Abstract
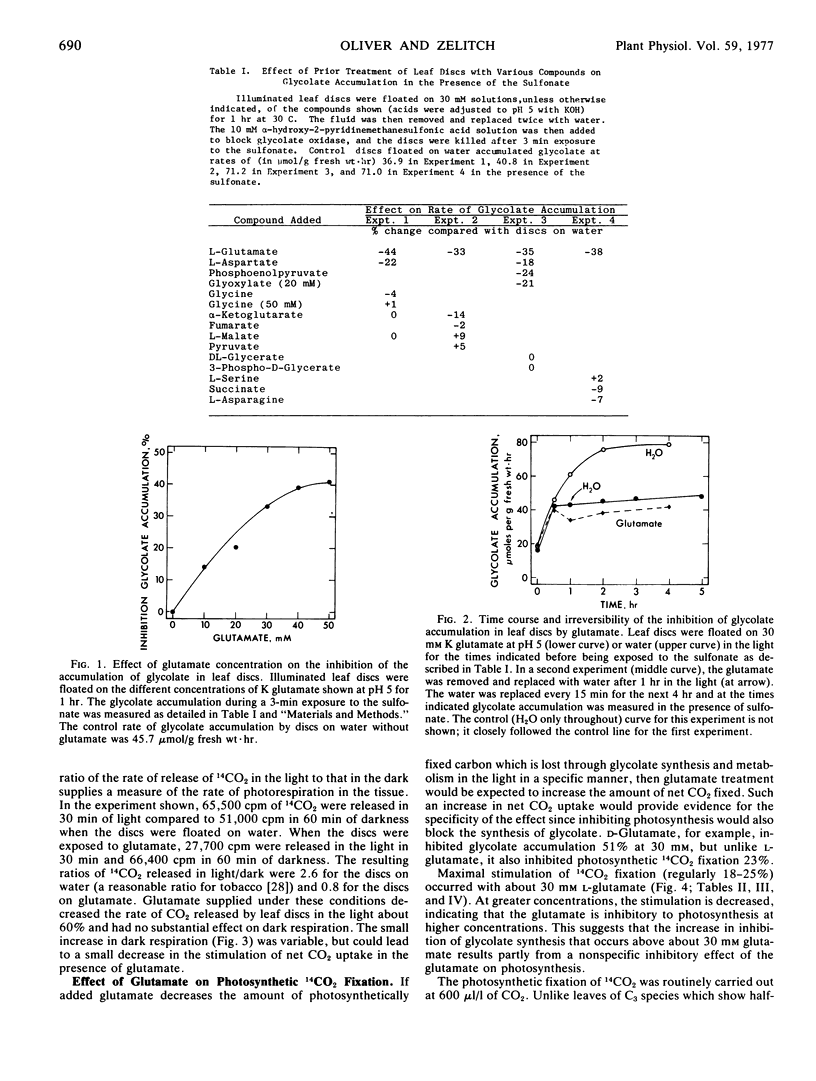
Experiments were undertaken to identify and characterize control mechanisms in tobacco leaf tissue which decrease the relative contribution of photorespiratory CO2 release and thereby increase net photosynthetic CO2 fixation. A number of metabolites were supplied to illuminated leaf discs and their effect on the inhibition of glycolate synthesis was measured. Glycolate accumulation, in the presence of α-hydroxy-2-pyridinemethanesulfonic acid, was inhibited in leaf discs previously floated on 30 mM solutions of either L-glutamate, L-aspartate, phospho-enolpyruvate, or glyoxylate. The effect of glutamate on glycolate synthesis, which was investigated in detail, was concentration- and time-dependent. Glycolate synthesis was inhibited about 40% by treating leaf discs with 30 mM glutamate, and the inhibition continued for more than 4 hours after the glutamate solution was removed.
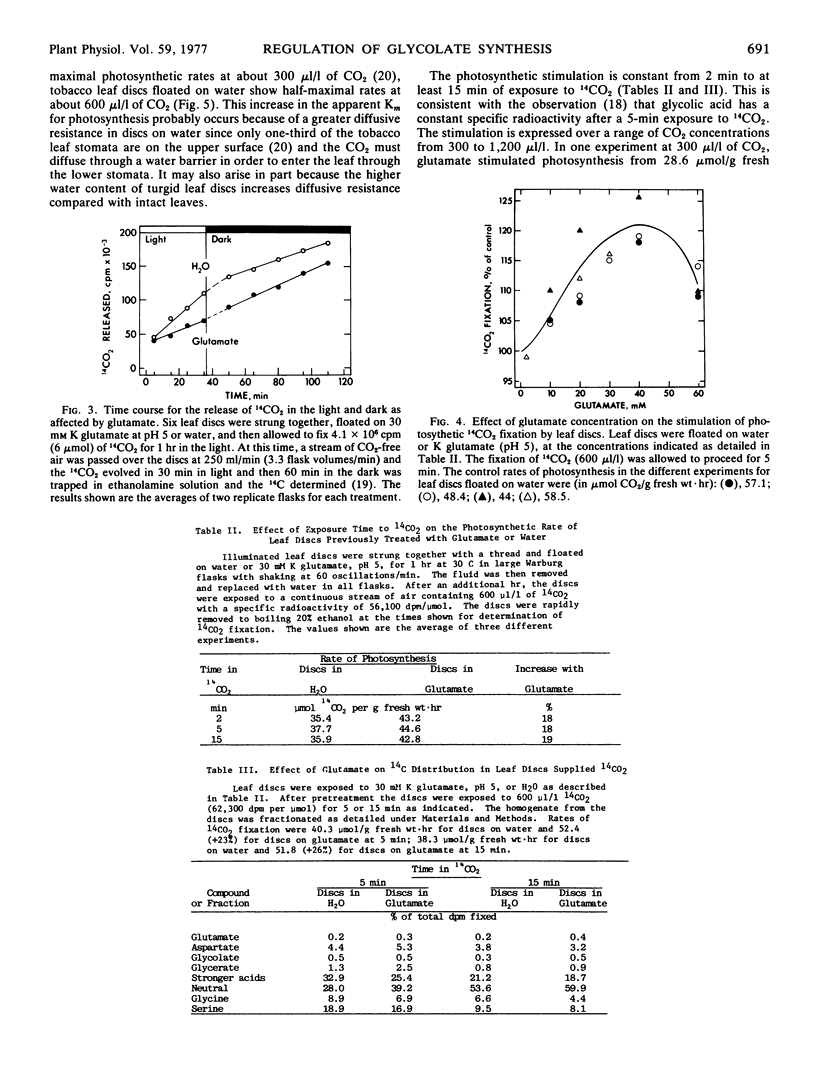
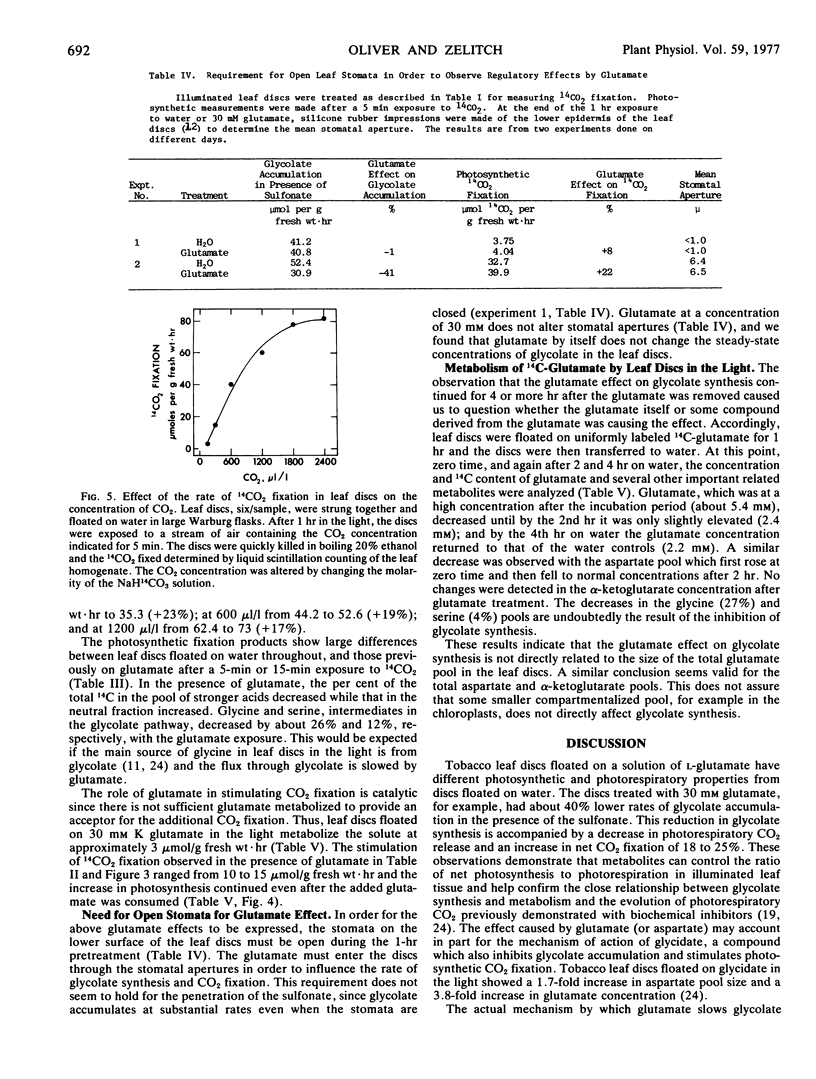
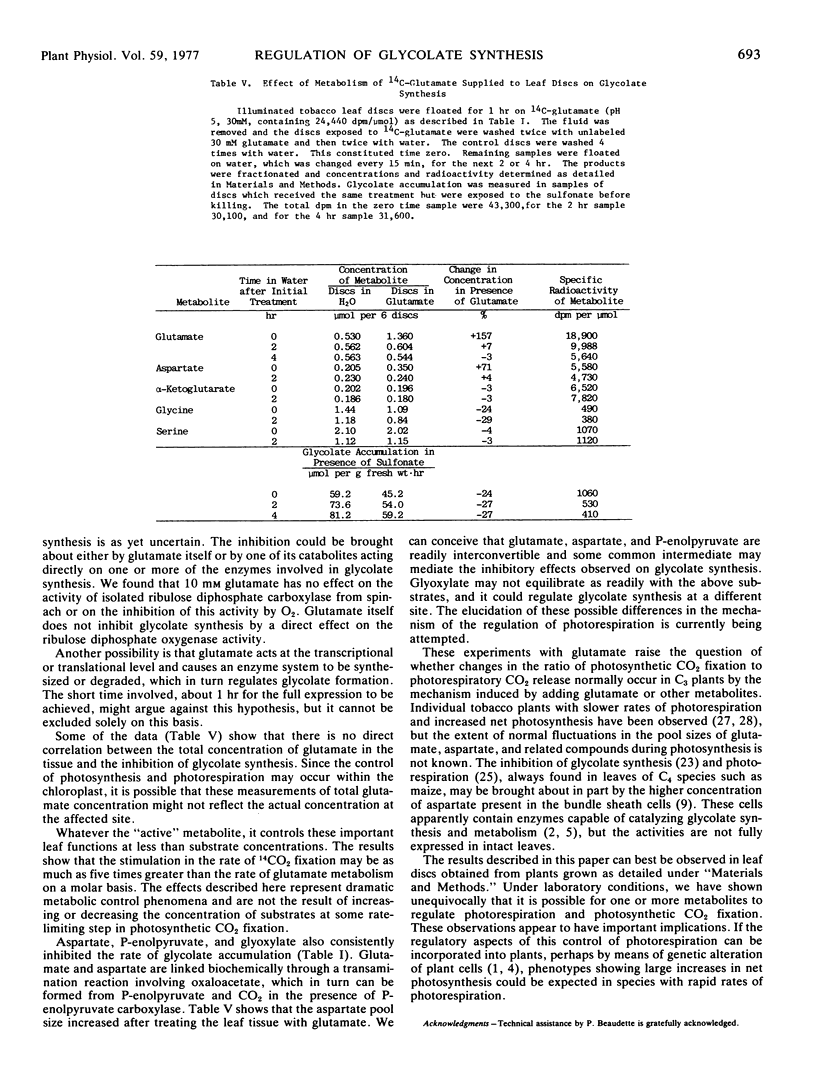
The glutamate inhibition of glycolate synthesis was accompanied by a marked decrease in the rate of photorespiratory CO2 release and by maximal increases of about 25% in net photosynthetic CO2 fixation. The products of 14CO2 fixation in leaf discs previously treated with glutamate showed a decrease in glycine (26%), serine (12%), and the stronger acids (18%), and an increase in the neutral compounds (26%) in comparison with discs floated only on water.
Data are presented which question whether a catabolite of glutamate or the amino acid itself is responsible for the results observed. These experiments support the view that a genetic selection strategy based on the metabolic control of photorespiration would result in large increases in net photosynthetic CO2 assimilation in species with high rates of photorespiration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlyn M. B., Zelitch I. Photoautotrophic growth and photosynthesis in tobacco callus cells. Plant Physiol. 1975 Dec;56(6):752–756. doi: 10.1104/pp.56.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Carlson P. S., Polacco J. C. Plant cell cultures: genetic aspects of crop improvement. Science. 1975 May 9;188(4188):622–625. doi: 10.1126/science.188.4188.622. [DOI] [PubMed] [Google Scholar]
- Chollet R. 14CO2 fixation and glycolate metabolism in the dark in isolated maize (Zea mays L.) bundle sheath strands. Arch Biochem Biophys. 1974 Aug;163(2):521–529. doi: 10.1016/0003-9861(74)90510-4. [DOI] [PubMed] [Google Scholar]
- Hatch M. D. The C 4 -pathway of photosynthesis. Evidence for an intermediate pool of carbon dioxide and the identity of the donor C 4 -dicarboxylic acid. Biochem J. 1971 Nov;125(2):425–432. doi: 10.1042/bj1250425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain Y., Gibbs M. Formation of glycolate by a reconstituted spinach chloroplast preparation. Plant Physiol. 1971 Sep;48(3):325–330. doi: 10.1104/pp.48.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N. C., Parlange J. Y. Analysis of operation and calibration of a ventilated diffusion porometer. Plant Physiol. 1970 Jul;46(1):175–177. doi: 10.1104/pp.46.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery H. B., Leavenworth C. S., Bliss C. I. THE PROBLEM OF SELECTING UNIFORM SAMPLES OF LEAVES. Plant Physiol. 1949 Jul;24(3):335–344. doi: 10.1104/pp.24.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I., Day P. R. The effect on net photosynthesis of pedigree selection for low and high rates of photorespiration in tobacco. Plant Physiol. 1973 Jul;52(1):33–37. doi: 10.1104/pp.52.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I., Day P. R. Variation in photorespiration. The effect of genetic differences in photorespiration on net photosynthesis in tobacco. Plant Physiol. 1968 Nov;43(11):1838–1844. doi: 10.1104/pp.43.11.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Investigation on photorespiration with a sensitive C-assay. Plant Physiol. 1968 Nov;43(11):1829–1837. doi: 10.1104/pp.43.11.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Pathways of carbon fixation in green plants. Annu Rev Biochem. 1975;44:123–145. doi: 10.1146/annurev.bi.44.070175.001011. [DOI] [PubMed] [Google Scholar]


