Abstract
Polycystic ovary syndrome (PCOS) is associated with increased risk of venous thromboembolism (VTE) and cardiovascular disease (CVD) in later life. We aimed to study the effect of liraglutide intervention on markers of VTE and CVD risk, in PCOS. In a double-blind, placebo-controlled, randomized trial, 72 overweight and/or insulin-resistant women with PCOS were randomized, in a 2:1 ratio, to liraglutide or placebo 1.8 mg/day. Endpoints included between-group difference in change (baseline to follow-up) in plasminogen activator inhibitor-1 levels and in thrombin generation test parameters: endogenous thrombin potential, peak thrombin concentration, lag time and time to peak. Mean weight loss was 5.2 kg (95% CI 3.0–7.5 kg, P < 0.001) in the liraglutide group compared with placebo. We detected no effect on endogenous thrombin potential in either group. In the liraglutide group, peak thrombin concentration decreased by 16.71 nmol/L (95% CI 2.32–31.11, P < 0.05) and lag time and time to peak increased by 0.13 min (95% CI 0.01–0.25, P < 0.05) and 0.38 min (95% CI 0.09–0.68, P < 0.05), respectively, but there were no between-group differences. There was a trend toward 12% (95% CI 0–23, P = 0.05) decreased plasminogen activator inhibitor-1 in the liraglutide group, and there was a trend toward 16% (95% CI −4 to 32, P = 0.10) reduction, compared with placebo. In overweight women with PCOS, liraglutide intervention caused an approximate 5% weight loss. In addition, liraglutide affected thrombin generation, although not significantly differently from placebo. A concomitant trend toward improved fibrinolysis indicates a possible reduction of the baseline thrombogenic potential. The findings point toward beneficial effects of liraglutide on markers of VTE and CVD risk, which should be further pursued in larger studies.
Keywords: polycystic ovary syndrome, liraglutide, GLP-1 analog, thrombin generation, plasminogen activator inhibitor-1, low-grade inflammation
Introduction
With a prevalence of 10%, polycystic ovary syndrome (PCOS) is the most common endocrine disorder among women of fertile age (1). The diagnosis is based on the three Rotterdam criteria: oligo-/amenorrhea, androgen excess and polycystic ovaries, where a minimum of two should be fulfilled and other etiologies excluded (2). The Rotterdam criteria encompass an ovarian dysfunction, but it is evident that PCOS also has a metabolic component, covering abdominal obesity, hypertension and dyslipidemia, as well as insulin resistance (3). The abdominal obesity is characterized by a state of low-grade inflammation. Visceral adipose tissue secretes pro-inflammatory adipokines promoting an inflammatory state, contributing to endothelial dysfunction, which is associated with an increased risk of cardiovascular disease (CVD) (4).
PCOS is associated with both CVD and venous thromboembolism (VTE). A recent meta-analysis found an odds ratio of 1.44 (1.13–1.84) for coronary heart disease in women with PCOS vs non-PCOS (5) and a cohort study, which included 87,000 participants, found 1.5-fold higher risk for VTE in women with PCOS than in controls (6). In general, cardiovascular events are rare in premenopausal women, which is why markers of low-grade inflammation and endothelial dysfunction are often used as measures of CVD risk in interventional studies in PCOS. Due to their association with atherosclerotic CVD, levels of von Willebrand factor (vWF), plasminogen activator inhibitor 1 (PAI-1) and high-sensitivity-CRP (hsCRP) are frequently used as surrogate CVD risk markers (7, 8, 9) and they are all found to be elevated in PCOS (10, 11).
Elevated thrombin generation, as measured by thrombin generation test, indicates hypercoagulability, as seen in users of combined oral contraceptives (12). Moreover, high thrombin generation is associated with first and recurrent VTE and possibly with coronary atherosclerosis (13, 14, 15). Thrombin generation has been found to be elevated in PCOS (12), but conflicting data exist (16). In a previous study, our group found high thrombin generation, PAI-1 and hsCRP to be linked to the overweight and insulin-resistant PCOS phenotype (17, 18). Thrombin generation seems to mainly be driven by overweight (19, 20) and is found to diminish with weight loss (21, 22, 23). The increased thrombin generation, together with hypofibrinolysis, as measured by elevated PAI-1 levels, could be a link to the increased risk of VTE in PCOS.
Lifestyle intervention and metformin are mainstays in the treatment of PCOS, aiming at reducing body weight and insulin resistance. In women with PCOS, metformin has been found to reduce PAI-1 activity and levels of hsCRP to a mild degree (24) and might to some extent diminish the increased thrombin generation induced by oral contraceptives, as recently investigated (12). Glucagonlike peptide-1 (GLP-1) analogs are commonly used in type 2 diabetes and obesity, where they promote weight loss, improve glycemic control and have been demonstrated to reduce plasma levels of cholesterol, PAI-1 and hsCRP (25, 26, 27). Glucagonlike peptide-1 analogs have been used in PCOS in smaller trials, all demonstrating weight loss, but none investigating the effect on thrombin generation or PAI-1 (28, 29, 30, 31, 32).
We hypothesized that intervention with the GLP-1 analog liraglutide, in overweight women with PCOS, would lead to a beneficial reduction in VTE and CVD risk markers: thrombin generation, vWF, PAI-1 and hsCRP, possibly due to a weight loss. To investigate this, we performed a randomized clinical trial (RCT) treating women with PCOS with either liraglutide or placebo for 26 weeks.
Subjects and methods
A randomized, placebo-controlled, double-blind clinical trial was conducted from March 2014 to December 2015 at Herlev Gentofte Hospital, University of Copenhagen, Denmark. Seventy-two women were randomized, in a 2:1-ratio, to 26 weeks of intervention with liraglutide or placebo 1.8 mg once daily. The study is registered at www.clinicaltrials.gov: Nbib2073929.
The study was approved by the Danish Data Protection Agency and the Ethics Committee of the Capital Region of Denmark (ID: H2-2013-142, EudraCT: 2013-003862-15) and performed in accordance with General Clinical Practice guidelines and the Declaration of Helsinki. After full explanation of the purpose and nature of all procedures used the participants gave oral and written informed consent, prior to screening.
Participants
Participants were enrolled from social media (www.facebook.com/PCOSkliniskforsoeg), from private practicing gynecologists and from our outpatient PCOS clinic, securing external validity. Inclusion and exclusion criteria are published elsewhere (33). In short, eligible women were ≥18 years, premenopausal and had PCOS according to Rotterdam criteria, i.e., minimum two of the three (1) oligo-/amenorrhea (cycle >35 days), (2) clinical (Ferriman–Gallwey score ≥8) or biochemical hyperandrogenism (total or free testosterone levels above reference: >1.8 nmol/L and >0.034 nmol/L, respectively) and (3) polycystic ovaries (≥12 follicles (2–9 mm) and/or volume >10 mL in at least one ovary) on transvaginal ultrasound. Other causes to bleeding irregularities and androgen excess were excluded. The women should have BMI ≥25 kg/m2 and/or insulin resistance defined as fasting plasma C-peptide >0.6 nmol/L at screening. In brief, exclusion criteria were pregnancy, breastfeeding, smoking >10 cigarettes/day, diabetes, hypertension, overt inflammatory disease, use of herbal medicine or medications known to affect the hemostatic system. The use of hormonal contraceptives within six weeks, injectable hormonal contraceptives within six months and antidiabetic or antihypertensive drugs within three months prior to randomization led to exclusion.
Intervention, randomization and blinding
Liraglutide/placebo was administered as a subcutaneous injection once daily: 0.6 mg the first week, 1.2 mg the second week and 1.8 mg for the rest of the study period (26 weeks in total). This dose was chosen as it caused weight loss and improved glycemic control in obese patients with type 2 diabetes (34). The participants registered compliance daily in a medical diary provided by the study personnel. The study drug (liraglutide and placebo) was delivered in identical prefilled pens, labeled with serial numbers and accompanied by a randomization list. As investigators and participants were blinded, an independent secretary instructed the investigators as to which serial numbers to supply each woman with. The participants were randomized in a 2:1 ratio (liraglutide:placebo), as we believed that this would facilitate the recruitment to the study.
Outcomes
Primary outcome was the difference between the groups in change from baseline to follow-up in endogenous thrombin potential (ETP) measured by thrombin generation test (TGT). Secondary outcomes were differences between groups in change from baseline to follow-up in other parameters of TGT (described in ‘Assays’ subsection) as well as plasma levels of vWF, PAI-1 and hsCRP.
Protocol
Each woman participated in four visits: screening, baseline (week 0), safety (week 8) and follow-up (week 26), previously described (33). At screening, informed consent was obtained after which a medical interview, physical examination, blood sample collection and transvaginal ultrasound were performed. At baseline and follow-up visits, participants had their blood drawn (between 08:00 and 10:00 h) after an overnight fast. After 15 min in a seated position, blood was drawn from an antecubital vein with a light tourniquet (40 mmHg). Blood was collected in citrated vacuum tubes and centrifuged for 20 min at 2000 g after which platelet poor plasma and serum were stored at −80°C until analysis. Weight, waist and hip circumference and blood pressure were measured in a standardized way. At the safety visit, participants had a brief physical examination and routine blood work done. Adverse events were registered at every visit, and participants were instructed to contact the investigators if they experienced any adverse effects.
Assays
Thrombin generation was assessed with a Calibrated Automated Thrombogram (Thrombinoscope BV, Maastricht, The Netherlands) using a fluorogenic method. After activation of the coagulation by adding tissue factor and phospholipids to platelet poor plasma, four parameters were measured: time to start of thrombin generation (lag time, min); peak thrombin concentration (nmol/L); time to peak (min) and area under the curve (endogenous thrombin potential (ETP), nmol/L × min) (35). Intra-assay CV: 3.4–5.7% for all parameters. Plasma levels of vWF-antigen were determined with a particle-enhanced immunoturbidimetric assay, HemosIL von Willebrand Factor Antigen kit using an ACL 9000 System (Instrumentation Laboratory, Milan, Italy), plasma levels of PAI-1 were determined with an ELISA (antibodies: Mon-I-1 and Mon-I-6) on Tecan Sunrise plate reader (Tecan, Basle, Switzerland) and plasma levels of hsCRP using a CardioPhase hsCRP kit on a BNII protein analyzer (Siemens Healthcare Diagnostics GmbH), with CV 4.6, 6.4 and 3.1%, respectively. All analyses were performed at Unit for Thrombosis Research, Department of Clinical Biochemistry, Hospital of South West Denmark, Esbjerg, Denmark. Baseline and follow-up samples from each participant were analyzed in the same batch. Plasma levels of insulin were determined using an electro-chemiluminescent immunoassay and a Cobas e411 reader (Roche Diagnostics GmbH), with an intra-assay CV of 2.1%. The Homeostasis Model Assessment of insulin resistance (HOMA2-IR) was calculated from fasting levels of insulin and glucose using an online HOMA calculator (www.dtu.ox.ac.uk/homacalculator). Glucose, HbA1c, cholesterol and triglyceride levels were assessed using routine analyses at the Department of Clinical Biochemistry, Herlev Gentofte Hospital, Denmark. Androgen levels were determined using liquid chromatography and double mass spectrometry at Rigshospitalet, Copenhagen, Denmark.
Statistics
A sample size calculation based on an estimated standard deviation of 130 units obtained from in-house data, declared 63 subjects, randomized 2:1, needed for 80% power to find a difference in effect size of 100 nmol/min of ETP. This effect size was supported by a previous study finding a similar reduction in ETP with a 5% reduction in BMI (22). To allow for drop-outs, 72 women were randomized. Distribution of data was checked using histograms and probability plots. Normally distributed data are presented as mean (s.d.), non-normally distributed data as median (p25–p75) and differences as mean (95% CI). Data were logarithmic transformed as appropriate, which is why some differences are presented as ratios. Fishers exact test was used for comparison of adverse effects between groups and paired t-test for quantification of effect (follow-up – baseline) in each group. In the initial protocol, we planned on calculating the between-group difference using an unpaired t-test on the intention-to-treat population. As a mixed model with maximum likelihood is a more optimal way of analyzing repeated measurements, we have chosen this statistic approach, and between-group differences in treatment effect are assessed using a repeated measurements mixed model (with maximum likelihood) with study drug as between-subjects effect and visit (time) as within-subject effect. Baseline data from all 72 women were included in the mixed model analyses, and missing data were assumed to be missing at random. Pre-specified subgroup analyses for Rotterdam phenotypes as well as for four metabolic subgroups using median HOMA2-IR and BMI 25 kg/m2 as cut-offs (HOMA2 < 2.3 + BMI < 25; HOMA2 < 2.3 + BMI > 25; HOMA2 > 2.3 + BMI < 25 and HOMA2 > 2.3 + BMI > 25) were performed using Mann–Whitney U test with Bonferroni correction. Associations between ETP and anthropometric, metabolic and endocrine parameters were assessed using univariate and multivariate linear regression analyses on baseline data. In analyses regarding hsCRP, estimates >10 mg/L were excluded as this indicated infection. Statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA). A two-sided P value <0.05 was considered significant.
Results
Participant flow, baseline data and adverse effects
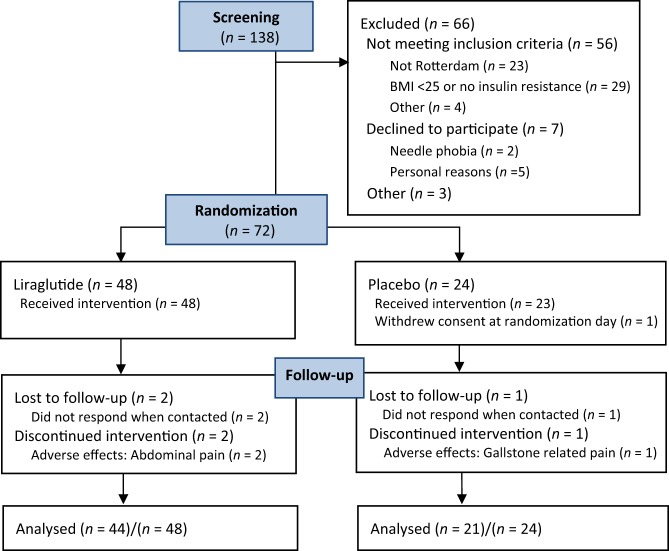
Of 138 women assessed for eligibility, 72 were included of which 48 were randomized to liraglutide and 24 to placebo (Fig. 1). Groups were comparable at baseline (Table 1). One woman in the placebo group withdrew her consent before starting treatment. Dropout ratio was 7/72 (9.7%) overall, 4/48 (8.3%) in the liraglutide and 3/24 (12.5%) in the placebo group. In the liraglutide group, the most prevalent adverse effect was nausea, mainly in the up-titration phase (Table 2). Gallstone-related pain was experienced by three (6.4%) women in the liraglutide group and one (4.4%) in the placebo group, and two women in the liraglutide group had a cholecystectomy. Due to the discontinuation of study drug during diagnosis and surgery, overall compliance for the two women was 64% and 87%, respectively. Self-reported median (p25–p75) compliance was 96% (89–99) in the active group and 93% (87–98) in the placebo group (P = 0.70). In the liraglutide group 1 (2%), 7 (15%) and 38 (83%) women completed the study at 0.6 mg/day, 1.2 mg/day and 1.8 mg/day, respectively. All women in the placebo group completed the study at the highest dose.
Figure 1.
Flow chart of included and excluded participants.
Table 1.
Baseline characteristics in the 72 PCOS women randomized.
| Liraglutide (n = 48) | Placebo (n = 24) | P | |
|---|---|---|---|
| Age (years) | 31.4 (24.6–35.6) | 26.2 (24.8–31.5) | 0.11 |
| Weight (kg) | 94.2 (15.4) | 91.3 (13.6) | 0.42 |
| BMI (kg/m2) | 33.3 (5.1) | 33.3 (4.6) | 0.98 |
| Waist (cm) | 102.6 (10.8) | 102.6 (11.1) | 0.99 |
| Waist/hip ratio | 0.91 (0.08) | 0.92 (0.10) | 0.75 |
| Systolic BP (mmHg)/diastolic BP (mmHg) | 123 (9)/79 (8) | 124 (9)/80 (7) | 0.55/0.75 |
| Smoking (<10 cigarettes/day) | 18.8% (9) | 33.3% (8) | 0.24 |
| First degree relative with diabetes | 31.3% (15) | 26.1% (6) (n=23) | 0.78 |
| Ethnicity | 0.68 | ||
| Caucasian | 93.8% (45) | 91.7% (22) | |
| Rotterdam phenotype | 0.54 | ||
| O/A + HA + PCO | 39.6% (19) | 45.8% (11) | |
| O/A + HA | 2.1% (1) | 8.3% (2) | |
| HA + PCO | 22.9% (11) | 16.7% (4) | |
| O/A + PCO | 35.4% (17) | 29.2% (7) | |
| Ferriman Gallway score | 7.0 (3.0–11.5) | 7.0 (2.5–12.5) | 0.90 |
| Total testosterone (nmol/L) | 1.23 (0.91–1.63) | 1.35 (0.95–1.93) | 0.46 |
| SHBG (nmol/L) | 31.0 (22.0–44.5) | 30.5 (23.0–37.5) | 0.68 |
| Total cholesterol (mmol/L) | 4.61 (0.80) | 4.67 (0.57) | 0.75 |
| LDL cholesterol (mmol/L) | 2.83 (0.71) | 2.99 (0.54) | 0.30 |
| HDL cholesterol (mmol/L) | 1.14 (0.25) | 1.09 (0.28) | 0.40 |
| Triglycerides (mmol/L) | 1.23 (0.90–1.63) | 1.15 (0.90–1.47) | 0.64 |
| HbA1c (mmol/mol) | 34.2 (2.8) | 34.6 (3.4) | 0.66 |
| HOMA2-IR | 2.29 (1.83–2.84) | 2.42 (1.91–3.20) | 0.25 |
| GFR (mL/min/1.73 m2) | 112.8 (13.3) | 117.5 (9.7) | 0.12 |
Values are presented as mean (s.d.), median (p25–p75).
Between-group differences at baseline were determined by un-paired t-test, Mann Whitney U-test, Chi2-test and Fisher exact test as appropriate.
BMI, body mass index; BP, blood pressure; O/A, oligo-/amenorrhea; HA, hyperandrogenism; PCO, polycystic ovaries; SHBG, sex hormone binding globulin; LDL, low density lipoprotein; HDL, high density lipoprotein; HbA1c, glycated hemoglobin; HOMA2-IR, homeostasis model assessment-estimated insulin resistance; GFR, glomerular filtration rate.
Table 2.
Adverse effects during 26 weeks liraglutide or placebo treatment.
| Liraglutide (n = 47) | Placebo (n=23) | P | |
|---|---|---|---|
| Nausea | 78.7 (37) | 13.0 (3) | <0.01 |
| Vomiting | 10.6 (5) | 0 (0) | 0.2 |
| Ructus/heartburn | 17.0 (8) | 0 (0) | 0.05 |
| Diarrhea | 10.6 (5) | 4.4 (1) | 0.7 |
| Constipation | 25.5 (12) | 0 (0) | <0.01 |
| Gastroenteritis | 10.6 (5) | 8.7 (2) | 1.0 |
| Epigastrial pain | 17.0 (8) | 0 (0) | 0.05 |
| Gallstone related pain | 6.4 (3) | 4.4 (1) | 1.0 |
| Cholecystectomy | 4.3 (2) | 0 (0) | 1.0 |
| Hypotension | 2.1 (1) | 0 (0) | 1.0 |
| Tachycardia | 2.1 (1) | 0 (0) | 1.0 |
| Syncope | 2.1 (1) | 0 (0) | 1.0 |
| Dizziness | 8.5 (4) | 0 (0) | 0.3 |
| Headache | 0 (0) | 13.0 (3) | <0.05 |
| Upper respiratory tract infection | 14.9 (7) | 17.4 (4) | 1.0 |
| Urinary tract infection | 4.3 (2) | 0 (0) | 1.0 |
| Hair loss | 2.1 (1) | 0 (0) | 1.0 |
| Rash at injection site | 6.4 (3) | 0 (0) | 0.6 |
| Joint pain | 2.1 (1) | 0 (0) | 1.0 |
Values are presented as % (n). Bold indicates P < 0.05. Adverse effects experienced at any point of the study period. Between-group differences were determined using Fishers exact test.
Anthropometric and metabolic measurements
Mean weight, BMI and waist circumference decreased significantly in the liraglutide group compared with the placebo group, −5.2 kg (95% CI −7.5 to −3.0, P < 0.0001), −1.8 kg/m2 (95% CI −2.7 to −1.0, P < 0.0001) and −5.7 cm (95% CI −9.3 to −1.9, P < 0.05), respectively. When compared to placebo, there was no effect of liraglutide on HOMA2-IR or on triglyceride and cholesterol levels (data not shown).
Thrombin generation, vWF, PAI-1 and hsCRP
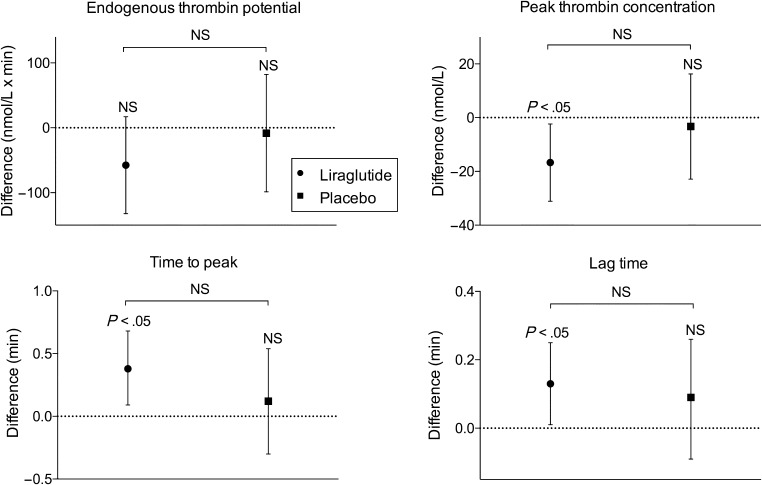
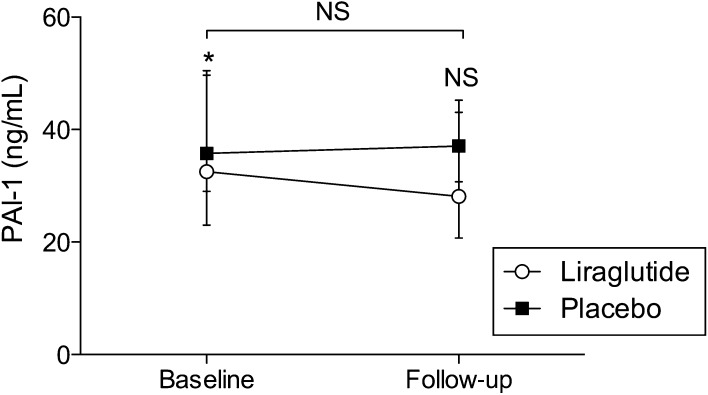
In the liraglutide group, peak thrombin concentration decreased by −16.71 nmol/L (95% CI −31.11 to −2.32, P < 0.05) and lag time and time to peak increased by 0.13 min (95% CI 0.01–0.25, P < 0.05) and 0.38 min (95% CI 0.09–0.68, P < 0.05), respectively (Fig. 2). There was no effect on the primary outcome ETP (Table 3). Levels of PAI-1 decreased 12% (95% CI 0–23, P = 0.05) over six months in the liraglutide group, and there was a trend toward decreased PAI-1 in the liraglutide group when compared with the placebo group (P = 0.10, Fig. 3). We observed no differences between subgroups with regard to effect on thrombin generation, vWF, PAI-1 or hsCRP levels.
Figure 2.
Change in thrombin generation parameters after intervention with liraglutide or placebo for 26 weeks. Dots represent mean change from baseline to follow-up in the liraglutide group and squares represent mean change from baseline to follow-up in the placebo group. Data are presented as mean (95% CI). Within-group comparisons demonstrated decreased peak thrombin concentration (top right), increased time to peak (bottom left) and increased lag time (bottom right), *P < 0.05. NS, non-significant.
Table 3.
Changes in pro-thrombotic and pro-inflammatory biomarkers from baseline to 26-week follow-up.
| Liraglutide | Placebo | Difference between groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n = 48) | Difference at six months (n = 44) | P | Baseline (n=24) | Difference at six months (n=21) | P | Mixed models (crude) | P | |
| ETP (µmol/L × min) | 1796 (332) | −57.6 (−132.3 to 17.2) | 0.13 | 1830 (285) | −8.2 (−98.7 to 82.3) | 0.85 | −47.3 (−161.9 to 67.3) | 0.41 |
| Peak thrombin (nmol/L) | 247.3 (41.1) | −16.7 (−31.1 to 2.3) | <0.05 | 245.7 (46.5) | −3.3 (−22.9 to 16.3) | 0.73 | −12.6 (−35.5 to 10.3) | 0.27 |
| Time to peak (min) | 7.36 (1.11) | 0.38 (0.09–0.68) | <0.05 | 7.86 (1.19) | 0.12 (−0.30 to 0.54) | 0.56 | 0.25 (−0.25 to 0.75) | 0.32 |
| Lag-time (min) | 3.33 (2.92–3.67) | 0.13 (0.01–0.25) | <0.05 | 3.59 (3.00–3.84) | 0.09 (−0.09 to 0.26) | 0.32 | 0.04 (−0.17 to 0.24) | 0.72 |
| vWF (% of normal) | 99.5 (87.5–122.5) | 1.64 (−3.3 to 6.6) | 0.51 | 123.0 (92.0–145.0) | −5.2 (−12.1 to 1.7) | 0.13 | 1.03 (0.96–1.11)a | 0.35 |
| hsCRP (mg/L)b | 2.05 (0.79–3.65) | 0.85 (0.70–1.03)a | 0.09 | 3.48 (1.17–4.32) | 0.75 (0.43–1.30)a | 0.29 | 1.02 (0.67–1.55)a | 0.92 |
Values are presented as mean (s.d.), median (p25–p75) and differences as mean (95%CI) or aratio from logarithmic transformed numbers. Bold indicates P < 0.05. Adjusting the mixed model for age, BMI and smoking at baseline did not alter the results significantly.
bliraglutide n = 45 (baseline), n = 42 (follow-up), placebo n = 20 (baseline), n = 18 (follow-up).
ETP, endogenous thrombin potential; PAI-1, plasminogen activator inhibitor 1; hsCRP, high sensitivity C-reactive protein and vWF = von Willebrand factor.
Figure 3.
Levels of PAI-1 at baseline and 26-week follow-up. Open circles represent the liraglutide group and black squares the placebo group. Data are presented as median (p25–p75) at baseline and follow-up. There was a trend toward decreased PAI-1 in the liraglutide group as compared with the placebo group, 16% (95% CI −4 to 32, P = 0.10). *P = 0.05. NS, non-significant; PAI-1, plasminogen activator inhibitor-1.
Associations between ETP and anthropometric, metabolic and endocrine variables
At baseline, ETP levels were associated with anthropometric and metabolic variables: BMI, waist circumference, diastolic blood pressure, HOMA2-IR, triglycerides, hsCRP and PAI-1, and reciprocally associated with HDL and SHBG. Including BMI in the model, only levels of PAI-1 remained associated with ETP. Including HOMA2-IR, triglycerides and hsCRP in the model, this association was no longer statistically significant.
Discussion
In this placebo-controlled randomized trial, investigating the effect of 26-week liraglutide intervention on VTE and CVD risk markers in overweight women with PCOS, we found a mean weight loss of 5.2 kg. Concomitantly, we observed significant improvements in thrombin generation parameters: decreased peak thrombin concentration, increased lag time and time to peak. However, the changes observed was not statically significant when compared with placebo, and liraglutide intervention appeared insufficient to influence the primary outcome, the total endogenous thrombin potential (ETP) in our population. In the liraglutide group, we demonstrated reduced PAI-1 levels and a trend toward a between-group difference, indicating improved fibrinolytic potential, and thus, a net beneficial effect on validated markers of VTE and CVD risk.
To our knowledge, no previous studies have focused on the effect of liraglutide on thrombin generation. An open-label, single-arm study investigating the effect of liraglutide (1.8 mg/day for six months) in 36 obese women with and without PCOS found improved endothelial function, seen as reduced levels of cell adhesion markers as well as slightly reduced clot lysis area, a complex measure of clot formation, density and lysis potential (29). Discrepancies might be explained by the single-armed design and their population having higher BMI and worse metabolic profile than ours. Moreover, clot formation is a measurement of platelet function and fibrin production, depending on thrombin concentration among numerous other factors (36), which is why our studies are not directly comparable.
Glintborg and coworkers recently studied the effect of metformin and combined oral contraceptives (COC) on thrombin generation in a RCT with 90 PCOS women (12). Thrombin generation, measured as ETP and peak thrombin concentration, increased after 12 months of intervention with COC, as well as with combined treatment (COC + metformin), but no changes were seen in the metformin-alone group despite an almost significant median weight loss of 3 kg (37). The increase in thrombin generation was smaller in the combined group compared with that in the COC group, suggesting a protective effect of metformin. Except from lower mean BMI, their population was comparable to ours and the same thrombin generation assay was used.
Our hypothesis of weight loss and improved glycemic control resulting in reduced thrombin generation has been confirmed in other populations. Bariatric surgery in 36 morbidly obese adults (with and without type 2 diabetes) resulted in weight loss (mean −32%), reduced insulin resistance as well as decreased ETP and peak thrombin concentration at two-year follow-up (21). Additionally, one year of lifestyle intervention in 27 overweight children caused reduced BMI as well as decreased ETP and peak thrombin concentration (22). In a RCT, Gram and coworkers found three months of daily endurance exercise to reduce BMI and ETP in 53 healthy, moderately overweight, young men, whereas there was no effect on peak thrombin concentration (23). Reasons for disagreement between our findings and the mentioned studies might be the excessive weight loss in the first (21), the lack of control group in the first and second (21, 22) and possibly a ‘healthier’ weight loss in the latter (23).
Intervention with GLP-1 analogs has previously been shown to affect the metabolic parameters in PCOS. In several smaller trials on women with PCOS, GLP-1 analogs were found to reduce well-known risk factors of CVD: body weight, waist circumference and visceral adipose tissue (28, 31, 32), whereas results regarding HOMA-IR are conflicting (28, 29, 30) and results on hsCRP are sparse and inconsistent (28, 29). In PCOS, metformin seems to attenuate endothelial dysfunction and low-grade inflammation, evaluated as levels of PAI-1 and hsCRP (24, 38, 39, 40). However, results are conflicting (41), and most of the studies are single-armed or open-labeled, testing metformin vs COC. Physical exercise was found to reduce BMI as well as levels of PAI-1 and hsCRP compared to baseline in a six-month RCT on 136 women with PCOS (42). However, the effect disappeared when compared with placebo or COC (42). Also, a lifestyle-induced weight loss of 8–11% was found to cause reduced levels of PAI-1 in an obese PCOS population (43). As most interventional studies on endothelial function and low-grade inflammation in PCOS lack a placebo arm, we cannot easily compare our findings.
Possibly GLP-1 analogs influence hemostasis in a direct way. The effect of GLP-1 analogs on hemostasis has been studied in vitro and in animal models. Human megakaryocytes have been found to express GLP-1 receptors and both human and murine blood incubated with the GLP-1 analog exenatide have been found to have reduced in vitro thrombus formation (44). Arterial thrombus formation decreased in mice treated with i.v. exenatide, suggesting GLP-1 analogs to reduce platelet aggregation by inhibiting the release of α- and dense granules (44). Moreover, liraglutide has been shown to attenuate high-glucose-mediated PAI-1 expression in human endothelial cells (45).
The anti-atherothrombotic potential of GLP-1-based therapies has been studied both in type 2 diabetes and obesity and have been found to reduce the levels of hsCRP and PAI-1 in both conditions (26, 27). In the recent LEADER trial including more than 9000 patients with type 2 diabetes and a concomitant cardiovascular condition, liraglutide reduced the occurrence of CVD events and the rate of CVD death, as compared with placebo, as an add-on medication (46). The LEADER population was large and therefore at considerably greater risk of CVD events than our, younger, population still being relatively healthy with regard to having obtained a major cardiovascular burden.
This study has several strengths: the placebo-controlled RCT design, the high external validity and the low dropout rate. However, there are some limitations. Only 83% of the women in the liraglutide group completed the study at the intended dose (1.8 mg/day). An explanation for finding unaltered thrombin generation and levels of hsCRP and vWF with liraglutide therapy could be that our population was relatively ‘metabolically healthy’. Worth noting is that the baseline fasting glucose and lipid levels were within normal range, despite the high mean BMI (33 kg/m2) and waist circumference (103 cm). Inclusion of women being at a higher risk of CVD, i.e., with even higher BMI, reduced glucose tolerance, overt diabetes or dyslipidemia, might have given different results. This might also have been obtained by using the National Institutes of Health criteria instead of the Rotterdam criteria (3) and including older women. Our lack of findings might be due to lack of power. The fact that some of the TGT parameters were altered in the liraglutide group indicates that there may be an effect of the treatment, but the population might be too small to identify it. Also, when looking at the CIs, we can neither confirm nor rule out a beneficial effect of liraglutide on thrombin generation.
In conclusion, in an overweight PCOS population, 26 weeks of liraglutide intervention caused minor alterations in thrombin generation parameters, but no difference in overall thrombin generation when compared to placebo. We observed a substantial weight loss and a trend toward improved fibrinolytic potential. Taken together, the results point toward beneficial effects on markers of VTE and CVD risk, which are promising, but need to be corroborated in larger studies. Liraglutide was well tolerated, although one should be aware of the risk of weight loss-related gallbladder stone attacks in a population of young overweight women.
Declaration of interest
M N, S F and S O S have nothing to disclose. C K and J F have given lectures at NovoNordisk sponsored symposia. C K is a member of a NovoNordisk Advisory board. J F is a member of NovoNordisk Advisory board with regard to liraglutide treatment in diabetes.
Funding
M N was supported by a grant from the University of Copenhagen throughout the study period. The study was investigator-initiated and funded by Novo Nordisk A/S, who contributed with study and placebo drug and with a grant, covering preparation of the study as well as expenses to laboratory measures. The funds were unconditioned in relation to study design, collection, analysis and interpretation of data as well as on writing the manuscript, but Novo Nordisk A/S had access to the manuscript prior to submission.
Author contribution statement
J F, S O S and C K initiated the study and all five authors contributed to the protocol. S F and M N conducted the study and collected the data. All authors contributed to intellectual interpretation of the results. M N performed the statistical analyses and produced the manuscript, which all co-authors have read and approved.
Acknowledgements
The authors gratefully acknowledge the women participating in this study, as well as the staff at the endocrine research laboratory and the Fertility clinic, Herlev Gentofte Hospital. The authors also thank statisticians Mathias Ejdrup Bredkjær and Tobias Wirenfeldt for the statistical guidance.
References
- 1.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Human Reproduction 2016. 31 2841–2855. ( 10.1093/humrep/dew218) [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003. consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility 2004. 81 19–25. ( 10.1016/j.fertnstert.2003.10.004) [DOI] [PubMed] [Google Scholar]
- 3.Daan NMP, Louwers YV, Koster MPH, Eijkemans MJC, de Rijke YB, Lentjes EWG, Fauser BC, Laven JS. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertility and Sterility 2014. 102 1444–1451.e3. ( 10.1016/j.fertnstert.2014.08.001) [DOI] [PubMed] [Google Scholar]
- 4.Molica F, Morel S, Kwak BR, Rohner-Jeanrenaud F, Steffens S. Adipokines at the crossroad between obesity and cardiovascular disease. Thrombosis and Haemostasis 2015. 113 553–566. ( 10.1160/TH14-06-0513) [DOI] [PubMed] [Google Scholar]
- 5.Zhao L, Zhu Z, Lou H, Zhu G, Huang W, Zhang S, Lui F. Polycystic ovary syndrome (PCOS) and the risk of coronary heart disease (CHD): a meta-analysis. Oncotarget 2016. 7 33715–33721. ( 10.18632/oncotarget.9553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird ST, Hartzema AG, Brophy JM, Etminan M, Delaney JAC. Risk of venous thromboembolism in women with polycystic ovary syndrome: a population-based matched cohort analysis. CMAJ 2013. 185 E115–E120. ( 10.1503/cmaj.120677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010. 375 132–140. ( 10.1016/S0140-6736(09)61717-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tofler GH, Massaro J, O’Donnell CJ, Wilson PWF, Vasan RS, Sutherland PA, Meigs JB, Levy D, D'Agostino RB. Plasminogen activator inhibitor and the risk of cardiovascular disease: the Framingham heart study. Thrombosis Research 2016. 140 30–35. ( 10.1016/j.thromres.2016.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonneveld MAH, de Maat MPM, Leebeek FWG. Von Willebrand factor and ADAMTS13 in arterial thrombosis: a systematic review and meta-analysis. Blood Reviews 2014. 28 167–178. ( 10.1016/j.blre.2014.04.003) [DOI] [PubMed] [Google Scholar]
- 10.Toulis KA, Goulis DG, Mintziori G, Kintiraki E, Eukarpidis E, Mouratoglou S-A, Pavlaki A, Stergianos S, Poulasouchidou M, Tzellos TG, et al. Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Human Reproduction Update 2011. 17 741–760. ( 10.1093/humupd/dmr025) [DOI] [PubMed] [Google Scholar]
- 11.Koiou E, Tziomalos K, Katsikis I, Dinas K, Tsourdi EA, Kandaraki EA, Delkos D, Papadakis E, Panidis D. Plasma von Willebrand factor antigen levels are elevated in the classic phenotypes of polycystic ovary syndrome. Hormones 2012. 11 77–85. [DOI] [PubMed] [Google Scholar]
- 12.Glintborg D, Sidelmann JJ, Altinok ML, Mumm H, Andersen M. Increased thrombin generation in women with polycystic ovary syndrome: a pilot study on the effect of metformin and oral contraceptives. Metabolism 2015. 64 1272–1278. ( 10.1016/j.metabol.2015.06.011) [DOI] [PubMed] [Google Scholar]
- 13.van Hylckama Vlieg A, Baglin CA, Luddington R, MacDonald S, Rosendaal FR, Baglin TP. The risk of a first and a recurrent venous thrombosis associated with an elevated D-dimer level and an elevated thrombin potential: results of the THE-VTE study. Journal of Thrombosis and Haemostasis 2015. 13 1642–1652. ( 10.1111/jth.13043) [DOI] [PubMed] [Google Scholar]
- 14.Eichinger S, Hron G, Kollars M, Kyrle PA. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D-dimer. Clinical Chemistry 2008. 54 2042–2048. ( 10.1373/clinchem.2008.112243) [DOI] [PubMed] [Google Scholar]
- 15.Borissoff JI, Joosen IA, Versteylen MO, Spronk HM, ten Cate H, Hofstra L. Accelerated in vivo thrombin formation independently predicts the presence and severity of CT angiographic coronary atherosclerosis. JACC: Cardiovascular Imaging 2012. 5 1201–1210. ( 10.1016/j.jcmg.2012.01.023) [DOI] [PubMed] [Google Scholar]
- 16.Burchall GF, Piva TJ, Linden MD, Gibson-Helm ME, Ranasinha S, Teede HJ. Comprehensive assessment of the hemostatic system in polycystic ovarian syndrome. Seminars in Thrombosis and Hemostasis 2016. 42 55–62. ( 10.1055/s-0035-1564837) [DOI] [PubMed] [Google Scholar]
- 17.Aziz M, Sidelmann JJ, Wissing MLM, Faber J, Skouby SO. Endogenous thrombin potential in polycystic ovary syndrome: the association to body mass index, insulin resistance, and inflammation. Gynecological Endocrinology 2015. 31 720–724. ( 10.3109/09513590.2015.1032930) [DOI] [PubMed] [Google Scholar]
- 18.Aziz M, Sidelmann JJ, Faber J, Wissing M-LM, Naver KV, Mikkelsen A-L, Nilas L, Skouby SO. Polycystic ovary syndrome: cardiovascular risk factors according to specific phenotypes. Acta Obstetricia et Gynecologica Scandinavica 2015. 94 1082–1089. ( 10.1111/aogs.12706) [DOI] [PubMed] [Google Scholar]
- 19.Beijers HJ, Ferreira I, Spronk HM, Bravenboer B, Dekker JM, Nijpels G, ten Cate H, Stehouwer CD. Body composition as determinant of thrombin generation in plasma: the Hoorn study. Arteriosclerosis, Thrombosis, and Vascular Biology 2010. 30 2639–2647. ( 10.1161/ATVBAHA.110.211946) [DOI] [PubMed] [Google Scholar]
- 20.Campello E, Zabeo E, Radu CM, Spiezia L, Gavasso S, Fadin M, Woodhams B, Vettor R, Simioni P. Hypercoagulability in overweight and obese subjects who are asymptomatic for thrombotic events. Thrombosis and Haemostasis 2015. 113 85–96. ( 10.1160/TH14-02-0156) [DOI] [PubMed] [Google Scholar]
- 21.Ay L, Kopp H-P, Brix J-M, Ay C, Quehenberger P, Schernthaner G-H, Pabinger I, Schernthaner G. Thrombin generation in morbid obesity: significant reduction after weight loss. Journal of Thrombosis and Haemostasis 2010. 8 759–765. ( 10.1111/j.1538-7836.2010.03766.x) [DOI] [PubMed] [Google Scholar]
- 22.Fritsch P, Kleber M, Schlagenhauf A, Laschnik B, Fritsch M, Muntean W, Mangge H, Reinehr T. Normalization of haemostatic alterations in overweight children with weight loss due to lifestyle intervention. Atherosclerosis 2011. 216 170–173. ( 10.1016/j.atherosclerosis.2011.01.042) [DOI] [PubMed] [Google Scholar]
- 23.Gram AS, Bladbjerg E-M, Skov J, Ploug T, Sjödin A, Rosenkilde M, Madsen DE, Stallknecht BM. Three months of strictly controlled daily endurance exercise reduces thrombin generation and fibrinolytic risk markers in younger moderately overweight men. European Journal of Applied Physiology 2015. 115 1331–1338. ( 10.1007/s00421-015-3106-z) [DOI] [PubMed] [Google Scholar]
- 24.Teede HJ, Meyer C, Hutchison SK, Zoungas S, McGrath BP, Moran LJ. Endothelial function and insulin resistance in polycystic ovary syndrome: the effects of medical therapy. Fertility and Sterility 2010. 93 184–191. ( 10.1016/j.fertnstert.2008.09.034) [DOI] [PubMed] [Google Scholar]
- 25.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012. 344 d7771 ( 10.1136/bmj.d7771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song X, Jia H, Jiang Y, Wang L, Zhang Y, Mu Y, Liu Y. Anti-atherosclerotic effects of the glucagon-like peptide-1 (GLP-1) based therapies in patients with type 2 diabetes mellitus: a meta-analysis. Scientific Reports 2015. 5 10202 ( 10.1038/srep10202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine 2015. 373 11–22. ( 10.1056/NEJMoa1411892) [DOI] [PubMed] [Google Scholar]
- 28.Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2008. 93 2670–2678. ( 10.1210/jc.2008-0115) [DOI] [PubMed] [Google Scholar]
- 29.Kahal H, Aburima A, Ungvari T, Rigby AS, Coady AM, Vince RV, Ajjan RA, Kilpatrick ES, Naseem KM, Atkin SL. The effects of treatment with liraglutide on atherothrombotic risk in obese young women with polycystic ovary syndrome and controls. BMC Endocrine Disorders 2015. 15 14 ( 10.1186/s12902-015-0005-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensterle M, Kravos NA, Pfeifer M, Kocjan T, Janez A. A 12-week treatment with the long-acting glucagon-like peptide 1 receptor agonist liraglutide leads to significant weight loss in a subset of obese women with newly diagnosed polycystic ovary syndrome. Hormones 2015. 14 81–90. [DOI] [PubMed] [Google Scholar]
- 31.Jensterle M, Salamun V, Kocjan T, Vrtacnik Bokal E, Janez A. Short term monotherapy with GLP-1 receptor agonist liraglutide or PDE 4 inhibitor roflumilast is superior to metformin in weight loss in obese PCOS women: a pilot randomized study. Journal of Ovarian Research 2015. 8 32 ( 10.1186/s13048-015-0161-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensterle M, Kocjan T, Kravos NA, Pfeifer M, Janez A. Short-term intervention with liraglutide improved eating behavior in obese women with polycystic ovary syndrome. Endocrine Research 2015. 40 133–138. ( 10.3109/07435800.2014.966385). [DOI] [PubMed] [Google Scholar]
- 33.Frøssing S, Nylander M, Kistorp C, Skouby S, Faber J. The LIPT-study: on risk markers of vascular thrombosis in polycystic ovary syndrome. A randomized, double-blind, placebo-controlled study of the effect of Liraglutide. Journal of Obesity and Weight Loss Therapy 2015. 5 254 ( 10.4172/2165-7904.1000254). [DOI] [Google Scholar]
- 34.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009. 373 473–481. ( 10.1016/S0140-6736(08)61246-5) [DOI] [PubMed] [Google Scholar]
- 35.Hemker HC, Al Dieri R, De Smedt E, Béguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thrombosis and Haemostasis 2006. 96 553–561. ( 10.1160/th06-07-0408) [DOI] [PubMed] [Google Scholar]
- 36.Wolberg AS. Thrombin generation and fibrin clot structure. Blood Reviews 2007. 21 131–142. ( 10.1016/j.blre.2006.11.001) [DOI] [PubMed] [Google Scholar]
- 37.Glintborg D, Altinok ML, Mumm H, Hermann AP, Ravn P, Andersen M. Body composition is improved during 12 months’ treatment with metformin alone or combined with oral contraceptives compared with treatment with oral contraceptives in polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2014. 99 2584–2591. ( 10.1210/jc.2014-1135) [DOI] [PubMed] [Google Scholar]
- 38.Diamanti-Kandarakis E, Paterakis T, Alexandraki K, Piperi C, Aessopos A, Katsikis I, Katsilambros N, Kreatsas G, Panidis D. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Human Reproduction 2006. 21 1426–1431. ( 10.1093/humrep/del003) [DOI] [PubMed] [Google Scholar]
- 39.Velazquez EM, Mendoza SG, Wang P, Glueck CJ. Metformin therapy is associated with a decrease in plasma plasminogen activator inhibitor-1, lipoprotein(a), and immunoreactive insulin levels in patients with the polycystic ovary syndrome. Metabolism 1997. 46 454–457. ( 10.1016/S0026-0495(97)90066-4) [DOI] [PubMed] [Google Scholar]
- 40.Morin-Papunen L, Rautio K, Ruokonen A, Hedberg P, Puukka M, Tapanainen JS. Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2003. 88 4649–4654. ( 10.1210/jc.2002-021688) [DOI] [PubMed] [Google Scholar]
- 41.Agarwal N, Rice SPL, Bolusani H, Luzio SD, Dunseath G, Ludgate M, Rees DA. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo-controlled, crossover trial. Journal of Clinical Endocrinology and Metabolism 2010. 95 722–730. ( 10.1210/jc.2009-1985) [DOI] [PubMed] [Google Scholar]
- 42.Orio F, Muscogiuri G, Giallauria F, Savastano S, Bottiglieri P, Tafuri D, Predotti P, Colarieti G, Colao A, Palomba S. Oral contraceptives versus physical exercise on cardiovascular and metabolic risk factors in women with polycystic ovary syndrome: a randomized controlled trial. Clinical Endocrinology 2016. 85 764–771. ( 10.1111/cen.13112) [DOI] [PubMed] [Google Scholar]
- 43.Thomson RL, Brinkworth GD, Noakes M, Clifton PM, Norman RJ, Buckley JD. The effect of diet and exercise on markers of endothelial function in overweight and obese women with polycystic ovary syndrome. Human Reproduction 2012. 27 2169–2176. ( 10.1093/humrep/des138) [DOI] [PubMed] [Google Scholar]
- 44.Cameron-Vendrig A, Reheman A, Siraj MA, Xu XR, Wang Y, Lei X, Afroze T, Shikatani E, El-Mounayri O, Noyan H, et al. Glucagon-like peptide-1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 2016. 65 1714–1723. ( 10.2337/db15-1141) [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Dear AE, Knudsen LB, Simpson RW. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. Journal of Endocrinology 2009. 201 59–66. ( 10.1677/JOE-08-0468) [DOI] [PubMed] [Google Scholar]
- 46.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2016. 375 311–322. ( 10.1056/NEJMoa1603827) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a