Abstract
New treatment options are needed for medullary thyroid carcinoma (MTC), a highly metastasizing neuroendocrine tumor that is resistant to standard radiotherapy and chemotherapy. We show that the following shikonin derivatives inhibit cell proliferation and cell viability of the MTC cell line TT: acetylshikonin, β,β-dimethylacrylshikonin, shikonin and a petroleum ether extract of the roots of Onosma paniculata containing several shikonin derivatives. The unsubstituted shikonin derivative was found to be the most effective compound with an IC50 of 1.1 µM. The cell viability of normal human skin fibroblasts, however, was not affected by the tested substances, indicating that shikonin derivatives might be selectively toxic for cancer cells. We further report that migration and invasion of TT cells were inhibited at non-toxic concentrations. Finally, shikonin was tested in vivo using the chick chorioallantoic membrane assay, where it significantly reduced tumor growth by inhibiting cell proliferation and inducing apoptosis. In summary, our results suggest that shikonin derivatives have the potential for the treatment of medullary thyroid carcinomas.
Keywords: medullary thyroid carcinoma, neuroendocrine tumor, shikonin, TT cells, CAM assay, apoptosis
Introduction
Medullary thyroid carcinomas (MTC) arise from the parafollicular C-cells of the thyroid and account for 5–10% of all thyroid cancers (1, 2). MTCs are calcitonin-producing tumors that occur sporadically in 70–80% of the cases. The remaining 20–30% are hereditary forms that are inherited in an autosomal-dominant pattern, either as part of multiple endocrine neoplasia syndrome type 2A (MEN2A) or 2B (MEN2B), or without any other associated endocrinopathies as familial medullary thyroid carcinoma (FMTC) (3, 4, 5).
MEN2 is characterized by germline mutations of the RET proto-oncogene. Almost all of them are missense mutations leading to increased function of the RET receptor tyrosine kinase. Oncogenic RET mutations of MEN2 are concentrated in a small sequence of the open reading frame and show striking correlations with the phenotype of the MEN2 variant. The most common variant, MEN2A, is mainly caused by mutations in the cysteine-rich portion of the extracellular domain (52% occur in codon 634), and additionally, predisposes to pheochromocytoma and to parathyroid hyperplasia. In MEN2B, the mutation is confined to one cytoplasmic amino acid substitution – Met918Thr. MEN2B has an earlier age of onset than MEN2A and a more aggressive course including pheochromocytoma, mucosal neuromas, megacolon and a Marfanoid habitus (3, 6, 7).
MTCs do not respond to conventional therapies like radiation or chemotherapy, and up to 80% of patients present with nodal metastases at the time of diagnosis, so that surgical removal of all neoplastic tissue is the best option. Recurrence, however, is common, frequently with metastases in the bones, lungs, liver and brain. To date, no effective treatment for distant metastases in MTC has been found (1, 8), and the search for new treatment options is thus highly warranted.
Roots of Boraginaceae genera Arnebia, Lithospermum and Onosma, in China also known as Zicao, are rich in shikonin constituents and have been used in traditional Chinese medicine for hundreds of years (9, 10). The medicinal relevance of the active compounds of their extracts was confirmed for the first time in 1978 (11). Since then, various pharmacological effects have been attributed to shikonin derivatives, including acceleration of wound healing, suppression of local acute inflammatory reactions, inhibition of angiogenesis and antimicrobial and cardioprotective activity (9, 12). Lately, numerous studies investigating anti-cancer effects of shikonin have been published. As reviewed by Andújar and coworkers (13), shikonin exerts its anti-tumor effects in a great variety of cancer cell lines through inhibition of cell proliferation and induction of apoptosis. Furthermore, shikonin has been shown to be effective in animal models (14, 15, 16) and in a clinical trial of late-stage lung cancer patients (17).
In the present study, we investigated for the first time the effect of a petroleum ether root extract of Onosma paniculata Bureau & Franchet on a cell line derived from multiple endocrine neoplasia syndrome type 2A (MEN2A). Additionally, we compared its active constituents acetylshikonin and β,β-dimethylacrylshikonin to the unsubstituted shikonin derivative.
Materials and methods
Cell culture
TT cells, obtained from the European Collection of Authenticated Cell Cultures (ECACC; Porton Down, Salisbury, UK), were cultivated in Ham’s F12 Nutrient Mixture (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS Gold; PAA Laboratories, Pasching, Austria). Cells were passaged at approximately 80% confluence to an initial cell number of 2 × 105 cells/mL using trypsin–EDTA (PAA Laboratories).
Normal human skin fibroblasts, HF-SAR (18), were cultured in EMEM (BioWhittaker, Lonza, Verviers, Belgium) supplemented with 2 mM l-glutamine (PAA Laboratories) and 10% FBS. Cells were passaged to an initial cell number of 1 × 105 cells/mL using Accutase (PAA Laboratories). All cells were kept in a humidified 5% CO2 atmosphere at 37°C.
Shikonin derivatives
As described previously (19, 20), shikonin derivatives were isolated from Onosma paniculata Bureau & Franchet. Briefly, a petroleum ether extract (EX) was prepared by exhaustive Soxhlet extraction and further fractionated using preparative HPLC. The main components were β,β-dimethylacrylshikonin (DMAS, 38.2%) and acetylshikonin (AS, 24.5%) as identified by NMR spectroscopy. For further comparison of the activity, shikonin (SHK) was purchased from Sigma-Aldrich.
Aliquots of the substances were freshly dissolved in DMSO (Sigma-Aldrich) every second week to ensure consistent bioactivity. Concentrations of DMSO after application of the compounds never exceeded 0.5%, which did not affect cell behavior as controlled by benchmark tests.
Growth inhibition assay
Aliquots (2 mL) of TT cells were seeded into 6-well plates (Sarstedt, Wiener Neudorf, Austria) at 2 × 105 cells/mL. After allowing the cells to adhere overnight, seven different concentrations of each substance were added. Seventy-two hours later, cells were detached with trypsin–EDTA (500 µL, 3 min). Then, 1.5 mL of FBS-containing medium was added and cells were counted in triplicates using a CASY1 Cell Counter & Analyzer TTC (Schärfe System, Reutlingen, Germany).
All assays were performed with at least three different passage numbers and with medium containing 10% FBS, but no antibiotics. IC50 values were calculated with Microsoft Excel 2010.
Cell viability assay
Cell viability of TT cells as well as normal human skin fibroblasts, HF-SAR, was assessed using the Cell Proliferation Reagent WST-1 (Roche Diagnostics). Cells were seeded into 96-well plates in aliquots of 100 µL with a cell density of 1 × 105 cells/mL and 3 × 104 cells/mL, respectively. After allowing the cells to adhere overnight, the medium was aspirated and replaced with medium supplemented with DMSO (solvent control) or different concentrations of shikonin derivatives as indicated. Samples were tested in 6 replicates after an incubation period of 72 h. Results are presented as percentage of solvent-treated control cells.
Cell morphology
Morphological changes occurring after application of shikonin were observed with a Nikon inverted microscope (Eclipse TE 300, Nikon). TT cells (2 mL) were seeded into 6-well plates at a density of 2 × 105 cells/mL. After allowing them to adhere overnight, cells were incubated with shikonin (IC50) and cell morphology was observed after 24 and 48 h. Images were taken with a Nikon 12-bit CCD camera (Nikon).
Monolayer wound-healing assay
TT cells (2 mL) were seeded into 6-well plates at a density of 5 × 105 cells/mL and grown to approximately 90% confluence. The medium was then removed and a scratch was created by scraping the cell monolayer with a p10 pipet tip. Debris was removed by washing the cells with 1 mL PBS, which was then replaced by 2 mL medium supplemented with either DMSO (solvent control) or the corresponding IC50/5 values of the shikonin derivatives. Reference points were created by marking the lid of the plate, and images were acquired with a phase-contrast microscope and a 12-bit CCD camera (Nikon). Cells were incubated at 37°C, 5% CO2 and observed periodically until the control cells closed the scratch. Experiments were performed with three different passages, and analysis was done using TScratch software (21).
Matrigel invasion assay
Matrigel invasion assays (BD Biosciences) followed the manufacturer’s protocol. Briefly, 1 × 105 TT cells were seeded per well, incubated with either DMSO as solvent control or the indicated concentration of shikonin. Invaded cells were detected with DAPI staining (Roche Diagnostics) and fluorescence microscopy (Leica DM 4000B; Leica) after 48 h.
Ex ovo chick chorioallantoic membrane assay
Fertilized white leghorn chicken eggs from a local hatchery were incubated at 37.6°C and 70% humidity (J. Hemel Breeding Machines, Germany). The eggshell was cracked on the third day after fertilization and the embryo was decanted to a sterile dish. On day 10, 1 × 106 TT cells were resuspended in 15 μL PBS and 5 μL Matrigel matrix (BD, Biosciences) and grafted in the center of a 5 mm silicon ring on the surface of the chorioallantoic membrane (CAM). Xenografts were treated topically every day, either with 2.2 µM shikonin (2 × IC50) in 10 μL PBS (n = 9) or with 0.02% DMSO in PBS (n = 12) for 3 days. On day 4 after seeding, the xenografts were photographed with a stereo microscope (Olympus SZX16), excised with the surrounding CAM, and then fixed in 4% paraformaldehyde followed by paraffin embedding and cutting of 5-μm sections.
Analysis of proliferation and apoptosis of CAM xenografts
The xenograft sections were processed and stained with an antibody against Ki-67 (clone MIB-1, 1:100; Dako) for the analysis of mitotically active cells. Apoptosis was assessed by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL; Abcam) according to the manufacturer’s instructions. The images were digitally recorded at a magnification of 400× with an Olympus BX53 microscope and an Olympus DP27 camera. Areas from the digitalized color photomicrographs were analyzed. Two examiners independently determined the percentage of positive cells.
Statistical analysis
All data are expressed as means ± standard deviation (s.d.) unless indicated otherwise. The level of significance between two groups was assessed by two-tailed unpaired Student’s t-test using the GraphPad Prism 4.0 statistics software (GraphPad Software). P values <0.05 were considered statistically significant.
Results
Shikonin derivatives exert anti-proliferative effects on TT cells
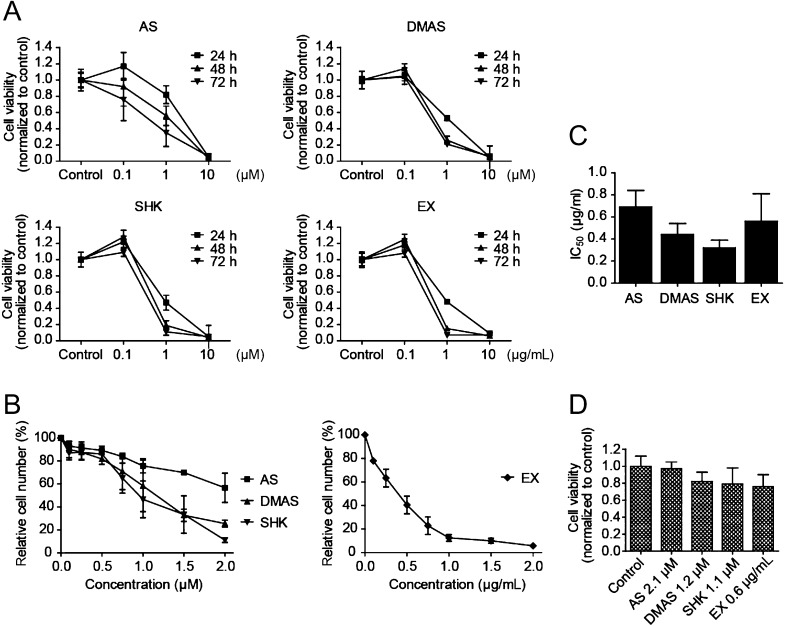
The effects of acetylshikonin (AS), β,β-dimethylacrylshikonin (DMAS), shikonin (SHK) and a petroleum ether extract of the dried roots of Onosma paniculata (EX) on the cell viability of medullary thyroid carcinoma cell line TT were examined at 0.1, 1 and 10 µM (for AS, DMAS and SHK) and 0.1, 1 and 10 µg/mL for the extract, respectively. Although the lowest concentration of the pure substances or the extract, respectively, had only a small effect, there was a pronounced reduction in cell viability at 1 µM or 1 µg/mL. 10 µM or 10 µg/mL, respectively, had already reduced cell viability drastically within 24 h (Fig. 1A). To determine the IC50 values of each substance, we incubated TT cells with 0.1–2 µM (for AS, DMAS and SHK) or 0.1–2 µg/mL (EX) for 72 h. A dose-dependent reduction of cell proliferation was seen for all examined compounds (Fig. 1B). Table 1 shows IC50 values as mean ± s.d. of at least three independent experiments. To allow for comparison of potency of the pure substances with the unfractionated extract, IC50 concentrations of acetylshikonin, β,β-dimethylacrylshikonin and shikonin were converted to µg/mL according to their molecular weights (Fig. 1C). The application of the extract, which is a mixture of several shikonin derivatives, did not produce lower IC50 values than application of the pure compounds. In fact, shikonin and β,β-dimethylacrylshikonin exhibited the lowest IC50 values on TT cells.
Figure 1.
Shikonin derivatives inhibit proliferation of TT cells in a dose-dependent manner but do not reduce cell viability in normal human skin fibroblasts. (A) TT cells were incubated with 0.1, 1 and 10 µM acetylshikonin (AS), β,β-dimethylacrylshikonin (DMAS) or shikonin (SHK) and 0.1, 1 and 10 µg/mL petroleum ether extract of Onosma paniculata roots (EX). Cell viability was reduced at 1 µM concentrations of acetylshikonin, β,β-dimethylacrylshikonin and shikonin and 1 µg/mL of the extract. Concentrations of 10 µM and 10 µg/mL had deleterious effects on cell viability. Experiments were performed three times in sextuplicates. (B) Cell proliferation of TT cells as assessed by cell counting was dose dependently inhibited by acetylshikonin, β,β-dimethylacrylshikonin, shikonin and the extract. Cell numbers are presented as percentage of control solvent-treated cells, n = 3–4. (C) Conversion of determined IC50 values (Table 1) from µM to µg/mL allows comparison of potency between pure substances and the unfractionated extract. Shikonin and β,β-dimethylacrylshikonin were found to reduce cell proliferation at lower concentrations than the extract. (D) Cell viability of HF-SAR skin fibroblasts, on the contrary, was not significantly reduced when cells were incubated with the IC50 concentrations of shikonin derivatives determined for TT cells. Results were normalized to control solvent-treated cells, n = 3.
Table 1.
IC50 values of shikonin derivatives for TT cells as determined through growth inhibition assays.
| Compound | IC50 (µM) | IC50 (µg/mL) |
|---|---|---|
| AS | 2.1 ± 0.4 | 0.69 ± 0.15 |
| DMAS | 1.2 ± 0.3 | 0.44 ± 0.10 |
| SHK | 1.1 ± 0.2 | 0.32 ± 0.07 |
| EX | – | 0.56 ± 0.25 |
Data are presented as mean ± s.d. of at least three independent experiments.
AS, acetylshikonin; DMAS, β,β-dimethylacrylshikonin; EX, petroleum ether extract of O. paniculata; SHK, shikonin.
Shikonin derivatives do not impair cell viability of skin fibroblasts
Normal HF-SAR human skin fibroblasts were incubated with shikonin derivatives at the IC50 concentrations determined for TT cells. Treatment with acetylshikonin did not affect the metabolic activity of HF-SAR cells, whereas treatment with β,β-dimethylacrylshikonin, shikonin and the extract caused a slight albeit statistically non-significant reduction in cell viability (Fig. 1D).
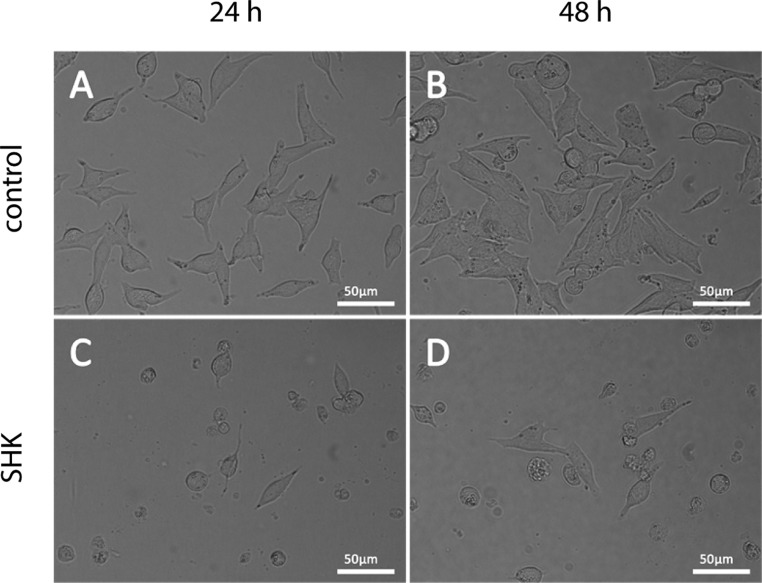
Shikonin derivatives induce morphological changes in TT cells
The reduction of cell viability and proliferation caused by treatment with shikonin derivatives was accompanied by morphological changes in TT cells. Incubation with the corresponding IC50 concentrations caused detachment and rounding of cells. Figure 2 shows images of TT cells treated with DMSO as solvent control and representative images after incubation with the IC50 concentration of shikonin derivatives.
Figure 2.
Treatment with shikonin induces morphological changes in TT cells. Cells were seeded and allowed to attach overnight before they were incubated with DMSO (A and B) or the corresponding IC50 concentration of shikonin (SHK) for 24 h (C) and 48 h (D). Scale bars, 50 µm.
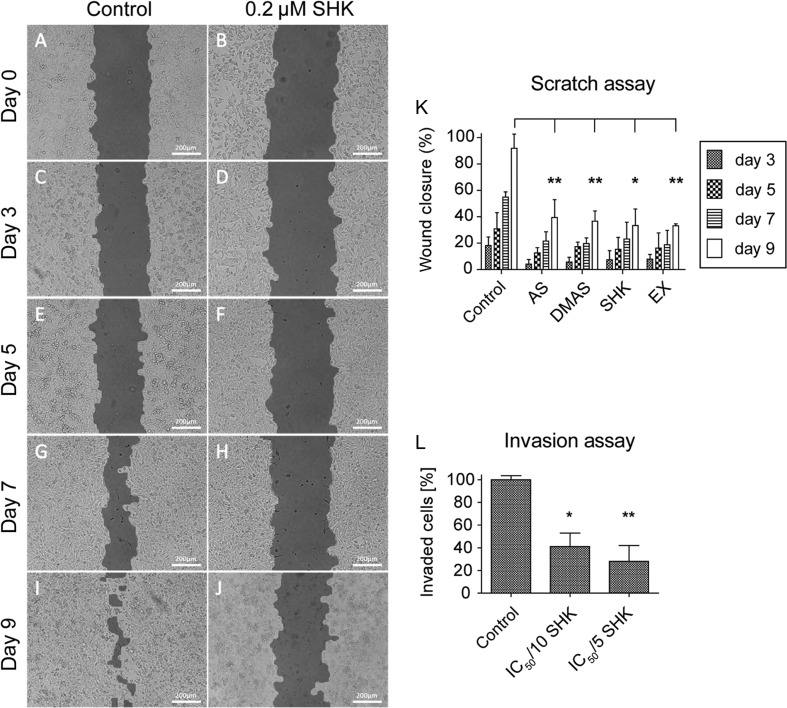
Cancer cell migration and invasion are inhibited at non-toxic concentrations
Migration of TT cells was assessed with wound-healing assays (Fig. 3A, B, C, D, E, F, G, H, I and J). Control solvent-treated TT cells were able to close the created gap within nine days, whereas treatment with shikonin derivatives at non-toxic concentrations (IC50/5) inhibited cell migration. Wound closure was significantly decreased (P < 0.05) after treatment with 0.2 µM shikonin, and very significantly (P < 0.01) after application of 0.4 µM acetylshikonin, 0.3 µM β,β-dimethylacrylshikonin or 0.15 µg/mL petroleum ether extract (Fig. 3K).
Figure 3.
Shikonin derivatives inhibit the migration and invasion of TT cells (A, B, C, D, E, F, G, H, I and J). In a monolayer wound-healing assay, solvent control-treated TT cells were able to close the gap (images on the left), whereas TT cells treated with the corresponding IC50/5 concentration of each compound failed to do so. Representative images show TT cells treated with DMSO (control) and the IC50/5, i.e. 0.2 µM shikonin (SHK, images on the right). Images were acquired immediately after the scratch was created (A and B), 3 days (C and D), 5 days (E and F), 7 days (G and H) and 9 days (I and J) later. Scale bars, 200 µm. (K) Statistical analysis of the scratch assay. All tested compounds showed similar efficiency on the inhibition of wound closure. (L) Treatment with 0.1 µM (IC50/10) shikonin reduced the invasion capacity of TT cells. AS, acetylshikonin; DMAS, β,β-dimethylacrylshikonin; SHK, shikonin; EX, O. paniculata extract; *P < 0.05, **P < 0.01, n = 3.
Additionally, the invasive behavior of TT cells was studied using Matrigel invasion assays. Invasion was significantly inhibited after application of 0.1 µM shikonin (IC50/10, P < 0.05) and 0.2 µM shikonin (IC50/5, P < 0.01) (Fig. 3L).
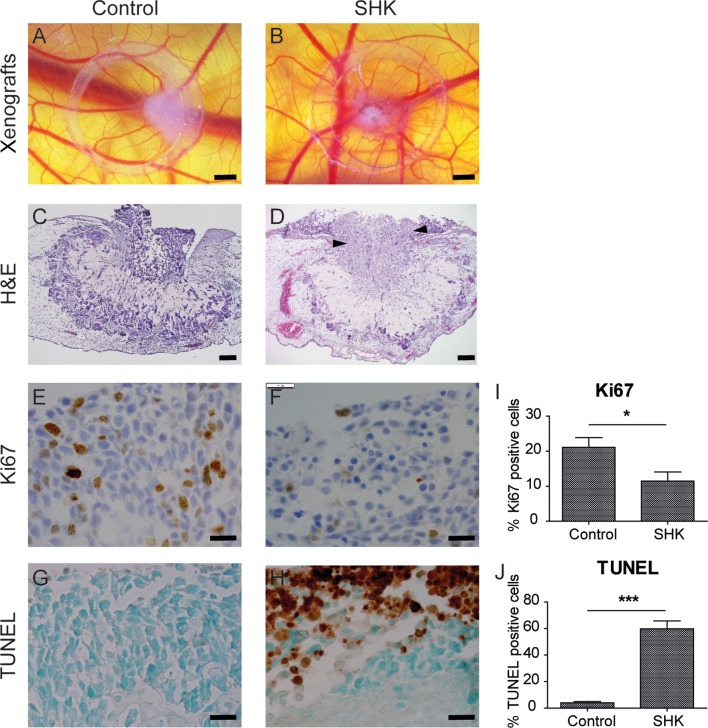
Shikonin inhibits cell proliferation and induces apoptosis in CAM xenografts
As the investigated shikonin derivatives showed promising anti-tumor effects in vitro, we next explored the effect of shikonin in a tumor xenograft of TT cells on chorioallantoic membranes (Fig. 4A and B). In this model of in vivo tumorigenesis, treatment with 2.2 µM shikonin (2 × IC50) gave cancer cells the appearance of an apoptotic phenotype without affecting the surrounding CAM (Fig. 4C and D). Immunohistochemical staining of cross-sections further revealed a significant (P < 0.05) decrease in proliferating cancer cells after shikonin treatment, as observed by Ki67 staining (Fig. 4E, F and I). Parallel sections were analyzed for TUNEL staining, which is an indicator of apoptosis. Treatment with shikonin highly significantly increased (P < 0.001) the number of TUNEL-positive TT cells compared to solvent control treatment (Fig. 4G, H and J), indicating that shikonin exerts its anti-tumorigenic effects through inhibition of cell proliferation as well as induction of apoptosis in cancer cells.
Figure 4.
Shikonin reduces cell proliferation and induces apoptosis in chicken chorioallantoic membrane xenografts (A, B, C, D, E, F, G and H). Representative images show TT xenografts treated with solvent control (control, left row, n = 12) or with 2.2 µM shikonin (SHK, right row, n = 9). (A and B) The CAM assay was used for in vitro testing of shikonin treatment of TT cells. Xenografts were photographed through a stereo microscope. Scale bars, 1 mm. (C and D) Hematoxylin–eosin (H&E) staining of cross-sections of TT grafts in the CAM. Arrows in (D) indicate apoptotic cells. Scale bars, 100 µm. (E, F, G and H) Immunohistochemical staining of TT cells grown on CAM using an antibody directed against the proliferation marker Ki67. Parallel samples were subjected to TUNEL staining. Scale bars, 20 µm. (I and J) Quantification of Ki67- and TUNEL-stained sections of TT grafts. The percentage of Ki67-positive cells was significantly (*P < 0.05) reduced after shikonin treatment, whereas the percentage of TUNEL-positive cells was highly significantly increased (***P < 0.001). Data are shown as mean ± s.e.m.
Discussion
Although medullary thyroid carcinoma is a rare hormone-producing tumor, it accounts for 13.4% of deaths attributable to thyroid cancers (22). As MTC does not respond to conventional therapy, novel treatment options are needed (1, 2, 8, 23) and compounds targeting the RET kinase are currently under investigation (24). Although promising results were achieved with other cancers, the response rates for MTC were low (25).
We investigated the effects of various shikonin derivatives, active compounds of the dried roots of Boraginaceae that are used as herbal anti-cancer medicine in China, on TT cells derived from a patient diagnosed with MEN2A (26). Shikonins are known to possess several pharmacological effects including anti-bacterial, wound-healing, anti-inflammatory and anti-cancer properties (9, 10, 11, 12, 13). The anti-cancer effects have been shown to be linked to fundamental cellular processes such as cell cycle progression, apoptosis (19, 20) and mitochondrial function (27, 28). Shikonins further affect main anti-apoptotic and pro-proliferative pathways such as MAPK and AKT signaling cascades (29, 30), but the effects on MTC cells with respect to migration and invasion as well as in vivo tumor growth have not yet been investigated. IC50 values on TT cells were found to be in the micromolar range with shikonin exhibiting the lowest IC50 of 1.1 µM. The potencies of the pure substances acetylshikonin, β,β-dimethylacrylshikonin and shikonin were compared with the unfractionated petroleum ether extract by converting the IC50 values from µM to µg/mL. Interestingly, application of the extract, which contains several shikonin derivatives (19), did not show better anti-tumor efficiency than single pure compounds. In fact, the IC50 of the extract (i.e. 0.56 ± 0.25 µg/mL) was found to be between the IC50 values determined for acetylshikonin (i.e. 0.69 ± 0.15 µg/mL) and β,β-dimethylacrylshikonin (i.e. 0.44 ± 0.10 µg/mL) as shown in Fig. 1C and Table 1. We so conclude that the main compounds of the root extract, i.e. acetylshikonin and β,β-dimethylacrylshikonin, are indeed the compounds responsible for the anti-tumor effects of the root extract but that there is no synergistic effect of the shikonin derivatives. Overall, shikonin derivatives seem to be highly efficient in the inhibition of medullary thyroid carcinoma cell proliferation as compared to e.g. papillary thyroid cancer cell lines where IC50 values of 4.20–11.50 µM have been reported (16).
In glioblastoma cells U87MG, various shikonin derivatives have been reported to have cytotoxic properties with IC50 values ranging from 3.61 µM to 51.97 µM (30). In combination with the tyrosine kinase inhibitor erlotinib, however, synergistic effects of e.g. acetylshikonin and β,β-dimethylacrylshikonin have been observed that were attributed to an inhibition of receptor tyrosine kinase phosphorylation and reduced phosphorylation of downstream signaling molecules (30). Whether synergy of shikonin derivatives with tyrosine kinase inhibitors could also be achieved in medullary thyroid carcinoma is thus of high interest for future research.
As the desired outcome in preclinical anti-cancer drug research is the induction of apoptosis in cancer cells but not in normal cells, we treated normal human skin fibroblasts with the shikonin derivatives at concentrations determined to reduce cell viability of cancer cells by 50%. We observed that these concentrations did not, or only slightly, reduce cell viability of HF-SAR cells, indicating selective cytotoxicity of shikonin and its derivatives on tumor cells.
Besides inducing apoptosis and inhibiting cell growth, the ability of anti-cancer drugs to inhibit cell invasion and metastasis is considered fundamental. Accordingly, we also examined the potential of shikonin derivatives to inhibit cell migration and invasion in vitro. We found that sub-lethal concentrations (IC50/5 and IC50/10) sufficed to inhibit cell migration and invasion of TT cells. As shown in Fig. 3K, we observed a comparative efficiency of all tested shikonin derivatives as the corresponding IC50/5 concentration reduced wound closure by 53% (acetylshikonin), 55% (β,β-dimethylacrylshikonin), 59% (shikonin) and 59% (extract), respectively.
Finally, we assessed the effects of shikonin treatment on TT xenografts. Although the application of shikonin did not affect the surrounding cells of the chicken chorioallantoic membrane, it caused a reduction of proliferating cancer cells. Concomitantly, there was a strong increase in TUNEL-stained cells after treatment with shikonin, indicating the induction of apoptosis in tumor cells. To the best of our knowledge, this is the first report of shikonin derivatives inducing apoptosis in an in vivo model of medullary thyroid carcinogenesis.
Conclusion
Shikonin and its derivatives display a wide spectrum of activity. As recently summarized by Andújar and coworkers (13), shikonin induces apoptosis in a variety of cancer cell lines but not in normal cell lines. In this study, we show that shikonin and its derivatives induce apoptosis and also inhibit cell migration and invasion in a cell line derived from medullary thyroid carcinoma, a tumor that is resistant to cytotoxic chemotherapy. Shikonin has, additionally, been shown to prevent cells from acquiring drug resistance (31), and thus, the targeted application of Onosma paniculata extracts and their active constituents could offer a new option in the treatment of MTC.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Austrian Science Fund (FWF, P27505), the Jubilee Fund of the Austrian National Bank Project #14394 and the Franz Lanyar Project #380.
Author contribution statement
R P, G S and N G T W designed the study. N K and R B provided the O. paniculata extract and extracted the pure shikonin compounds. C H and N G T W performed the experiments. All authors participated in the writing of the manuscript.
Acknowledgements
The authors thank Eugenia Lamont (Medical Editor and Translator, Medical University of Graz, Graz, Austria) for critically reading the manuscript. We gratefully thank SFL Technologies (Stallhofen, Austria) for providing us with the Olympus SZX16 stereomicroscope.
References
- 1.Schlumberger M, Carlomagno F, Baudin E, Bidart JM, Santoro M. New therapeutic approaches to treat medullary thyroid carcinoma. Nature Clinical Practice Endocrinology and Metabolism 2008. 4 22–32. ( 10.1038/ncpendmebib717) [DOI] [PubMed] [Google Scholar]
- 2.Prokopakis E, Doulaptsi M, Kaprana A, Velegrakis S, Vlastos Y, Velegrakis G. Treating medullary thyroid carcinoma in a tertiary center. Current trends and review of the literature. Hippokratia 2014. 18 130–134. [PMC free article] [PubMed] [Google Scholar]
- 3.Mulligan LM, Eng C, Healey CS, Clayton D, Kwok JB, Gardner E, Ponder MA, Frilling A, Jackson CE, Lehnert H, et al. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nature Genetics 1994. 6 70–74. ( 10.1038/ng0194-70) [DOI] [PubMed] [Google Scholar]
- 4.Eng C, Mulligan LM, Smith DP, Healey CS, Frilling A, Raue F, Neumann HP, Pfragner R, Behmel A, Lorenzo MJ, et al. Mutation of the RET protooncogene in sporadic medullary thyroid carcinoma. Genes, Chromosomes and Cancer 1995. 12 209–212. ( 10.1002/gcc.2870120308) [DOI] [PubMed] [Google Scholar]
- 5.Flicker K, Ulz P, Höger H, Zeitlhofer P, Haas OA, Behmel A, Buchinger W, Scheuba C, Niederle B, Pfragner R, et al. High-resolution analysis of alterations in medullary thyroid carcinoma genomes. International Journal of Cancer 2012. 131 E66–E73. ( 10.1002/ijc.26494) [DOI] [PubMed] [Google Scholar]
- 6.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nature Reviews Cancer 2005. 5 367–375. ( 10.1038/nrc1610) [DOI] [PubMed] [Google Scholar]
- 7.Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nature Reviews Cancer 2014. 14 173–186. ( 10.1038/nrc3680) [DOI] [PubMed] [Google Scholar]
- 8.Fialkowski EA, Moley JF. Current approaches to medullary thyroid carcinoma, sporadic and familial. Journal of Surgical Oncology 2006. 94 737–747. ( 10.1002/jso.20690) [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Yang L, Oppenheim JJ, Zack Howard OM. Cellular pharmacology studies of shikonin derivatives. Phytotherapy Research 2002. 16 199–209. ( 10.1002/ptr.1100) [DOI] [PubMed] [Google Scholar]
- 10.Papageorgiou VP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angewandte Chemie International Edition 1999. 38 270–300. () [DOI] [PubMed] [Google Scholar]
- 11.Papageorgiou VP. Wound healing properties of naphthaquinone pigments from Alkanna tinctoria. Experientia 1978. 34 1499–1501. ( 10.1007/BF01932375) [DOI] [PubMed] [Google Scholar]
- 12.Andújar I, Ríos JL, Giner RM, Recio MC. Pharmacological properties of shikonin – a review of literature since 2002. Planta Medicine 2013. 79 1685–1697. ( 10.1055/s-0033-1350934) [DOI] [PubMed] [Google Scholar]
- 13.Andújar I, Recio MC, Giner RM, Ríos JL. Traditional Chinese medicine remedy to jury: the pharmacological basis for the use of shikonin as an anticancer therapy. Current Medicinal Chemistry 2013. 20 2892–2898. ( 10.2174/09298673113209990008) [DOI] [PubMed] [Google Scholar]
- 14.Gong K, Li W. Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: a potential new treatment for hepatocellular carcinoma. Free Radical Biology and Medicine 2011. 51 2259–2271. ( 10.1016/j.freeradbiomed.2011.09.018) [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Zhou P, Huang H, Chen D, Ma N, Cui QC, Shen S, Dong W, Zhang X, Lian W, et al. Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. International Journal of Cancer 2009. 124 2450–2459. ( 10.1002/ijc.24195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Q, Ji M, Guan H, Shi B, Hou P. Shikonin inhibits thyroid cancer cell growth and invasiveness through targeting major signaling pathways. Journal of Clinical Endocrinology and Metabolism 2013. 98 1909–1917. ( 10.1210/jc.2013-2583) [DOI] [PubMed] [Google Scholar]
- 17.Guo XP, Zhang XY, Zhang SD. Clinical trial on the effects of shikonin mixture on later stage lung cancer. Chinese Journal of Modern Developments in Traditional Medicine 1991. 11 598–599, 580. [PubMed] [Google Scholar]
- 18.Wolf C, Lederer K, Pfragner R, Schauenstein K, Ingolic E, Siegl V. Biocompatibility of ultra-high molecular weight polyethylene (UHMW-PE) stabilized with alpha-tocopherol used for joint endoprostheses assessed in vitro. Journal of Materials Science Materials in Medicine 2007. 18 1247–1252. ( 10.1007/s10856-006-0098-6) [DOI] [PubMed] [Google Scholar]
- 19.Rinner B, Kretschmer N, Knausz H, Mayer A, Boechzelt H, Hao XJ, Heubl G, Efferth T, Schaider H, Bauer R. A petrol ether extract of the roots of Onosma paniculatum induces cell death in a caspase dependent manner. Journal of Ethnopharmacology 2010. 129 182–188. ( 10.1016/j.jep.2010.02.006) [DOI] [PubMed] [Google Scholar]
- 20.Kretschmer N, Rinner B, Deutsch AJA, Lohberger B, Knausz H, Kunert O, Blunder M, Boechzelt H, Schaider H, Bauer R. Naphthoquinones from Onosma paniculata induce cell-cycle arrest and apoptosis in melanoma cells. Journal of Natural Products 2012. 75 865–869. ( 10.1021/np2006499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebäck T, Schulz MMP, Koumoutsakos P, Detmar M. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques 2009. 46 265–274. ( 10.2144/000113083) [DOI] [PubMed] [Google Scholar]
- 22.Jiménez C, Hu MI-N, Gagel RF. Management of medullary thyroid carcinoma. Endocrinology and Metabolism Clinics of North America 2008. 37 481–496, x–xi. ( 10.1016/j.ecl.2008.03001) [DOI] [PubMed] [Google Scholar]
- 23.Niederle B. Screening for medullary carcinoma of the thyroid. British Journal of Surgery 2014. 101 1625–1626. ( 10.1002/bjs.9652) [DOI] [PubMed] [Google Scholar]
- 24.Gild ML, Bullock M, Pon CK, Robinson BG, Clifton-Bligh R. Destabilizing RET in targeted treatment of thyroid cancers. Endocrine Connections 2016. 5 10–19. ( 10.1530/EC-15-0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halperin DM, Phan AT, Hoff AO, Aaron M, Yao JC, Hoff PM. A phase I study of imatinib, dacarbazine, and capecitabine in advanced endocrine cancers. BMC Cancer 2014. 14 561 ( 10.1186/1471-2407-14-561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlomagno F, Salvatore D, Santoro M, de Franciscis V, Quadro L, Panariello L, Colantuoni V, Fusco A. Point mutation of the RET proto-oncogene in the TT human medullary thyroid carcinoma cell line. Biochemical and Biophysical Research Communications 1995. 207 1022–1028. ( 10.1006/bbrc.1995.1287) [DOI] [PubMed] [Google Scholar]
- 27.Wiench B, Eichhorn T, Paulsen M, Efferth T. Shikonin directly targets mitochondria and causes mitochondrial dysfunction in cancer cells. Evidence-Based Complementary and Alternative Medicine 2012. 2012 726025 ( 10.1155/2012/726025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang X, Zhang C, Wei J, Fang Y, Zhao R, J Y. Apoptosis is induced by shikonin through the mitochondrial signaling pathway. Molecular Medicine Reports 2016. 13 3668–3674. ( 10.3892/mmr.2016.4967) [DOI] [PubMed] [Google Scholar]
- 29.Zhao Q, Assimopoulou AN, Klauck SM, Damianakos H, Chinou I, Kretschmer N, Rios JL, Papageorgiou VP, Bauer R, Efferth T. Inhibition of c-MYC with involvement of ERK/JNK/MAPK and AKT pathways as a novel mechanism for shikonin and its derivatives in killing leukemia cells. Oncotarget 2015. 6 38934–38951. ( 10.18632/oncotarget.5380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Q, Kretschmer N, Bauer R, Efferth T. Shikonin and its derivatives inhibit the epidermal growth factor receptor signaling and synergistically kill glioblastoma cells in combination with erlotinib. International Journal of Cancer 2015. 137 1446–1456. ( 10.1002/ijc.29483) [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Xie J, Pan Q, Wang B, Hu D, Hu X. Anticancer agent shikonin is an incompetent inducer of cancer drug resistance. PLoS ONE 2013. 8 8–14. ( 10.1371/journal.pone.0048156) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a