Abstract
Based on high-voltage electron microscopic (HVEM) data of fixed cultured cells, an elaborate three-dimensional network of filaments, including and interconnecting other elements of the cytoskeleton, was observed in cells some half a century ago. Despite many attempts and comparative studies, this “microtrabecular lattice” (MTL) of the cytoplasmic ground substance could not be established as a genuine component of the eukaryotic cell, and is mostly considered today as a sample-preparation artifact of protein adherence and cross-linking to the cytoskeleton. Here we elaborate on the provocative idea that recent observations of hydrogel-forming phase transitions of repetitive regions of intrinsically disordered proteins (IDPs) bear resemblance in creation, organization and physical appearance to the MTL. We review this phenomenon in detail, and suggest that phase transitions of actin regulatory proteins, neurofilament side-arms and other proteins could generate non-uniform spatial distribution of cytoplasmic material in the vicinity of the cytoskeleton that might even give rise to fixation phenomena resembling the MTL. Whether such hydrogel formation by IDPs is a general physical phenomenon, will remain to be seen, nevertheless, the underlying organizational principle provokes novel experimental studies to uncover the ensuing higher-level regulation of cell physiology, in which the despised and long-forgotten concept of MTL might give some interesting leads.
Keywords: intrinsic protein disorder, unstructured protein, hydrogel transition, sol-gel transition, phase transition, low-complexity region, multivalent
Introduction: The Micrortabecular Lattice
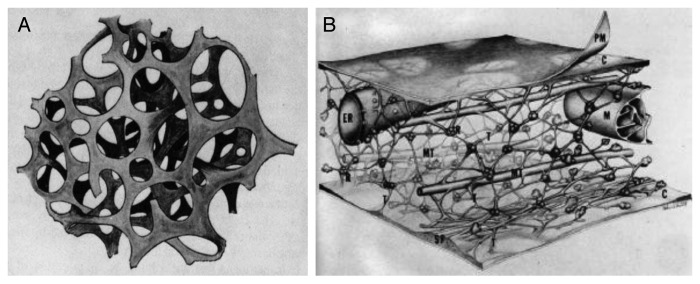
The concept of the microtrabecular lattice (MTL) arose from high-voltage electron microscopic (HVEM) studies of cultured cells following chemical (glutaraldehyde/osmium fixation, alcohol/acetone dehydration, critical-point drying) or non-chemical (freezing) fixation.1-4 In the 1970s, Porter and colleagues consistently observed a highly interwoven 3D scaffold of filamentous structures in cells, which contained and interconnected membarenous (e.g., endoplasmic reticulum) and non-membraneous (e.g., polysomes, microfilaments and microtubules) organelles of the cell, reviving much earlier views of the reticular theory of the protoplasm (Fig. 1A). The MTL was consistently reported to be made up of filaments 3–6 (up to 10) nm in diameter and varied length sometimes reaching 100–200 nm, supporting other cytoplasmic structures (Fig. 1B).
Figure 1.
Images of the microtrabecular lattice. The microtrabecular lattice was observed in HVEM images of cells following chemical or non-chemical fixation. Microtrabeculae were described as an irregular 3D lattice of filaments 3–6 nm in diameter, interconnecting membraneous and nomembraneous organelles in the cell including cytoskeletal components. (A) Its foundation was in the reticular theory of the protoplasm (living contents of a cell) based on earlier concepts and observations (original figure adapted from ref. 46). (B) Artistic rendering of the mircotrabecular lattice of cytoplasmic ground substance, shown as an interwoven 3D meshwork of filaments connecting organelles and other components of the cytoskeleton (adapted from ref. 4).
The MTL system appeared to divide the cell into a protein-rich polymerized phase and a water-rich fluid phase, which overall seemed to be responsible for the gelatinous consistency of the cytoplasm. MTL was observed in many different cell types and under many different conditions, showing some ultrastructural variations upon the application of different fixation protocols. The nonrandom distribution of individual fibers (microtrabeculae) relative to other structures in the cytoplasm made these cytoskeletal elements appear as integral components of an organized cytoplasm. It was even suggested that microtrabeculae are not static, they have the capacity to shorten and elongate, providing the force for intracellular motion. For almost two decades, however, MTL mostly remained a cytological observation4: when the lattice itself (or its components) were isolated and identified, its molecular composition showed close resemblance to the cytoskeleton.3 In accord, some doubts have always persisted whether microtrabeculae were real physical entities that represented the fundamental organization of the cytoplasm, or, rather, they arose as condensation artifacts of the cell.4,5
In EM, it was always appreciated that structures appearing due to fixation are in fact artifacts, because harsh conditions required to fix and stain the cell inevitably cause proteins in solution to condense around organelles and cytoskeletal fibers. In this sense, MTL was always thought as an artifact induced during critical point drying, which causes coagulation of proteins around distorted microfilaments.6,7 The question, rather, was “equivalence or preservation,” i.e., how much images observed in micrographs represent what was there in the cell before fixation: (1) a thickened and fixed version of the cytoskeleton itself, (2) something that contained and supported the cytoskeleton or (3) more than the cytoskeleton.4 Many approaches were taken to clarify on this artifactual nature of MTL, such as experiments with different fixation protocols and cell types, even with cytoskeleton-less erythrocytes and homogeneous protein solutions.
A major blow to the concept, however, came from freeze-etching (freeze-fracture) experiments, in which fixed cells without crosslinking showed structures consistent with the now-accepted three major components of the cytoskeleton (microfilaments, intermediate filaments and microtubules), but not with MTL fibers.5 The whole issue eventually settled around the view that microtrabecules are actually bundles of actin and intermediate filaments woven into a complex and characteristic fabric, crosslinking accessory proteins decorating and thickening cytoskeletal filaments.8 Slowly, the MTL concept became replaced by a more modern view of the cytoskeleton, which forms a three-dimensional reticulum of fibrous protein polymers throughout the cytoplasm, leaving space for relatively more soluble proteins to rather freely diffuse, while still forming transient associations with each other and the meshwork itself.5
Phase Transition and Hydrogel Formation of Polymers
Physical chemistry of sol-gel phase transitions
Putting aside for a second if MTL was real and whether recent observations of sol-gel transitions of multivalent intrinsically disordered proteins (IDPs) provide some late justification to the concept, it should be made clear that the ability of multivalent chemicals to undergo phase transitions constitutes a basic principle of polymer chemistry.9,10 Both multivalent small molecules and polymers can realize multiple interactions, of various types (both covalent or non-covalent, such as metal coordination and hydrogen bonds), which may lead to the formation of polymolecular entities. The supramolecular polymers that arise this way are reversible and dynamic materials that can modify their constitution by exchanging, recombining, and incorporating components. The resulting sharp sol-gel transitions that depend on the physical properties of the monomeric species, such as its concentration, valency and binding affinity, cause the sudden appearance of novel chemical and physical features. Depending on the balance of factors, the polymer can assume distinct physical forms varying from a phase-separated liquid to a crystalline solid.
Although somewhat less generally acknowledged and appreciated, similar multivalency of interactions is also often exploited in biological systems,11,12 where it may also cause phase transitions tuning biological activity, such as the potency of bacterial toxin inhibitors,13 binding affinity in carbohydrate-lectin systems14 or cadherin clustering in cell adhesion.15 Multivalency also seems to be critical, although less studied, in intracellular signal transduction systems.11 As detailed in this paper, sol-gel transitions emerging from multivalency, modest affinity and intrinsic flexibility appear to be critical in an ever increasing number of biological systems (Table 1) and emerge as a novel physical regulatory and organizational principle of cell physiology.
Table 1. Hydrogel-forming proteins.
| Protein | UniProt code | Function | Length | IUPred disorder (%) | References |
|---|---|---|---|---|---|
| NCK | P16333 | actin signaling | 377 | 12.73 | 23 |
| WASP | P42768 | actin signaling | 502 | 76.10 | 23 |
| nephrin | O60500 | actin signaling | 1241 | 19.90 | 23 |
| NF-H | P12036 | neurofilament subunit | 1026 | 72.71 | 21 |
| Nsp-1 | P14907 | nuclear pore complex | 823 | 81.65 | 22 |
| FUS | P35637 | RNA-binding protein | 526 | 93.92 | 25 |
| hnRNP-A1 | P09651 | RNA-binding protein | 372 | 54.03 | 25 |
| integrin-binding protein | designed | integrin binding | 251 | 100.00 | 26 |
| average | 639,75 | 63.88 |
Engineered hydrogels in practical applications
At the intersection of biology and biotechnology, these observations also open new avenues in protein engineering. The underlying physical and chemical principles foster a growing interest in creating bioinspired materials and biomaterials for a variety of applications in biomedicine.16-18 Often these are based on polymers that have extensive cross-linking potential, such as multifunctional hydrogel particles made from poly(ethylene glycol) for the multiplexed measurement of proteins,19 or various organic monomers mixed with triethylene glycol dimethacrylate (TEGDMA) cross-linker as a nonfouling and bio-inert coating of implants in contact with bodily fluids.20
In addition to chemical polymers, engineered proteins are also increasingly used and utilized in a variety of applications. Their application usually relies on two features: multivalency for multiple cross-linking and internal flexibility to enable the formation of an interwoven 3D lattice. In recent applications, leucine-zipper containing proteins (as tissue engineering scaffolds16), calmodulin (in microfluidic gates16), elastin-like peptides (in drug delivery16), nucleoporins (as artificial nanopores17), neurofilament proteins (as lubricants and adhesives17), and stem-cell differentiation factors sonic hedgehog (SHH) and ciliary neurotrophic factor (CNTF, as cell adhesive 3D patterned scaffolds18) have been described. The multivalency required for phase transition is either encoded in the protein itself, or is developed by chemical and/or photophysical means. It is hardly by chance that two of the proteins utilized for their potency in practical applications (nucleoporins and neurofilements) have been recently reported to undergo phase transition under natural conditions,21,22 which is of potential significance for their physiological functions. Such examples are discussed in detail next.
Phase Transitions of IDPs
Hydrogel (sol-gel) transitions have been reported recently in several biological systems (Table 1): the Non-catalytic region of tyrosine kinase adaptor protein-Wiskott-Aldrich Syndrome Protein (Nck/WASP),23 Nuclear pore complex (NPC) component nucleoporins,24 RNA-binding proteins involved in the formation of RNA granules,25 neurofilament side-arms,21 and modular constructs containing coiled-coil regions and integrin recognition elements.26 The systems are biologically unrelated, yet they show significant similarities that allow potential generalizations. In all the cases the proteins can adhere to each other due to their extreme flexibility (structural disorder, Fig. 2 and Table 1) through multiple contacts (multivalency, Fig. 3).
Figure 2.
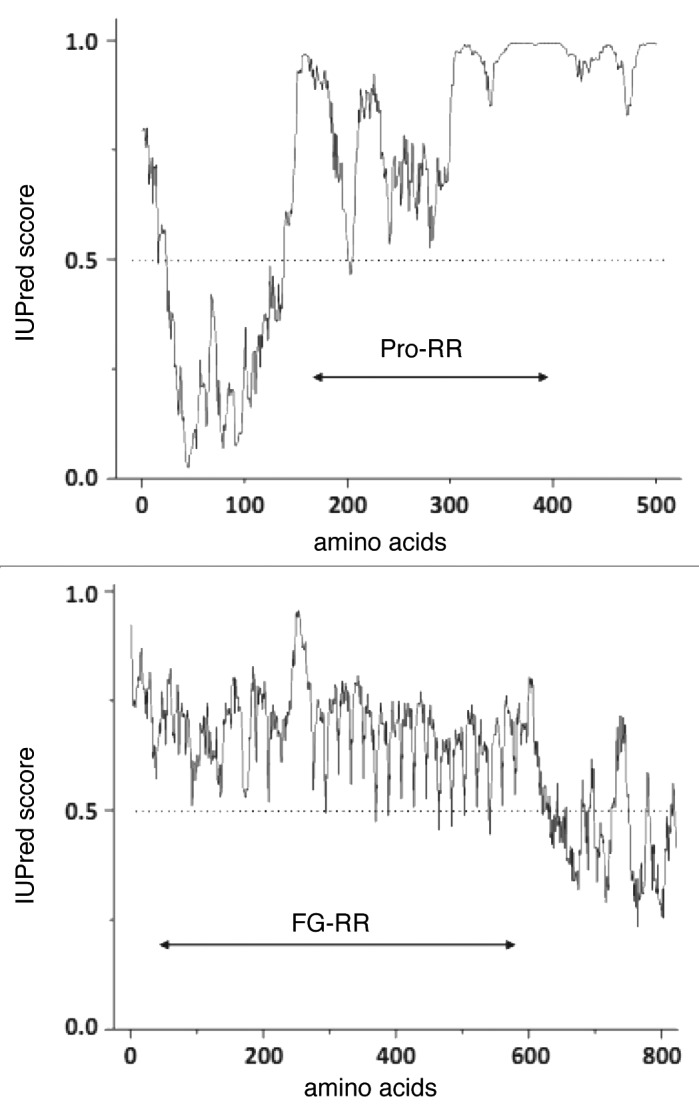
Predicted structural disorder of proteins undergoing sol-gel transition. Recently described proteins forming hydrogels appear to have little in common, with the exception of their multivalency and prevalent structural disorder. This latter is demonstrated by bioinformatic predictions using the IUPred algorithm.47,48 Two illustrative proteins described in the paper (human N-WASP (P42768)) and yeast nucleoporin NSP1 (P14907)) appear to be significantly disordered (residues with IUPred score above 0.5 are thought to fall into locally disordered regions, further predictions are given in Table 1). It is of note that the hydrogel-forming repetitive regions (proline-rich region Pro-RR in WASP and FG-repeat region FG-RR in nucleoporin) fall into completely disordered local regions.
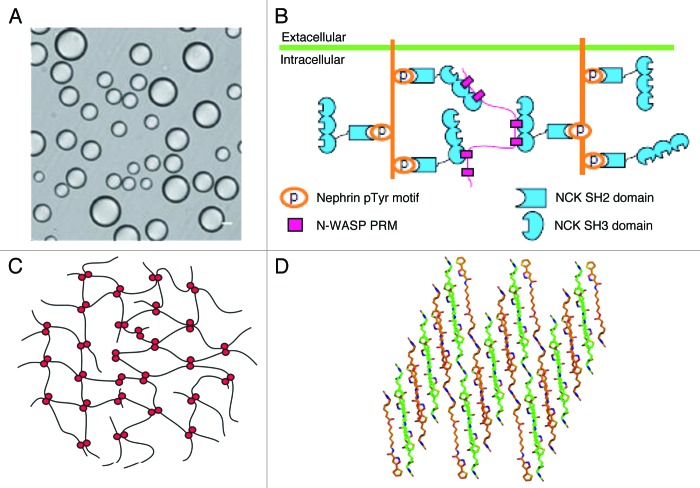
Figure 3.
Phase transition by low-complexity regions of IDPs. Liquid droplets formed of multivalent SH3-PRM (NCK/WASP) system, observed by differential interference contrast microscopy (A) probably form as a result of extensive cross-linking of three multivalent proteins (B): nephrin (multiple pTyr residues), NCK (an SH2 domain binding pTyr-motifs and three SH3 domains binding Pro-rich motifs) and N-WASP (with a long Pro-rich region with multiple Pro-rich motifs, figure adapted from ref. 23). Formation of similar gel phases has been described in other protein systems, in which the underlying multivalent interaction is chemically different, as exemplified by multiple distributed hydrophobic interactions of nucleoporins (C, adapted from ref. 24) and amyloid-type phase separation of RNA-binding proteins (D, adapted from ref. 25).
NCK-WASP system
Repetitive signaling proteins that contain complementary binding regions undergo phase transitions of potential physiological significance. Initially, it was shown that two classes of engineered proteins either composed of multiple Src-homology 3 (SH3) domains or their Pro-rich motif (PRM) ligand sequences, readily formed spherical droplets of 1 to 50 mircometer in diameter that phase-separated from the bulk solution.23 The droplets (Fig. 3A) contain copolymers of the two proteins, their formation depends on the valency of the partners, and they can be abolished by high-affinity monovalent ligands. Their formation was also demonstrated by fluorescent fusion constructs in live cells, whereas photobleaching experiments showed a thousand-fold reduced diffusion of constituents within the droplets.
A similar phase transition was observed in the natural signaling system composed of nephrin, NCK and N-WASP (Table 1). All three proteins are multivalent: the cytoplasmic tail of the transmembrane protein nephrin contains three phosphorylated Tyr (pTyr) residues, which can anchor three NCK molecules via their SH2 domains. In turn, NCK contains three SH3 domains, which can bind the six PRMs in the proline-rich region (Pro-RR) of N-WASP. In accord, the three proteins can form large dynamic supramolecular complexes, which is conducive of sol-gel transition within the droplet phase (Fig. 3B). It was found that the multivalency of proteins is necessary for the phase transition,23 the physiological importance of which might be manifested in a switch-like increase in actin-polymerizing Arp2/3 activity concordant to nephrin–NCK–N-WASP droplet formation.
Nucleoproins of the nuclear pore complex
The link between hydrogel formation and function is even more direct in the nuclear pore complex (NPC). The function of NPC is to mediate and regulate nucleocytoplasmic shuttling in the cell. The NPC is a size-selective sieving device that permits rapid passage of small (macro)molecules and cargoes bound to nuclear transport receptors (NTRs, karyopherins), but suppresses nucleocytoplasmic fluxes of macromolecules of the size above the threshold of about 30–40 kDa. Yeast NPC is made up of about 450 copies of 30 different protein subunits, termed nucleoporins (Nups).27 Thirteen Nups in yeast contain long intrinsically disordered regions (IDRs, Fig. 2, Table 1) dominated by up to 40 short clusters of hydrophobic amino acids (such as FSFG or GLFG, thus termed FG Nups).
AFM compressibility measurements and associations of FG-domain coated beads suggest that Nups can form a cohesive meshwork of filaments by transient and distributed hydrophobic interactions mediated by Phe residues of the FG repeats.28 Such inter-repeat interactions of Nups (Fig. 3C), such as Nup49p, Nup57p, Nup145p, and Nup116p, cross-link FG-repeat domains into reversible hydrogels.24 The gels are highly elastic and stable, yet they are highly dynamic to let rapid passage either of small molecules by passive diffusion or larger ones mediated by rapid association-dissociation of NTR-cargo complexes. The physiological relevance of these observations follows from several observations. The estimated local concentration of FG Nup regions in the NPC is above the critical concentration for forming a gel phase, thus the entire permeability barrier is probably organized in such a hydrogel structure.24 Phe → Ser mutants of FG Nups cannot form a hydrogel and cannot complement for the deletion of the wild-type protein, i.e., FG-repeat domains are essential for viability.24 In addition, the FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes.22
RNA-binding proteins
A range of RNA-binding proteins also form hydrogels. In eukaryotic cells, the localization and translation of mRNA is often regulated by RNA-protein assemblies termed RNA granules. The proteins in granules often have hnRN-K homology (KH) or RNA recognition motif (RRM) RNA-binding domains and low complexity regions (termed as such because their amino acid composition is dominated by a few residues and/or their sequence is repetitive in nature29,30). Many constituents of RNA granules can be precipitated from cell lysates by a small molecule (biotinylated isoxazole, b-isox), and these proteins (actually, their low-complexity regions) can undergo concentration-dependent phase transitions to a hydrogel-like state.25 Prominent proteins involved in phase transition (FUS, TIA1, FMRP, CIRBP, TDP43, CPEB2, RBM3, hnRNAP1, Sup35 and hnRNPA2) are predicted to be highly disordered (compare Table 1), and are also involved in heterotypic interaction with the hydrogel formed by other RNA-binding proteins (e.g., potentially homotypic hydrogel composed exclusively of hnRNPA2, for example, traps hnRNPA1, CIRBP and RBM3 avidly, Sup35 moderately and FUS, TIA1 and TDP43, among others, weakly).25
The structural basis of hydrogel formation is the polymerization of low-complexity regions of proteins to amyloid-like fibers of cross-β structure, as shown by X-ray diffraction and EM studies (Fig. 3D). As opposed to well-known physiologic and pathologic amyloid fibers,31 however, these hydrogel-forming amyloids are highly dynamic, they readily appear in the presence of a microcrystalline template and dissociate upon changes in protein concentration, temperature and the addition of mild denaturants. It was suggested that the phase transition based on low-complexity sequences of regulatory proteins might be a general phenomenon, representing a novel spatial organizing principle for cellular structures that are not membrane bound.25
Neurofilaments
Besides microtubules and actin (micro-) filaments, neurofilaments (NFs, intermediate filaments in other cells) comprise the third major cytoskeletal constituent of vertebrate cells. The filaments are built as mixtures of various proportions of three major subunits of different molecular weight, low (NF-L, about 60 kDa), medium (NF-M, about 100 kDa) and high (NF-H, about 120 kDa) Mw subunits. All three subunits have a short, disordered N-terminal head domain, a hydrophobic helical body (rod) region and a C-terminal intrinsically disordered side-arm or projection domain of variable length.32 In humans, this domain is about 100 amino acids in NF-L, 500 amino acids in NF-M and 600 amino acids in NF-H. The side-arms are of low-complexity, highly charged, repetitive, and intrinsically disordered (Table 1). These properties are particularly conspicuous in the case of NF-H, which is built of 30 copies of 6–8-residue repeats of the KSPEKA consensus motif. The self-assembly of NF fibrils in the cytoskeleton is driven by the helical rod region forming extensive coiled-coil regions typically comprising 32 subunits.32 From this hydrophobic core, the side-arms project radially and exert a long-range repulsive force of entropic origin, as demonstrated in AFM experiments.33 Such an “entropic brush” mechanism might ensure that a uniform and highly dynamic spacing is maintained in the cytoskeleton.34
Nerofilament side-arms, however, also do something else. It was observed in small-angle X-ray scattering (SAXS) and AFM experiments that they can also attract each-other. Although the overall charge of side-arms is negative due to frequent Glu (and phospho-Ser) residues, abundant positive charges make them behave as polyampholites, which can attract each other and form transient cross-bridges21 supporting a spaced, aligned liquid crystal hydrogel structure. The hydrogel has more than one stable structures, as, upon the application of external pressure, it converts to a more collapsed state. The apparent behavior of the gel phase is also strongly dependent on the ratio of the different subunits, with homopolymer networks forming rather stiff structures. The transition to the hydrogel structure could be satisfactorily described by an electrostatic model of attractive “handshaking” interactions between matching oppositely charged residues of side-arms.21
Integrin-binding constructs
The completely unrelated nature of the above examples and engineered hydrogels suggest that multivalency and protein disorder, under appropriate conditions, can inevitably lead to the formation of hydrogels by phase transitions. This simple principle is further tested by artificial integrin-binding intrinsically disordered protein constructs (Table 1), which might even model critical phase transitions in cell-cell communication.15
Hydrogels with integrin binding activity could be created from a range of proteins that combine three functionalities, two leucine zipper associating domains of opposite charge (acidic and basic), flanking a long disordered linker region containing nine repeats of AGAGAGPEG and three RGD integrin binding sequences.26 The proteins can self-assemble into stable hydrogels at appropriate concentrations, pH and temperature. Association occurs through the formation of coiled-coil bundles driven by opposite charges, which cross-link individual proteins into a loose meshwork. The lengthy disordered linker region remains largely disordered in the hydrogel, providing accessibility of the RGD motifs to interaction partner(s), due to which the hydrogel supports the adhesion, spreading, and polarization of fibroblast cells. Hydrogels provide close to physiological environment to cell growth, as demonstrated by confocal microscopic images of cells showing focal adhesion complexes and organized actin stress fibers in cells on protein coated surfaces. The appearance of these cellular entities was conditional on the presence of RGD motifs in the engineered proteins. It is concluded that such hydrogel-forming bioactive proteins might be exploited in cell culture applications,26 and they may even be indicative of 2D phase transitions of multivalent cell-surface cadherin receptors operating in the formation of cellular contacts.15
General Features of Hydrogel Formation
Despite the lack of biological relatedness of the above systems, there are characteristic physical similarities which might suggest that this is a general biological phenomenon. In all the examples discussed, gel formation relies on the formation of a complex network of crosslinked polymer phase, enabled by repetitive/multivalent and highly flexible (disordered) proteins.
These interactions are conditional on multivalency of binding between proteins of repetitive binding elements: [G/S]Y[G/S] repeats in the low-complexity regions of RNA-binding proteins,25 FG repeats of nuclear pore proteins,24 repetitive Pro-rich region and multiple SH3 domains in NCK/WASP,23 multiple coiled-coil dimerization regions and integrin-binding RGD motifs in integrin-binding proteins26 and highly repetitive polyampholitic regions in neurofilament side-arms.21 The multi-dentate nature of the proteins ensures that their association can be spatially extended practically indefinitely, forming the physical basis of transition from the microscopic to macroscopic physical range. The exact chemical/physical nature of the interaction hardly matters, and actually shows large variations among the examples discussed (Table 1, Fig. 3). In two cases interaction is ensured by strict binding complementarity (SH3 domains and Pro-rich motifs in NCK/WASP (Fig. 3B), and coiled-coil regions in modular integrin-binding constructs). More loose, distributed associations between multiple hydrophobic regions operate in others, such as in nucleoporins (Fig. 3C). Electrostatics provides the basis of extended network of interactions in neurofilament side-arms. In the case of RNA-binding proteins, the ultrastructural basis of network formation is the appearance of amyloid-like fibrils (Fig. 3D), which makes it rather similar in appearance to self-templating physiological prions.35
As suggested, structural disorder also seems to unite the distinct phenomena. Bioinformatic predictions (Table 1, Fig. 2) demonstrate a high incidence of structural disorder in the proteins noted in hydrogel transitions (average: 63.88%), and identification of the exact regions involved (FG repeat regions of Nups, Pro-rich region in WASP, side-arms of NFs and low-complexity regions in RNA-binding proteins, cf. Fig. 2) points to the compulsory involvement of structural disorder. It is hardly of coincidence that IDPs frequently contain repetitive elements,36,37 and due to their open and flexible nature they may also be conducive of the formation of rather dynamic and extendable systems. Although not directly related to hydrogel formation, the repetitive, multidentate and disordered nature of elastic proteins, such as elastin, also forms the basis of their self-organization into a fibrillar, highly hydrated and elastic phase that provides elasticity to tissues.38 Because IDPs/IDRs abound in higher eukaryotes,39,40 they tend to correlate with low-complexity regions29,30 and often they harbor sequence elements tandemly repeated,36,37 the sol-gel transition observed in a few cases thus far might be a rather general phenomenon.
In all the systems, the formation of the hydrogel phase seems to be involved in the functioning of the proteins. The function of NPC is to regulate nucleocytoplasmic transport, and hydrogel formation by FG-Nups is directly involved in creating a permeability barrier of the expected properties.22 In the case of RNA-binding proteins, it has been hypothesized that part of their function is to physically segregate mRNA into RNA granules, thereby spatially regulating translation.25 Interactions between neurofilament side-arms ensure regulated, dynamic and rather uniform spacing in the cytoskeleton.21 Phase transition within the NCK/WASP system regulates the availability of the Arp2/3-binding region of WASP, thereby regulating Arp2/3 activity and actin polymerization.23 Integrin-binding proteins26 and cadherins15 mediate regulated and physical cell-cell interactions.
If formation of hydrogel phases turns out to be regulated, it may also provide a basic device that would allow the cell to manipulate its physiological state. In the NSK-WASP system it was suggested that phosphorylation of Tyr-residues in nephrin and Pro-RR in WASP may directly regulate formation of the hydrogel phase.23 Phosphorylation of neurofilament side-arms also directly regulates their interactions via altering their charge distributions, and the hydrogel actually has more than one stable physical states, which may undergo regulated transitions.21 In the case of RNA-binding proteins, hydrogel formation is very sensitive to environmental conditions, such as temperature, protein concentration and the presence of the small-molecule inducer. Therefore, the hydrogel is highly dynamic and readily dissociates, which suggests that it may be amenable for regulation.25 In all, studying and understanding the existence and regulation of these gel phases might help unravel a novel layer of regulation of cell physiology.
Microtrabecular Lattice: Real at Last?
Whether these hydrogel phases appearing as a results of polymerization of multivalent proteins are truly connected with observations interpreted some 50 y ago as the MTL, is an exciting, inspiring and thought-provoking possibility, even if it turns out not to be true. The overlaps of the two distinct phenomena at least raise some conceptual parallels, as follows.
The ultrastructural appearance of MTL and hydrogels show considerable similarities: as shown, modern hydrogels come in several guises, such as oily droplets and extended gels (Fig. 3A) supported by multiple distributed interactions and cross-β structure between filaments (Fig. 3B–3D). This is not that different from what microtrabecules appeared to look like (Fig. 1). For example, structures similar to microtrabecular lattice could be observed by treating isolated actin-crosslinking proteins (α-actin, filamin) in a way similar to fixing whole cells.8 In addition, the MTL was described lately as a highly cross-linked and distorted version of the cytoskeleton, imparting to the cytoplasm its gelatinous consistency, and restraining other organelles and proteins from Brownian motion in living cells, very much like hydrogels do.23 Both MTL-restrained cytoplasm and hydrogels are described to be in a liquid jelly-like state: cohesive, plastic, deformable, and more or less rigid. Both organizational principles could deal with the paradox of the cytoplasm, i.e., that it shows at the same time the characteristics of liquids (fluidity) and solids (elasticity).
Irrespective of whether the MTL is revived by these novel observations and ideas, though, it should be taken as a conceptual lead to approach and interpret the cited - and possibly many more - observations of sol-gel transitions as a novel regulatory and organizing principle in the cytoplasm. The first steps have already been taken by the noted studies demonstrating their existence in vitro in the case of distinct proteins (Table 1). The real test of their importance, of course, is the demonstration of their in vivo existence. As evidenced by the eventual doom of the MTL concept, the transient and dynamic nature of IDP hydrogels might represent a special obstacle to be overtaken by the full arsenal of novel cell-biological structural techniques developed in the past half a century. Fluorescence studies, might have the spatial resolution and detection sensitivity required to reach this goal: it was demonstrated, for example, that fluorescence resonance energy transfer (FRET) is sufficiently sensitive to capture the interaction of IDPs in live cells.41 Hydrogel formation in cells was actually demonstrated by fluorescence approaches in the case of the NCK/WASP system.23 Cryo-electron tomography might also represent a possibility, because it has the power of resolving ultrastructural building blocks in the cytoplasm. Even the presence and structural characteristic of enigmatic prions has been demonstrated using this technique.35
Should the phase transition of IDPs appear as a general phenomenon, understanding its cellular implications is of immense interest. An obvious consequence is the physical segregation of part of the cytoplasm, i.e., the appearance of novel compartments in the cell. Compartmentalization is a unique device of eukaryotic cells, which ensures the physical and chemical separation of their components, thereby increasing complexity of regulation of their metabolism.42 The hydrogel phase enabled by IDPs/IDRs might enable such physical and/or kinetic compartmentalization. As suggested,43 signaling is dominated not by random encounters of freely diffusible proteins, rather by macroscopic complexes. Model calculations of the number of possible arrangements of the interactome also suggests that a random collision model for the interactome is untenable, the operation of the cell must be dominated by a hierarchic assembly process driven by assembly pathways and dedicated transport mechanisms.44 The gel phases described might regulate the movement of regulatory proteins in and out of organized subcellular domains, and impart transitions between distinct states. Segregating material into physically separate phases may generate nonlinearity in signaling, connect disparate length scales in the cell, and contribute to creating cellular bodies and other two- and three-dimensional compartments.23 As also raised in conjunction with the importance of MTL, cell physiology might be basically affected by altered availability, activity, and degradation of proteins by their transient associations and retarded diffusion. As shown convincingly for the NCK/WASP and other systems, the association and transition process can be regulated by post-translational modifications.23
The appearance of a transient novel gel phase might also have a profound effect on the physical behavior of the cell. It is noted that “cellular bodies,” i.e., subcellular compartments that are not membrane bounded (e.g., Cajal bodies, P bodies) are enriched in multivalent proteins and nucleic acids, within which the physical properties of a polymer could impart micrometer-scale structural and dynamic organization and could control their chemistry (for example, catalysis, molecular interactions or structural rearrangements). It may even present a way of information transfer over larger distances, and connect the length scale of molecules (nanometers) to cells (micrometers).
Conclusion
As suggested by all the examples discussed, the capacity of proteins to undergo (regulated) phase transitions may be an important regulatory device of the cell. The transition is enabled by multiple distributed interactions between repetitive/low complexity regions of proteins, which tend to by structurally disordered. Sequence analysis of the proteome shows the prevalence of structural disorder39,40 and a high preponderance of repetitive regions.36,37,45 The function of disordered proteins is traditionally thought to stem directly from their disorder (entropic chains) or from molecular recognition. The hydrogel transition described in these papers can be considered as a variant of the two, enabled by their extended homo- and heterotypic interactions.
By the limited evidence available to date, this phase transition phenomenon seems conceptually connected with the extinct MTL concept. Whereas today it is considered as an artifact of fixation, it was thought to arise via cross-linking of elements of cytoskeleton (microfilaments and intermediate filaments) and proteins in their proximity, perhaps already in loose association with them. It is of note that several hydrogel-forming IDPs are related to the cytoskeleton (actin regulatory proteins and neurofilament side-arms). That is, even if the MTL system as suggested decades ago does not exist, distinct yet coherent observations of phase transitions of repetitive disordered IDPs/IDRs calls for a revival/extension of our concepts of the physical inhomogeneity of cytoplasmic constituents. The long-gone concept of the microtrabecular lattice calls for appropriate approaches for their visualization and characterization in living cells, in order to uncover thus far unappreciated physical means of regulation of cell physiology.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Odysseus grant G.0029.12 from Research Foundation Flanders (FWO).
References
- 1.Byers HR, Porter KR. . Transformations in the structure of the cytoplasmic ground substance in erythrophores during pigment aggregation and dispersion. I. A study using whole-cell preparations in stereo high voltage electron microscopy. J Cell Biol 1977; 75:541 - 58; http://dx.doi.org/ 10.1083/jcb.75.2.541; PMID: 264122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolosewick JJ, Porter KR. . Stereo high-voltage electron microscopy of whole cells of the human diploid line, WI-38. Am J Anat 1976; 147:303 - 23; http://dx.doi.org/ 10.1002/aja.1001470305; PMID: 988741 [DOI] [PubMed] [Google Scholar]
- 3.Schliwa M, van Blerkom J, Porter KR. . Stabilization and the cytoplasmic ground substance in detergent-opened cells and a structural and biochemical analysis of its composition. Proc Natl Acad Sci U S A 1981; 78:4329 - 33; http://dx.doi.org/ 10.1073/pnas.78.7.4329; PMID: 6945586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolosewick JJ, Porter KR. . Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol 1979; 82:114 - 39; http://dx.doi.org/ 10.1083/jcb.82.1.114; PMID: 479294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heuser J. . Whatever happened to the ‘microtrabecular concept’?. Biol Cell 2002; 94:561 - 96; http://dx.doi.org/ 10.1016/S0248-4900(02)00013-8; PMID: 12732437 [DOI] [PubMed] [Google Scholar]
- 6.Ris H. . The cytoplasmic filament system in critical point-dried whole mounts and plastic-embedded sections. J Cell Biol 1985; 100:1474 - 87; http://dx.doi.org/ 10.1083/jcb.100.5.1474; PMID: 4039327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolosewick JJ, Condeelis J. . Fine structure of gels prepared from an actin-binding protein and actin: comparison to cytoplasmic extracts and cortical cytoplasm in amoeboid cells of cortical cytoplasm in amoeboid cells of Dictyostelium discoideum. J Cell Biochem 1986; 30:227 - 43; http://dx.doi.org/ 10.1002/jcb.240300305; PMID: 3700494 [DOI] [PubMed] [Google Scholar]
- 8.Heuser JE, Kirschner MW. . Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol 1980; 86:212 - 34; http://dx.doi.org/ 10.1083/jcb.86.1.212; PMID: 6893451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen RJ, Benedek GB. . Equilibrium and kinetic theory of polymerization and the sol-gel transition. J Phys Chem 1982; 86:3696 - 714; http://dx.doi.org/ 10.1021/j100216a005 [DOI] [Google Scholar]
- 10.Lehn J-M. . Supramolecular polymer chemistry—scope and perspectives. Polym Int 2002; 51:825 - 39; http://dx.doi.org/ 10.1002/pi.852 [DOI] [Google Scholar]
- 11.Pawson T, Nash P. . Assembly of cell regulatory systems through protein interaction domains. Science 2003; 300:445 - 52; http://dx.doi.org/ 10.1126/science.1083653; PMID: 12702867 [DOI] [PubMed] [Google Scholar]
- 12.Cohen P. . The regulation of protein function by multisite phosphorylation--a 25 year update. Trends Biochem Sci 2000; 25:596 - 601; http://dx.doi.org/ 10.1016/S0968-0004(00)01712-6; PMID: 11116185 [DOI] [PubMed] [Google Scholar]
- 13.Sisu C, Baron AJ, Branderhorst HM, Connell SD, Weijers CA, de Vries R, et al. . The influence of ligand valency on aggregation mechanisms for inhibiting bacterial toxins. Chembiochem 2009; 10:329 - 37; http://dx.doi.org/ 10.1002/cbic.200800550; PMID: 19034953 [DOI] [PubMed] [Google Scholar]
- 14.Dam TK, Oscarson S, Roy R, Das SK, Pagé D, Macaluso F, et al. . Thermodynamic, kinetic, and electron microscopy studies of concanavalin A and Dioclea grandiflora lectin cross-linked with synthetic divalent carbohydrates. J Biol Chem 2005; 280:8640 - 6; http://dx.doi.org/ 10.1074/jbc.M412827200; PMID: 15632152 [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Vendome J, Shapiro L, Ben-Shaul A, Honig B. . Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature 2011; 475:510 - 3; http://dx.doi.org/ 10.1038/nature10183; PMID: 21796210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banta S, Wheeldon IR, Blenner M. . Protein engineering in the development of functional hydrogels. Annu Rev Biomed Eng 2010; 12:167 - 86; http://dx.doi.org/ 10.1146/annurev-bioeng-070909-105334; PMID: 20420519 [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan N, Kumar S. . Ordered and disordered proteins as nanomaterial building blocks. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2012; 4:204 - 18; http://dx.doi.org/ 10.1002/wnan.1160; PMID: 22231983 [DOI] [PubMed] [Google Scholar]
- 18.Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, Morshead CM, Shoichet MS. . Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat Mater 2011; 10:799 - 806; http://dx.doi.org/ 10.1038/nmat3101; PMID: 21874004 [DOI] [PubMed] [Google Scholar]
- 19.Appleyard DC, Chapin SC, Srinivas RL, Doyle PS. . Bar-coded hydrogel microparticles for protein detection: synthesis, assay and scanning. Nat Protoc 2011; 6:1761 - 74; http://dx.doi.org/ 10.1038/nprot.2011.400; PMID: 22015846 [DOI] [PubMed] [Google Scholar]
- 20.Dobbins SC, McGrath DE, Bernards MT. . Nonfouling hydrogels formed from charged monomer subunits. J Phys Chem B 2012; 116:14346 - 52; http://dx.doi.org/ 10.1021/jp307588b; PMID: 23189949 [DOI] [PubMed] [Google Scholar]
- 21.Beck R, Deek J, Safinya CR. . Structures and interactions in ‘bottlebrush’ neurofilaments: the role of charged disordered proteins in forming hydrogel networks. Biochem Soc Trans 2012; 40:1027 - 31; http://dx.doi.org/ 10.1042/BST20120101; PMID: 22988859 [DOI] [PubMed] [Google Scholar]
- 22.Frey S, Görlich D. . A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 2007; 130:512 - 23; http://dx.doi.org/ 10.1016/j.cell.2007.06.024; PMID: 17693259 [DOI] [PubMed] [Google Scholar]
- 23.Li P, Banjade S, Cheng H-C, Kim S, Chen B, Guo L, et al. . Phase transitions in the assembly of multivalent signalling proteins. Nature 2012; 483:336 - 40; http://dx.doi.org/ 10.1038/nature10879; PMID: 22398450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frey S, Richter RP, Görlich D. . FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 2006; 314:815 - 7; http://dx.doi.org/ 10.1126/science.1132516; PMID: 17082456 [DOI] [PubMed] [Google Scholar]
- 25.Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, et al. . Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012; 149:753 - 67; http://dx.doi.org/ 10.1016/j.cell.2012.04.017; PMID: 22579281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mi L, Fischer S, Chung B, Sundelacruz S, Harden JL. . Self-assembling protein hydrogels with modular integrin binding domains. Biomacromolecules 2006; 7:38 - 47; http://dx.doi.org/ 10.1021/bm050157p; PMID: 16398496 [DOI] [PubMed] [Google Scholar]
- 27.Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, et al. . The molecular architecture of the nuclear pore complex. Nature 2007; 450:695 - 701; http://dx.doi.org/ 10.1038/nature06405; PMID: 18046406 [DOI] [PubMed] [Google Scholar]
- 28.Patel SS, Belmont BJ, Sante JM, Rexach MF. . Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell 2007; 129:83 - 96; http://dx.doi.org/ 10.1016/j.cell.2007.01.044; PMID: 17418788 [DOI] [PubMed] [Google Scholar]
- 29.Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, Dunker AK. . Sequence complexity of disordered protein. Proteins 2001; 42:38 - 48; http://dx.doi.org/; PMID: 11093259 [DOI] [PubMed] [Google Scholar]
- 30.Wootton JC. . Non-globular domains in protein sequences: automated segmentation using complexity measures. Comput Chem 1994; 18:269 - 85; http://dx.doi.org/ 10.1016/0097-8485(94)85023-2; PMID: 7952898 [DOI] [PubMed] [Google Scholar]
- 31.Chiti F, Dobson CM. . Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 2006; 75:333 - 66; http://dx.doi.org/ 10.1146/annurev.biochem.75.101304.123901; PMID: 16756495 [DOI] [PubMed] [Google Scholar]
- 32.Janmey PA, Leterrier JF, Herrmann H. . Assembly and structure of neurofilaments. Curr Opin Colloid Interface Sci 2003; 8:40 - 7; http://dx.doi.org/ 10.1016/S1359-0294(03)00010-4 [DOI] [Google Scholar]
- 33.Brown HG, Hoh JH. . Entropic exclusion by neurofilament sidearms: a mechanism for maintaining interfilament spacing. Biochemistry 1997; 36:15035 - 40; http://dx.doi.org/ 10.1021/bi9721748; PMID: 9424114 [DOI] [PubMed] [Google Scholar]
- 34.Mukhopadhyay R, Hoh JH. . AFM force measurements on microtubule-associated proteins: the projection domain exerts a long-range repulsive force. FEBS Lett 2001; 505:374 - 8; http://dx.doi.org/ 10.1016/S0014-5793(01)02844-7; PMID: 11576531 [DOI] [PubMed] [Google Scholar]
- 35.Saibil HR, Seybert A, Habermann A, Winkler J, Eltsov M, Perkovic M, et al. . Heritable yeast prions have a highly organized three-dimensional architecture with interfiber structures. Proc Natl Acad Sci U S A 2012; 109:14906 - 11; http://dx.doi.org/ 10.1073/pnas.1211976109; PMID: 22927413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lise S, Jones DT. . Sequence patterns associated with disordered regions in proteins. Proteins 2005; 58:144 - 50; http://dx.doi.org/ 10.1002/prot.20279; PMID: 15476208 [DOI] [PubMed] [Google Scholar]
- 37.Tompa P. . Intrinsically unstructured proteins evolve by repeat expansion. Bioessays 2003; 25:847 - 55; http://dx.doi.org/ 10.1002/bies.10324; PMID: 12938174 [DOI] [PubMed] [Google Scholar]
- 38.Rauscher S, Baud S, Miao M, Keeley FW, Pomès R. . Proline and glycine control protein self-organization into elastomeric or amyloid fibrils. Structure 2006; 14:1667 - 76; http://dx.doi.org/ 10.1016/j.str.2006.09.008; PMID: 17098192 [DOI] [PubMed] [Google Scholar]
- 39.Pancsa R, Tompa P. . Structural disorder in eukaryotes. PLoS One 2012; 7:e34687; http://dx.doi.org/ 10.1371/journal.pone.0034687; PMID: 22496841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. . Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol 2004; 337:635 - 45; http://dx.doi.org/ 10.1016/j.jmb.2004.02.002; PMID: 15019783 [DOI] [PubMed] [Google Scholar]
- 41.Sakon JJ, Weninger KR. . Detecting the conformation of individual proteins in live cells. Nat Methods 2010; 7:203 - 5; http://dx.doi.org/ 10.1038/nmeth.1421; PMID: 20118931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ovádi J, Srere PA. . Macromolecular compartmentation and channeling. Int Rev Cytol 2000; 192:255 - 80; http://dx.doi.org/ 10.1016/S0074-7696(08)60529-X; PMID: 10553282 [DOI] [PubMed] [Google Scholar]
- 43.Gibson TJ. . Cell regulation: determined to signal discrete cooperation. Trends Biochem Sci 2009; 34:471 - 82; http://dx.doi.org/ 10.1016/j.tibs.2009.06.007; PMID: 19744855 [DOI] [PubMed] [Google Scholar]
- 44.Tompa P, Rose GD. . The Levinthal paradox of the interactome. Protein Sci 2011; 20:2074 - 9; http://dx.doi.org/ 10.1002/pro.747; PMID: 21987416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlin S, Brocchieri L, Bergman A, Mrazek J, Gentles AJ. . Amino acid runs in eukaryotic proteomes and disease associations. Proc Natl Acad Sci U S A 2002; 99:333 - 8; http://dx.doi.org/ 10.1073/pnas.012608599; PMID: 11782551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dahlgren U, Kepner WA. A text-book of the principles of animal histology. New York: The Macmillan Company, 1908. [Google Scholar]
- 47.Dosztányi Z, Csizmók V, Tompa P, Simon I. . The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J Mol Biol 2005; 347:827 - 39; http://dx.doi.org/ 10.1016/j.jmb.2005.01.071; PMID: 15769473 [DOI] [PubMed] [Google Scholar]
- 48.Dosztányi Z, Csizmok V, Tompa P, Simon I. . IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 2005; 21:3433 - 4; http://dx.doi.org/ 10.1093/bioinformatics/bti541; PMID: 15955779 [DOI] [PubMed] [Google Scholar]