis by Isolated Chloroplasts during Photosynthesis. Plant Physiol. 1972 Aug;50(2):228–234. doi: 10.1104/pp.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes A. C., Neyra C. A., Hageman R. H. Nitrite assimilation and amino nitrogen synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Mar;53(3):411–415. doi: 10.1104/pp.53.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. F., Gurman A. W., Bryan J. K. Changes in Enzyme Regulation during Growth of Maize: I. Progressive Desensitization of Homoserine Dehydrogenase during Seedling Growth. Plant Physiol. 1975 Jun;55(6):991–998. doi: 10.1104/pp.55.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelis M., Miflin B. J., Pratt H. M. A chloroplast-localized diaminopimelate decarboxylase in higher plants. FEBS Lett. 1976 Apr 15;64(1):197–200. doi: 10.1016/0014-5793(76)80282-7. [DOI] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J. The location of nitrite reductase and other enzymes related to amino Acid biosynthesis in the plastids of root and leaves. Plant Physiol. 1974 Oct;54(4):550–555. doi: 10.1104/pp.54.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton I. L., Pfuderer P., Stringer C. D., Hartman F. C. Isolation and characterization of rabbit muscle triose phosphate isomerase. Biochemistry. 1970 Dec 8;9(25):4952–4958. doi: 10.1021/bi00827a019. [DOI] [PubMed] [Google Scholar]
- O'neal D., Hew C. S., Latzko E., Gibbs M. Photosynthetic carbon metabolism of isolated corn chloroplasts. Plant Physiol. 1972 Apr;49(4):607–614. doi: 10.1104/pp.49.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]



