Abstract
Objective
Immune cells play a critical role in atherosclerosis. Co-stimulatory and co-inhibitory molecules of the tumor necrosis factor receptor and CD28 immunoglobulin superfamilies shape T cell and B cell responses, but also have a major effect on antigen presenting cells and non-immune cells.
Approach and Results
Pharmacological inhibition or activation of co-stimulatory and co-inhibitory molecules as well as genetic deletion demonstrated their involvement in atherosclerosis. This review highlights recent advances in understanding how co-stimulatory and co-inhibitory pathways shape the immune response in atherosclerosis.
Conclusion
Insights gained from co-stimulatory and co-inhibitory molecule function in atherosclerosis may inform future therapeutic approaches.
Graphical Abstract
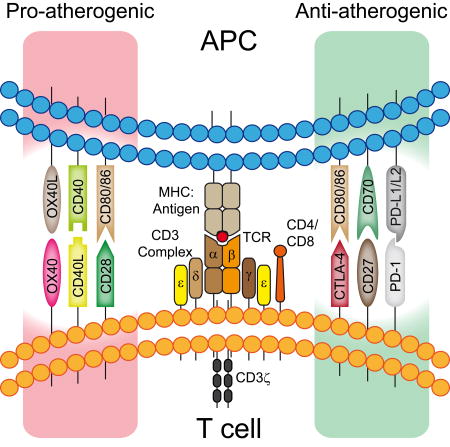
1. A brief introduction to atherosclerosis
Atherosclerosis is a chronic inflammatory disease of the artery wall. The resulting clinical events are the leading cause of death worldwide.1 The factors contributing to atherosclerosis are multifaceted, encompassing environmental and genetic risk factors, perturbed cholesterol homeostasis, and a lingering immune response that all influence atherogenesis, plaque progression, vascular dysfunction and ultimately plaque rupture or erosion, the proximal causes of major adverse cardiovascular events (MACE).2–5 Dyslipidemia and endothelial dysfunction promote the increased influx and retention of lipoprotein particles including low-density lipoprotein (LDL).6 Adhesion molecules are expressed by activated endothelial cells (EC) preferentially at sites of disturbed blood flow.7, 8 Furthermore, the modification of retained lipoproteins (e.g. oxidation) can induce a low-grade immune response involving activation activating endothelial cells.5, 6 Adhering leukocytes, predominately inflammatory monocytes, transmigrate into the subendothelial space, and contribute to the pro-inflammatory micromilieu by secretion of chemokines that further increase recruitment of monocytes, neutrophils, and lymphocytes from the circulation.9 Some monocytes differentiate into macrophages that scavenge the trapped lipoprotein particles and transform into foam cells. In addition to their recruitment, macrophages can also undergo local proliferation. Yet, their egress may be prevented by retention signals, thus also contributing to growth of the atherosclerotic lesion.10, 11 As the capacity to clear or store lipids is exceeded in these cells, they can undergo apoptosis. When the uptake of apoptotic cells (efferocytosis) fails these cells undergo secondary necrosis and form the acellular necrotic core of atherosclerotic lesions. Retained lipoprotein particles such as LDL undergo oxidation and other modifications. This makes them ligands for scavenger receptors (SR) like CD36, SR-A, SR-B and toll-like receptors (TLRs), which activate various pro-inflammatory signaling pathways that induce co-stimulatory molecules. Modified LDL presents lipid neoepitopes, the role of which in atherogenesis is poorly understood.12, 13
Dendritic cells (DC) are key players in bridging innate and adaptive immunity. Lymph node and spleen DCs are most capable in presenting antigen to naïve T cells. A network of vascular DCs is found in the arterial intima of healthy individuals and the frequency of DCs in arteries increases further during the course of atherosclerosis.6, 14 DCs can also take up lipids and contribute to foam cell formation.15, 16 Although foam cells seem not to leave the progressive atherosclerotic lesion, monocyte-derived DCs are able to leave atherosclerotic lesions in regression.17 Most monocyte-derived cells in atherosclerotic lesions express high levels of major histocompatibility complex class II (MHC-II), which is required for presentation of peptide antigens to CD4+ T cells. These mechanisms provide the basics for activation of T cells in atherosclerotic lesions and recall responses to both, model antigens and atherosclerosis antigens, which have been demonstrated in mouse arteries using multiphoton imaging.18, 19 B cells found in the adventitia also express MHC-II.20, 21 In general, B cells can process soluble and membrane-associated antigen after their antigenic activation via the B cell receptor. In vivo imaging demonstrated CCR7-dependent migration of antigen-specific B cells22 to the B cell T cell boundary in lymph nodes after antigen-encounter where these cells engaged in interactions with antigen-specific T cells for up to 60 min.23 B cells are important antigen-presenting cells, but less able to present antigen to naïve T cells than DCs.24 The reconstitution of μmt−/− mice lacking B cells with B cells derived from MhcII−/− mice resulted in an impaired antigen-specific T cell response, demonstrating that antigen presentation by B cells contributes to T cell activation.25 The B cell specific deletion of MHC-II did not alter numbers of T cells, but reduced the frequency of activated CD4+ and CD8+ T cells in a mouse model of lupus which was accompanied by amelioration of disease and improved kidney function.26 Similarly, mice were protected from experimental autoimmune encephalitis and displayed reduced Th1 and Th17 responses when B cells were devoid of MHC-II expression.27 However, the role of antigen presentation by B cells in atherosclerosis is unknown.
Antibodies specific for plaque-restricted antigens such as oxLDL were detected in human atherosclerotic plaques.13 Further antigens detected by antibodies in atherosclerosis are HSP60 and HSP65.28 Lipid peroxidation-derived neoepitopes are found on the surface of oxidized (ox)LDL.29 Spectratyping analysis of the T cell receptor (TCR) repertoire in atherosclerotic lesions revealed a limited variety of TCRs, which is the hallmark of an oligoclonal T cell response.30 Unfortunately, TCR spectratyping allows no conclusions about the nature of the antigenic epitopes. The presence of oligoclonal T cells in atherosclerotic lesions indicates the presence of an adaptive immune response mounted against atherosclerosis-relevant antigens. Such a response usually requires the migration of antigen presenting cells (APC) such as DCs carrying plaque-derived antigens to lymph nodes, although direct proof for such activity in atherosclerosis is still missing. DCs can foster the development of atherosclerosis by modulating the differentiation of effector T cells.18 Furthermore, ex vivo aorta cultures demonstrated substantial interactions between antigen-loaded DC and antigen-specific T cells within the aortic lesion.19 Recent evidence suggests peptide moieties of apolipoprotein B (ApoB)-100 as major atherosclerosis-specific antigens that are MHC-II restricted.31–33 To produce an effective immune response, CD4+ T cells must recognize antigens in the presence of co-stimulatory signals, which are the subject of this review.
2. CD4+ T cells in atherosclerosis
Most lymphocytes in murine atherosclerotic lesions are CD4+ T cells, whereas human atherosclerotic lesions have equal numbers of CD4+ and CD8+ T cells.34, 35 Many lesional CD4+ T cells secrete interferon gamma (IFNγ), and mice deficient for the Th1 lineage transcription factor T-bet or the Th1 cytokine IFNγ display reduced atherosclerotic development.36, 37 Many of these cells express both T-bet and low levels of the transcription factor Foxp3, suggesting that these cells may derive from regulatory T cells (Tregs).38, 39 The contribution of Th2 cells in atherogenesis is unclear. The key cytokines produced by Th2 cells are IL-4, IL-5, IL-10, and IL-13, all of which can contribute to atheroprotection.40, 41 However, depending on the atherosclerotic mouse model studied, IL-4 either enhanced or decreased atherosclerotic lesion formation.40, 42 Similarly, the role of Th17 cells in atherosclerosis is controversial, as some studies ascribe Th17 cells a pro-atherogenic function whereas others suggest an athero-protective role for IL-17, the hallmark cytokine of Th17 cells.43–46
Tregs, a subset of CD4+ T cells, suppress the proliferation of effector CD4+ T cells in response to antigen presentation. Tregs are subdivided into natural Tregs (nTregs) and induced Tregs (iTregs). nTregs are generated in the thymus by selection against self-antigens if the signal strength provided by the T cell receptor is low to intermediate47, 48, whereas iTregs are generated in response to transforming growth factor beta (TGFβ) in the periphery.49 Tregs are potent anti-atherogenic cells and exert their function by multiple effector mechanisms50, most prominently by secretion of the anti-inflammatory cytokines IL-10 and TGFβ. Deficiency of these cytokines either systemically or in T cells was shown to be pro-atherogenic.51, 52 The adoptive transfer of Tregs ameliorated atherosclerosis53 whereas depletion of Tregs exacerbated atherosclerosis.54 However, this was accompanied by altered liver lipid metabolism, which makes the data difficult to interpret.54 In vitro, both differentiation of Th17 cells and Tregs from naïve T cells requires the cytokine TGFβ. Atherosclerotic lesions of hyperlipidemic Ldlr−/− mice demonstrated a significant loss of Tregs at advanced stages of atherosclerosis.55 Additionally, emerging data suggest that Tregs may convert to Th17 cells in mice and humans as atherosclerosis progresses.38, 39, 56–58 Tregs in atherosclerotic lesions also can acquire a Th1-like phenotype associated with IFNγ production and loss of suppressive capacity.31, 32 However, more work is needed to provide conclusive evidence and underlying mechanisms of Treg plasticity in atherosclerosis.
3. Co-stimulatory pathways in atherosclerosis
A functional T cell response requires not only recognition of MHC:antigen complexes via the antigen-specific TCR but also the integration of co-stimulatory signals exceeding a certain threshold.59 These signals are important during different stages of a T cell response including clonal expansion, skewing towards T cell effector phenotypes and enhancing T cell survival in primary and secondary immune reactions. Two main families of co-stimulatory molecules are instrumental for these processes: the Ig-like CD28 family and the tumor necrosis factor receptor superfamily (TNFRSF). In general, the expression and functionality of co-stimulatory and co-inhibitory is conserved between mice and men. Important examples of functional differences between species will be given below.60
3.1 CD28 – CD80/CD86
CD28 on naïve T cells binds to CD80 and CD86 on APCs. Although CD80 is constitutively expressed on many APCs, CD86 is strongly induced by inflammatory stimuli such as TLR agonists derived from pathogens (e.g. LPS).61 Interactions of CD28 with CD80/CD86 are essential to induce a T cell response including proliferation of effector cells and memory formation. Of note, almost all murine CD4+ and CD8+ T cells express CD28, whereas only 80% of human CD4+ T cells and 50% of CD8+ T cells express CD28.62 The absence of CD28 stimulation during an antigen-specific stimulus renders T cells anergic, leading to irreversible unresponsiveness to their cognate antigen.63 The activation of T cells occurs physiologically only when the T cell receives a TCR and co-stimulatory signal. Superagonistic CD28 antibodies were thought to have beneficial effects and advanced to clinical trials, but they caused a severe cytokine storm in healthy volunteers, probably by the activation of tissue memory CD4+ T cells.64 This cytotoxic effect was not apparent in rodents where a Treg response was favored after transient lymphocytosis.65 The expression of CD80 and CD86 is increased on monocyte-derived DC of patients with cardiovascular disease.66 Expression of CD80 and CD86 strongly correlates with lesional inflammation and plaque vulnerability.67, 68 Similarly, atherosclerotic lesions of hypercholesterolemic mice exhibited T cells, DC, and macrophages expressing CD28 and CD80/CD86, respectively.69
Combined deficiency of CD80 and CD86 in atherosclerotic Ldlr−/− mice reduced atherosclerotic burden, which was accompanied by decreased abundance of MHC-II-expressing APC in atherosclerotic lesions and a reduced Th1 response.69 However, lethally-irradiated Ldlr−/− mice transplanted with Cd28−/− or Cd80−/−Cd86−/− bone marrow developed 2-fold larger lesions compared to control mice transplanted with wildtype bone marrow. This accelerated lesion development was likely caused by impaired Treg development and uncontrolled T cell effector response.53 Another costimulatory molecule of the CD28 superfamily is CD83. DC in human atherosclerotic lesions express CD83 and the content of CD83+ mature DC increased significantly in unstable plaques as compared to stable ones.70, 71 CD83 regulates B cell activation and germinal center responses72 as well as CD4+ T cell development.73, 74 Although largely expressed by activated APCs, the costimulatory function of CD83 seems to be dispensable for murine T cell activation whilst CD83-stimulated human monocytes suppressed T cell responses.75, 76 However, the role and function of CD83 in cardiovascular disease is so far unidentified.
3.2 TNFSF and TNFRSF members
3.2.1 CD40 – CD40L
CD40 (TNFRSF5) expression was first discovered on APC, especially B cells.77 However, a plethora of immune and non-immune cells expresses CD40 with different functions exerted upon activation by its ligand CD40L (CD154, TNFSF5).78, 79 The co-stimulatory CD40-CD40L axis is an important master regulator of immune processes. Among others, germinal center formation and especially immunoglobulin class switching in B cells is highly-dependent on CD40L expression by T cells. DCs receiving CD40L-mediated signals are more potent in antigen presentation and inducing a T cell response.80–83. CD40 is mediating T cell memory formation and induces the expression of inflammatory cytokines (TNFα, IL-1α, IL-1β, IL-6, IL-8, and IL-12), chemokines (CCL2, CCL3, CCL5) and matrix-degrading enzymes by monocytes and macrophages.84–88 CD40-CD40L interactions also drive the expression of co-stimulatory molecules such as CD80, CD86, and CD70. Moreover, CD40-CD40L promotes CD8+ T cell activation even without the need for CD4+ T cell help.84, 89
A genetic polymorphism in the 5′ UTR of CD40 was enriched in a case-control study of Chinese patients with acute coronary syndrome and ischemic stroke.90, 91 Furthermore, the rs1535045-T allele of the CD40 locus positively correlated with cardiovascular disease and plasma cholesterol levels in a Chinese Han population.92
Pharmacological inhibition of CD40-CD40L interactions or global deficiency reduced atherosclerotic burden accompanied by stable lesion formation in murine models of atherosclerosis.93–96 CD40L expressed by activated thrombocytes fosters recruitment of monocytes to the inflamed endothelium and is important for platelet-leukocyte aggregate formation, which can contribute to atheroprogression.97, 98 Furthermore, platelet CD40L expression reduced the abundance of atheroprotective Tregs, further contributing to inflammation.98 CD40 expression by platelets sustained atherosclerosis by increasing adhesion molecule expression of EC and subsequent increased adhesion of leukocytes.99
Pharmacological inhibition of CD40-CD40L interactions is an attractive target, but clinical trials were discontinued because of severe thromboembolic complications as discussed below.
On a cautionary note, transplantation of bone marrow deficient for co-stimulatory or inflammatory molecules into Ldlr−/− mice often yields results opposing studies using the corresponding compound deficient mice or respective blocking antibodies.53, 69, 93, 100, 101
In particular, lesion size between Ldlr−/− mice Ldlr−/− Cd40−/− mice was comparable102 whereas transplantation of Cd40−/− bone marrow into Ldlr−/− mice reduced lesion formation.103 Apoe−/− mice deficient for CD40 also harbored smaller lesions as compared to Apoe−/− control mice which is likely based on defective tumor necrosis factor receptor-associated factor (TRAF)6 signaling.103 The underlying cause for the observed discrepancies is not clear and might be based on the varying kinetics of atherosclerosis between the mouse models involved. Apoe−/− mice develop spontaneous atherosclerosis whereas Ldlr−/− mice need to receive a cholesterol-enriched diet. The comparison of mouse models of atherosclerosis is beyond the scope of this review and has been summarized elsewhere.104, 105
3.2.2 CD27 – CD70
The co-stimulatory molecules CD27 (TNFRSF7) and CD70 (TNFSF7) play important roles during the establishment of long-term T cell immunity.106 Naïve T cell express CD27, which is increased after TCR engagement with cognate peptide MHC complexes. CD27 is lost from the cell surface by proteolytic shedding, but long-lived central memory cells express CD27 again.107 Apart from T cells, CD27 is found on NK cells, activated B cells, and hematopoietic stem cells.106, 108 CD70, the ligand for CD27, is expressed on T cells, B cells, and activated DC.109 Whereas antigen-stimulated T cells and B cells transiently increase CD70 expression, APCs in the intestine and medullary thymic epithelial cells constitutively express CD70.110 The development of effector T cells appears independent of the presence of CD27 whereas thymic output of nTregs is reduced in Cd27−/− mice.110, 111 CD27 and CD70 interactions play an important role in mounting fulminant CD4+ and CD8+ T cell responses including memory formation at effector and priming sites.110, 112
Proper CD27/CD70 signaling is needed for B cell proliferation and plays an important role during the process of Ig synthesis.113 Insufficient CD70 triggering on B cells leads to an impaired germinal center formation, thereby affecting the humoral immune response.114 However, B cells from Cd27−/− mice still undergo class switching and Ig maturation in aged mice, thus, other factors contribute and compensate for CD27 defects which are only present during early phases. In contrast, human CD27+ B cells produced a higher amount of Ig, IL-10, and displayed enhanced survival.115–117 In accordance, humans carrying mutations in the CD27 gene suffer from a severe immunodeficiency characterized by hypogammaglobulinemia, dysregulated lymphoproliferation and increased susceptibility for infections with Epstein-Barr virus (EBV).118–120 Co-stimulation of CD70 via CD27 induced B cell proliferation but impaired terminal differentiation and Ig secretion of human and murine B cells, although stimulation of CD70 via soluble CD27 resulted in increased Ig secretion.113, 121, 122 Thus, soluble and membrane bound CD27 interacting with CD70 exert species-specific effects and seem to contribute to a germinal center reaction in mice. In humans, CD27 promotes terminal B cell differentiation and CD70 might downregulate humoral immunity.
Mice deficient for CD27 demonstrated a reduction in the proliferative capacity of antigen-specific T cells which is not dependent on cell cycle entry.123 CD27 acts anti-apoptotic in various manners, thus contributing to T cell survival and memory formation. T cells stimulated in vitro by CD27 signaling increase expression of the anti-apoptotic molecule B-cell lymphoma-extra large (Bcl-xL).124 Moreover, CD27 acts indirect on antigen-experienced CD8+ T cells as deficiency for CD27 reduced production of the autocrine growth factor IL-2, thus limiting survival and proliferation of all T cells in non-lymphoid tissue.125 Additionally, the pharmacological inhibition of CD27/CD70 interactions with a blocking antibody increased FasL expression on CD4+ T cells which in turn induced apoptosis of virus-specific CD8+ T cells.126 Early work addressing the role of CD27 and CD70 on atherosclerosis demonstrated an atheroprotective role for stimulation of CD70.127 Chronic CD70 overexpression in B cells continuously induced CD27 signaling on T cells, leading to a predominant, presumably pro-atherogenic Th1 response. However, this was accompanied by increased rate of apoptosis among pro-atherogenic Ly6C+ monocytes, thus reducing macrophage abundance in atherosclerotic lesions. Furthermore, constitutive CD27/CD70 signaling led to immunopathology characterized by the conversion of naïve T cells into IFNγ-producing effector T cells leading to the progressive loss of B cells.127, 128 Thus, the model of B cell-restricted CD70 overexpression is of limited use to determine the precise roles of CD27 and CD70 in atherosclerosis.
Macrophages are the main lesional CD70-expressing cells.129 Macrophages deficient for CD70 displayed a unique phenotype characterized by enhanced M1 and M2 marker expression, yet were less viable and incompetent in mounting a proper inflammatory response.129 Furthermore, Cd70−/− macrophages were less efficient in scavenging and cholesterol efflux, thereby contributing pathogenically to atherosclerosis progression. Accordingly, deficiency of CD70 resulted in exacerbated atherosclerosis.129 Of note, CD70 and Apoe compound mutant mice were protected from hypertension and renal damage due to reduced accumulation of CD4+ and CD8+ effector memory T cells.130
3.2.3. GITR – GITRL
Tregs express significant levels of GITR (TNFRSF18). Stimulation with its ligand (TNFSF18, GITRL) reduces their suppressive capacity.131 However, naïve T cells also express GITR at low levels, which is increased upon activation.132 Moreover, mast cells, APCs, NK cells, and granulocytes express GITR.133 GITR expression on human immune cells is more restricted and expression has been described in Tregs, NK cells and macrophages.134 The latter cells also demonstrated GITRL expression upon TLR signaling whereas antigen recognition drives GITRL expression in T cells.133 Functional differences between species-specific GITR-GITRL interactions have been reviewed elsewhere.134 Furthermore, EC express GITRL, which is enhanced upon LPS treatment, suggesting potential interaction of T cells and EC via this co-stimulatory axis during inflammation.135, 136 In human atherosclerotic lesions, plaque-resident macrophages demonstrate GITR and GITRL expression.137 Interestingly, GITR stimulation of macrophages increased expression of matrix metalloproteinase (MMP)-9, which co-localized with GITR expression in atherosclerotic lesions, suggesting that GITR-GITRL interactions exert plaque destabilizing effects.137 In mice, chronic GITR stimulation is atheroprotective.138 Ldlr−/− mice transplanted with bone marrow from B cell-restricted GITRL overexpressing mice enhanced thymic generation and lesional abundance of Tregs. Although deficiency for GITR is protective in murine models of asthma, experimental colitis, and collagen-induced arthritis, the exact role of GITR in atherosclerosis needs to be clarified.139
3.2.4 OX40 – OX40L
OX40 (CD134/TNFRSRF4) and its ligand (OX40L/CD252/TNFSF4) are predominantly expressed on activated CD4+ and CD8+ T cells whereas naïve and resting memory T cells express neither OX40 nor OX40L.140 Besides, other immune cells such as neutrophils and NK cells express OX40 constitutively whereas OX40L is inducible among others on APC, EC, and smooth muscle cells (SMC).140 OX40 and OX40L expression is important for expansion of antigen-specific T cells to mount a functional T cell response and to form memory.141, 142 OX40 also functions as a negative regulator of Tregs, thus further increasing the pro-inflammatory response.143 Additionally, OX40 interactions with OX40L drive B cell activation and immunoglobulin production and play a role in macrophage activation.144, 145 Plaque-resident macrophages express OX40L in mice and humans, whereas lesional T cells were positive for OX40.146, 147 Of note, a single nucleotide polymorphism of the OX40 gene in intron 5 was significantly associated with myocardial infarction.148 Spontaneous mutations in the Tnfsf4 locus of healthy C57BL/6 mice are associated with susceptibility for atherosclerosis. 146 The minor allele of the single nucleotid polymorphism rs3850641 of TNFSF4 is associated with an increased risk for women to develop myocardioal infarction.29 However, another group could not confirm associations between theTNFSF4 genotype and an increased risk to develop carotid aretry disease or stroke.147 Genetic disruption or pharmacological inhibition of this co-stimulatory dyad attenuated atherosclerotic development and even caused regression of established atherosclerotic lesions, including a reduced neovascularization of the vasa vasorum. 29,149, 150
3.2.5 CD30 – CD30L
CD30 (TNFRSF8) and CD30L (TNFSF8, CD153) are expressed by activated T cells and B cells. Furthermore, mature DC, macrophages, and mast cells demonstrate CD30L expression.151 Co-stimulation via CD30-CD30L induces T cell proliferation and activation and is important for long-lived CD8+ T cell memory formation.152 The global absence of CD30 in mice reduced secondary humoral responses by an impaired induction of follicular germinal center responses accompanied by reduced antibody production.153, 154 Pharmacological CD30L blockage efficiently prevented development of spontaneous/type I diabetes mellitus in NOD mice.80 Furthermore, CD30L blockage reduced atherosclerotic burden in Ldlr−/− mice presumably by reducing overall T cell proliferation in the spleen and lymph nodes without affecting the humoral response.155
3.2.6 4-1BB – 4-1BBL
The co-stimulatory molecule 4-1BB (TNFRSF9, CD137) is expressed on activated CD4+ T cells, CD8+ T cells, NK cells, resting monocytes, and DC whereas its ligand is predominantly expressed on APC.156 Mice deficient for 4-1BB have less NK and NKT cells whereas T cell development is not affected.157, 158 Furthermore, 4-1BB deficiency increased the number of myeloid progenitor cells and mature DCs, yet reduced the survival of the latter cell type.159, 160 Of note, while 4-1BBL reduced human NK cell activity in co-cultures with tumor cell lines, it activated murine NK cells and led to enhanced killing activity.161 In addition, Tnfrsf9−/− mice mounted a stronger antigen-specific T cell response although DC functionality is impaired.162 Presumably, these effects would contribute to progression of atherosclerosis. However, stimulation of 4-1BB with an agonistic antibody increased atherosclerotic burden accompanied by enhanced lesional inflammation whereas 4-1BB deficiency attenuates atherosclerosis in mice.163, 164 Advanced atherosclerotic lesions accumulated more macrophages and T cells when 4-1BB was lacking.165 Lesions in these mice showed signs of vulnerability, accompanied by reduced SMC survival and collagen production.165 Interestingly, macrophage glucose metabolism is regulated by the interaction of 4-1BBL with its receptor, leading to increased metabolic activity and cell proliferation.166 Thus, intervention in 4-1BB-4-BBL interactions might display a valuable therapeutic option in macrophage-driven diseases such as atherosclerosis.
3.2.7 LIGHT – HEVM
HVEM (herpes virus entry mediator, TNFRSF14) is expressed by resting T cells and APCs whereas its main ligand, LIGHT (TNFSF14), is expressed by activated T cells, monocytes, DCs, and NK cells.167, 168 HVEM can also interact with lymphotoxin-α, BTLA (B and T lymphocyte attenuator), and CD160.169 Interactions with LIGHT and lymphotoxin-α contribute to T cell activation and cytokine production whereas ligation of HVEM to BTLA and CD160 promotes co-inhibitory effects.169 The contribution of HVEM and LIGHT to atherosclerosis is not fully understood. EC and macrophages in atherosclerotic lesions express HVEM and LIGHT and both transcripts were elevated in aortas of atherosclerotic Apoe−/− mice.170 HVEM signaling induced the production of MMP-1, -9, and -13 by monocytic cells in vitro and the staining of MMPs overlapped with HVEM in human atherosclerotic lesions suggesting that HVEM signaling contributes to plaque destabilization and rupture.171 Further pro-atherogenic features of this co-stimulatory axis involve the adhesion of platelets to EC and contribute to atheroprogression by guiding leukocyte adhesion to the inflamed endothelium.172, 173 Furthermore, LIGHT expressed by platelets induces signals in EC and monocytes that increase the expression of adhesion molecules and chemokines.174 The increased expression of pro-atherogenic inflammatory mediators such as IL-8 and MCP-1 depends on a LIGHT-mediated induction of proteinase-activated receptor 2.170 T cell-restricted overexpression of LIGHT induced hyperlipidemia by a substantial reduction of hepatic lipase expression.175 In the liver hepatic lipase is surface expressed and promotes uptake of lipoproteins containing cholesterol and triglycerides, hydrolyzing the latter. However, the mechanisms underlying how T cell-mediated LIGHT expression alters liver metabolism is not understood.
4. Co-inhibitory pathways shaping atherosclerosis
4.1 PD-1 – PD-L1/PD-L2
The CD28-superfamily also includes the co-inhibitory molecule PD-1 (CD279) which binds to PD-L1 (CD274) and PD-L2 (CD273). Whereas PD-L1 expression is broadly found on APCs and tissue cells of non-hematopoietic origin, especially in the presence of innate inflammatory stimuli, PD-L2 expression is mainly restricted to APCs.176 Furthermore, PD-L1 can be expressed in vitro by vascular SMC and vascular EC in vitro and in vivo.177–179 Interestingly, in vitro incubation of vascular ECs with oxLDL increased PD-L1 expression. 180 This led to a strong induction of anti-inflammatory cytokine production by co-cultured Tregs displaying a potential atherosclerosis counterbalancing mechanism.180 Furthermore, hypercholesterolemia promoted PD-L1 expression on splenic macrophages and DCs of Ldlr−/− mice.181 On the other hand, circulating T cells and myeloid DC from patients with coronary artery disease demonstrated reduced PD-1 and PD-L1 expression compared to healthy individuals.182 Deficiency of PD-1 or its ligands increased CD4+ and CD8+ T cell activation and their influx into atherosclerotic lesions which accelerated atherosclerosis in Ldlr−/− mice.181, 183 Similarly, the administration of a PD-1 blocking antibody to Ldlr−/− mice exacerbated atherosclerosis and lesional T cell infiltration.183 The overall increased T cell activation in atherosclerotic Pd1−/− mice did not favor a certain subtype, but enhanced abundance and response of pro- and anti-atherogenic subsets, suggesting that the pro-inflammatory compartment outcompetes immunosuppression by Tregs.184 PD-1 and Tim-3 expression defines highly exhausted CD8+ T cells. In vitro re-stimulation of PD-1+Tim-3+ CD8+ T cells isolated from human atherosclerotic lesions demonstrated skewing towards an anti-inflammatory cytokine profile, which was reverted by applying PD-1- and Tim-3-blocking antibodies, suggesting that these particular CD8+ T cells in lesions are of regulatory nature whereas other reports attribute CD8+ T cells pro-atherogenic function.185,149,150 Monoclonal antibodies to PD1 and PD-L1 and PD-L2 are now widely used in immunotherapy of cancer patients.186 It should be considered that these treatments may increase cardiovascular risk.
4.2 CTLA-4 – CD80/CD86
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, CD152) competes with CD28 for binding to CD80 and CD86. However, CTLA-4 decreases immune responses and functions as an immune checkpoint regulator. The clinical targeting of this molecule in tumor malignancies is discussed below. Effector T cells increase surface abundance of CTLA-4 after activation whereas Tregs express CTLA-4 constitutively, which probably represents one of their main immunosuppressive effector mechanisms.187 The impact of a genetic CTLA-4 deficiency on atherosclerosis is unknown since mice deficient for CTLA-4 succumb to an autoimmune lymphoproliferative disorder.188 Application of CTLA-4 blocking antibodies resulted in a dramatic increase of atherosclerotic burden in hypercholesterolemic mice.189 Conversely, the application of a CTLA-4-Ig fusion protein, which mimics CTLA-4 function, prevented CD80/CD86-CD28 interactions accompanied by reduced T cell activation. Such treatment also resulted in limited neointima formation and reduced homocysteine-accelerated atherosclerosis.189, 190 In line with these results, transgenic mice constitutively expressing CTLA-4 on T cells were protected from atherosclerosis.191 Similar to the effects of blocking PD1/PDL1/PDL2 in cancer patients, CTLA4 blockade may trigger pro-atherosclerotic effects.
5. Potential therapeutic applications in atherosclerosis
5.1 Targeting CD40/CD40L interactions
The modulation of CD40/CD40L interactions was clinically tested in a variety of chronic inflammatory diseases and cancer.78 However, pharmacological interference with antagonistic or agonistic antibodies triggered severe side effects. Patients suffering from lupus glomerulonephritis experienced a marked reduction of hematuria when treated with a blocking anti-CD40L-antibody.155 However, this trial was prematurely discontinued as anti-CD40L-antibody treatment caused thromboembolic events and myocardial infarctions, likely by the destabilization of platelet aggregates.192, 193 Also, agonistic or antagonistic anti-CD40 antibodies failed in diverse clinical disorders as the treatment was not efficient, or side effects including thrombocytopenia, neutropenia, and pleural effusion led to the discontinuation of trials.194–196 An alternative therapeutic strategy harnesses interactions of CD40L with Mac-1, also known as CD11b/CD18 integrin, which is abundantly expressed of neutrophils, NK cells, monocytes, and macrophages.197 The intraperitoneal application of a small peptide prevented interaction of CD40L with Mac-1 and reduced atherosclerotic burden in Ldlr−/− mice, potentially by reducing leukocyte recruitment to the inflammatory site.198 Disrupting CD40-Mac-1 interactions did not prevent functional CD40-CD40L interactions and spared thrombotic events.198 A different therapeutic approach targets the interaction of CD40 with its downstream signaling interaction partner TRAF6. Ablation of CD40-TRAF6 interactions led to more stable and smaller atherosclerotic lesions, accompanied by reduced monocyte influx into the arterial wall.103 Furthermore, a small compound designed to prevent CD40-TRAF6 interactions increased survival of mice with induced sepsis and improved insulin resistance in obese mice.199, 200 However, the efficacy of this compound in atherosclerosis has yet to be tested.
5.2 Anti-CD27/CD70 antibodies in cancer and cardiovascular implications
Hematologic malignancies and solid tumors feature high CD70 expression.201–205 The constitutive activation of effector T cells by persistent antigen and constitutive signaling via CD27/CD70 interaction leads to exhaustion, demonstrated in patients suffering from B cell non-Hodgkin’s lymphoma.206 Exhausted T cells are less cytotoxic and incapable of attacking the tumor. The pharmacological inhibition of CD27/CD70 interaction by blocking-CD70 antibodies represents a promising therapeutic strategy in the treatment of such malignancies.207, 208 Furthermore, the opsonization of CD70 expressing tumor cells by these antibodies could induce antibody-dependent cellular cytotoxicity and phagocytosis, thus directly attacking the tumor cells and contributing to tumor regression. Alternatively, agonistic CD27 modulation by the CDX-1127 (varlilumab) antibody under clinical evaluation in patients suffering from B cell malignancies, melanoma, and renal cell carcinoma.209, 210 Such agonistic CD27 stimulation successfully re-activated exhausted effector T cells, leading to a profound anti-tumor immune response and tumor regression in mice with a transgene expressing human CD27.211 As CD70 deletion exacerbates atherosclerosis, potential side effects on the cardiovascular system by pharmacological modulation of CD27/CD70 interactions require consideration.129
5.3 Anti-CTLA-4 and anti-PD-1 antibodies in oncotherapy and their potential cardiovascular effects
The co-inhibitory molecules CTLA-4 and PD-1 regulate T cell activation, however, in different ways. The blockage of both immune checkpoint mediators is desirable in advanced tumor malignancies, since cytotoxic anti-tumor T cells are reactivated and not suppressed anymore. Dual blockage with the monoclonal antibodies ipilimumab and nivolumab, targeting CTLA-4 and PD-1 respectively, has been tested in clinical trials.212 Recently, the Checkmate-67 phase III study demonstrated a 55% response rate when both antibodies were used to treat patients suffering from advanced melanoma.213 Furthermore, patients receiving treatment with both antibodies showed a median progression free survival of almost 12 months, 2–4 times higher compared to treatment with one or the other antibody. Data on the overall survival of the dual-treated patients are not yet published. Although an overall enhanced anti-tumor T cell response is highly desirable, uncontrolled T cell activity could exacerbate atherosclerosis and cardiovascular disease. Indeed, as pointed out above, pharmacological blockage or deficiency of CTLA-4 or PD-1 increased atherosclerotic burden. Overall, it has to be considered whether a patient suffering from advanced tumor malignancies can gain expanded lifetime when undergoing immune checkpoint modulating therapy at the potential cost of accepting a higher risk for cardiovascular complications.
6. Open questions and future directions
Past research has identified co-stimulatory or co-inhibitory molecules as important modulators of immune response mediating effects on various cell types. Functional studies by genetic deletions or pharmacological manipulation have shown that these molecules significantly contribute to atherosclerosis. More research is needed to evaluate expression patterns of all these molecules on immune and non-immune cells in health and disease, especially during atherosclerosis progression. Additionally, not much is known about how immune cells integrate signals received by various co-stimulatory and co-inhibitory pathways. Understanding the integration of co-stimulatory pathways in chronic inflammatory conditions such as atherosclerosis will help to develop new, tailored therapeutic approaches. The clinical success of blocking PD1, PD-L1, PD-L2, and/or CTLA-4 in cancer support the general feasibility of manipulating co-inhibitory and co-stimulatory pathways. Whether such approaches will succeed in curbing major adverse cardiovascular events is the subject of ongoing and future studies.
Table 1.
Expression profile and effects of co-stimulatory molecules in atherosclerosis
| Co-stimulatory Molecule | Expression on resting cells | Expression on activated cells | Effect on atherosclerosis | |
|---|---|---|---|---|
| Protein | Gene | |||
| CD28 | Cd28 | Naïve T cells, Eosinophils, Basophils, Treg | T cells |
Unclear: Increased atherosclerosis in Ldlr−/− mice transplanted with Cd28−/−, Cd80−/−, or Cd86−/− BM53 Reduced atherosclerosis in Cd80−/− Cd86−/− Ldlr−/− mice69 |
| CD80 | Cd80 | ++: APC | +++: APC | |
| CD86 | Cd86 | +: APC | +++: APC | |
|
| ||||
| CTLA-4/CD152 | Ctla4 | Tregs | Tregs, effector T cells |
Clear: CTLA-4 blocking antibodies189 increased atherosclerosis CTLA-4-Ig fusion protein190 reduced atherosclerosis |
|
| ||||
| CD40 | Tnfrsf5 | +++: B cells, SMC; ++Platelets; +: Macrophages, Neutrophils, EC | +++: T cells, APCs, Platelets, Neutrophils, EC | Clear: Pharmacological inhibition94–96 or global deficiency93 reduced atherosclerosis |
| CD40L | Tnfsf5 | ++: B cells; +: Macrophages, DC, Neutrophils, EC, SMC | +++: T cells, B cells, Macrophages, Platelets; +: DC, EC, Neutrophils | |
|
| ||||
| CD27 | Tnfrsf7 | naive T cells, NK cells, murine HSC | human memory B cells, murine centroblasts, memory T cells | Unclear: CD70 deficiency129 and overexpression127 reduced atherosclerosis by limiting macrophage function or survival of Ly6Chi monocytes, respectively |
| CD70 | Tnfsf7 | MTEC, APC subset in lamina propria | APC | |
|
| ||||
| GITR | Tnfrsf18 | +++: Treg; +: naive CD4 T cells, Mast cells, APC | ++Macrophages | Clear: Chronic GITRL overexpression is atheroprotective by Treg expansion138 |
| GITRL | Tnfsf18 | +: EC | +++: EC, T cells, APC | |
|
| ||||
| OX40/CD134 | Tnfrsf4 | Neutrophils, NK cells | Effector CD4 and CD8 T cells | Clear: Genetic ablation150 or pharmacological inhibition149 attenuates atherosclerosis |
| OX40L/CD252 | Tnfsf4 | APC, EC, SMC | Effector CD4 and CD8 T cells, Macrophages | |
|
| ||||
| CD30 | Tnfrsf8 | Activated T and B cells | Insufficient data: Pharmacological CD30L blockage reduced atherosclerosis155 | |
| CD30L | Tnfsf8 | B cells, MTEC | Activated T and B cells, DC, Macrophages, Mast cells, Granulocytes | |
|
| ||||
| 4-1BB/CD137 | Tnfrsf9 | Monocytes, DC, B cells, FDC, NK cells, Granulocytes | Activated CD4 and CD8 T cells, EC | Clear: Agonistic 4-1BB stimulation163 increased atherosclerosis whereas genetic deficiency attenuated atherosclerosis164. Later stage lesions in 4-1BB KO mice show vulnerable lesions165 |
| 4-1BBL/CD137L | Tnfsf9 | APC | ||
|
| ||||
| HVEM/CD270 | Tnfrsrf14 | T cells, APC | EC, Macrophages | Insufficient data: Not fully understood, signaling induces pro-inflammatory mediator expression and altered liver metabolism170, 171 |
| LIGHT/CD258 | Tnfsf14 | Monocytes, NK cells, DC | T cells, EC, Macrophages | |
|
| ||||
| PD-1/CD279 | Pdcd1 | Myeloid DC, pro-B cells, Treg | Myeloid DC, T cells | Clear: Genetic deficiency of Pd1 or Pd-l1/Pd-l2181 or pharmacological inhibition of Pd-1183 increased atherosclerosis in Ldlr−/− mice |
| PD-L1/CD274 | Pdcd1lg1 | +: vascular EC, vascular SMC | T cells, NK cells, Macrophages, Myeloid DC, B cell, Epithelial cells, and vascular EC | |
| PD-L2/CD273 | Pdcd1lg2 | DC | DC | |
APC: Antigen Presenting Cell, BM: Bone Marrow, DC: Dendritic Cell, EC: Endothelial Cell, FDC: Follicular DC, HSC: Hematopoietic Stem Cell, SMC: Smooth Muscle Cell, NK cell: Natural Killer cell, +: mild expression, ++: intermediate expression, +++: strong expression
Acknowledgments
Sources of Funding
The original research underlying this review was funded by the National Institutes of Health and National Heart, Lung, and Blood Institute (Klaus Ley: R01-HL115232, R01-HL118676, P01-HL088093, R01-HL126543, R01-HL121697, P01-HL055798) and Deutsche Forschungsgemeinschaft (Norbert Gerdes: SFB-1123-A5).
Acknowledgement: None
List Of Abbreviations
- APC
Antigen Presenting Cell
- ApoB
Apolipoprotein B
- Bcl-XL
B-cell lymphoma-extra large
- BTLA
B and T Lymphocyte Attenuator
- CTLA-4
Cytotoxic T-lymphocyte-associated Protein 4
- DC
Dendritic Cell
- EC
Endothelial Cell
- GITR
Glucocorticoid-induced TNFR-related Protein
- HVEM Herpes
Virus Entry Mediator
- IFNγ
Interferon gamma
- Ig
Immunoglobulin
- iTreg
Induced Treg
- LDL
Low-density Lipoprotein
- LPS
Lipopolysaccharide
- MACE
Major Adverse Cardiovascular Events
- MHC
Major Histocompatibility Complex
- nTreg
Natural Treg
- oxLDL
oxidized LDL
- SMC
Smooth Muscle Cell
- SR
Scavenger Receptor
- TCR
T Cell Receptor
- TGFβ
Transforming Growth Factor beta
- TLR
Toll-like Receptor
- TNFRSF
Tumor Necrosis Receptor Superfamily
- TNFSF
Tumor Necrosis Factor Superfamily
- Treg
Regulatory T cell
Footnotes
Disclosure: None
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalanuria AA, Nyquist P, Ling G. The prevention and regression of atherosclerotic plaques: emerging treatments. Vasc Health Risk Manag. 2012;8:549–561. doi: 10.2147/VHRM.S27764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 2014;9:73–102. doi: 10.1146/annurev-pathol-020712-163936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 6.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XQ, Nigro P, World C, et al. Thioredoxin interacting protein promotes endothelial cell inflammation in response to disturbed flow by increasing leukocyte adhesion and repressing Kruppel-like factor 2. Circ Res. 2012;110:560–568. doi: 10.1161/CIRCRESAHA.111.256362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steffensen LB, Mortensen MB, Kjolby M, et al. Disturbed Laminar Blood Flow Vastly Augments Lipoprotein Retention in the Artery Wall: A Key Mechanism Distinguishing Susceptible From Resistant Sites. Arterioscler Thromb Vasc Biol. 2015;35:1928–1935. doi: 10.1161/ATVBAHA.115.305874. [DOI] [PubMed] [Google Scholar]
- 9.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 10.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Gils JM, Derby MC, Fernandes LR, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palinski W, Rosenfeld ME, Yla-Herttuala S, et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yla-Herttuala S, Palinski W, Butler SW, et al. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 14.Millonig G, Niederegger H, Rabl W, et al. Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler Thromb Vasc Biol. 2001;21:503–508. doi: 10.1161/01.atv.21.4.503. [DOI] [PubMed] [Google Scholar]
- 15.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulson KE, Zhu SN, Chen M, et al. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 17.Llodra J, Angeli V, Liu J, et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonasson L, Holm J, Skalli O, et al. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985;76:125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koltsova EK, Garcia Z, Chodaczek G, et al. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galkina E, Kadl A, Sanders J, et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohanta SK, Yin C, Peng L, et al. Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis. Circ Res. 2014;114:1772–1787. doi: 10.1161/CIRCRESAHA.114.301137. [DOI] [PubMed] [Google Scholar]
- 22.Okada T, Miller MJ, Parker I, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garside P, Ingulli E, Merica RR, et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 24.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 25.Crawford A, Macleod M, Schumacher T, et al. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 26.Giles JR, Kashgarian M, Koni PA, et al. B Cell-Specific MHC Class II Deletion Reveals Multiple Nonredundant Roles for B Cell Antigen Presentation in Murine Lupus. J Immunol. 2015;195:2571–2579. doi: 10.4049/jimmunol.1500792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molnarfi N, Schulze-Topphoff U, Weber MS, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wick G, Jakic B, Buszko M, et al. The role of heat shock proteins in atherosclerosis. Nat Rev Cardiol. 2014;11:516–529. doi: 10.1038/nrcardio.2014.91. [DOI] [PubMed] [Google Scholar]
- 29.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulsson G, Zhou X, Tornquist E, et al. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:10–17. doi: 10.1161/01.atv.20.1.10. [DOI] [PubMed] [Google Scholar]
- 31.Tse K, Gonen A, Sidney J, et al. Atheroprotective Vaccination with MHC-II Restricted Peptides from ApoB-100. Front Immunol. 2013;4:493. doi: 10.3389/fimmu.2013.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura T, Tse K, McArdle S, et al. Atheroprotective vaccination with MHC-II-restricted ApoB peptides induces peritoneal IL-10-producing CD4 T cells. Am J Physiol Heart Circ Physiol. 2017 doi: 10.1152/ajpheart.00798.2016. ajpheart 00798 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredrikson GN, Soderberg I, Lindholm M, et al. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23:879–884. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- 34.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 35.van Dijk RA, Duinisveld AJ, Schaapherder AF, et al. A change in inflammatory footprint precedes plaque instability: a systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J Am Heart Assoc. 2015:4. doi: 10.1161/JAHA.114.001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buono C, Binder CJ, Stavrakis G, et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta S, Pablo AM, Jiang X, et al. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, McArdle S, Gholami A, et al. CCR5+T-bet+FoxP3+ Effector CD4 T Cells Drive Atherosclerosis. Circ Res. 2016;118:1540–1552. doi: 10.1161/CIRCRESAHA.116.308648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butcher MJ, Filipowicz AR, Waseem TC, et al. Atherosclerosis-Driven Treg Plasticity Results in Formation of a Dysfunctional Subset of Plastic IFNgamma+ Th1/Tregs. Circ Res. 2016;119:1190–1203. doi: 10.1161/CIRCRESAHA.116.309764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol. 2002;22:456–461. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- 41.Cardilo-Reis L, Gruber S, Schreier SM, et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erbel C, Chen L, Bea F, et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 44.Smith E, Prasad KM, Butcher M, et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taleb S, Romain M, Ramkhelawon B, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gistera A, Robertson AK, Andersson J, et al. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5:196ra100. doi: 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- 47.Klein L, Jovanovic K. Regulatory T cell lineage commitment in the thymus. Semin Immunol. 2011;23:401–409. doi: 10.1016/j.smim.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Klein L, Kyewski B, Allen PM, et al. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spitz C, Winkels H, Burger C, et al. Regulatory T cells in atherosclerosis: critical immune regulatory function and therapeutic potential. Cell Mol Life Sci. 2016;73:901–922. doi: 10.1007/s00018-015-2080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 52.Robertson AK, Rudling M, Zhou X, et al. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 54.Klingenberg R, Gerdes N, Badeau RM, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maganto-Garcia E, Tarrio ML, Grabie N, et al. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–195. doi: 10.1161/CIRCULATIONAHA.110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potekhina AV, Pylaeva E, Provatorov S, et al. Treg/Th17 balance in stable CAD patients with different stages of coronary atherosclerosis. Atherosclerosis. 2015;238:17–21. doi: 10.1016/j.atherosclerosis.2014.10.088. [DOI] [PubMed] [Google Scholar]
- 57.Ait-Oufella H, Tedgui A. Regulatory T-Cell Plasticity: Another Layer of Complexity in Atherosclerosis. Circ Res. 2016;118:1461–1463. doi: 10.1161/CIRCRESAHA.116.308805. [DOI] [PubMed] [Google Scholar]
- 58.Kyaw T, Toh BH, Bobik A. Foxp3+CD4+ Regulatory T-Cell Subtypes and Atherosclerosis. Circ Res. 2016;119:1151–1153. doi: 10.1161/CIRCRESAHA.116.309999. [DOI] [PubMed] [Google Scholar]
- 59.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 60.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 61.Ville S, Poirier N, Blancho G, et al. Co-Stimulatory Blockade of the CD28/CD80–86/CTLA-4 Balance in Transplantation: Impact on Memory T Cells? Front Immunol. 2015;6:411. doi: 10.3389/fimmu.2015.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 63.Wells AD, Walsh MC, Bluestone JA, et al. Signaling through CD28 and CTLA-4 controls two distinct forms of T cell anergy. J Clin Invest. 2001;108:895–903. doi: 10.1172/JCI13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 65.Hunig T. Manipulation of regulatory T-cell number and function with CD28-specific monoclonal antibodies. Adv Immunol. 2007;95:111–148. doi: 10.1016/S0065-2776(07)95004-X. [DOI] [PubMed] [Google Scholar]
- 66.Dopheide JF, Sester U, Schlitt A, et al. Monocyte-derived dendritic cells of patients with coronary artery disease show an increased expression of costimulatory molecules CD40, CD80 and CD86 in vitro. Coron Artery Dis. 2007;18:523–531. doi: 10.1097/MCA.0b013e3282eff1ad. [DOI] [PubMed] [Google Scholar]
- 67.de Boer OJ, Hirsch F, van der Wal AC, et al. Costimulatory molecules in human atherosclerotic plaques: an indication of antigen specific T lymphocyte activation. Atherosclerosis. 1997;133:227–234. doi: 10.1016/s0021-9150(97)00135-4. [DOI] [PubMed] [Google Scholar]
- 68.Muller A, Mu L, Meletta R, et al. Towards non-invasive imaging of vulnerable atherosclerotic plaques by targeting co-stimulatory molecules. Int J Cardiol. 2014;174:503–515. doi: 10.1016/j.ijcard.2014.04.071. [DOI] [PubMed] [Google Scholar]
- 69.Buono C, Pang H, Uchida Y, et al. B7-1/B7-2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:2009–2015. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- 70.Yilmaz A, Lochno M, Traeg F, et al. Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis. 2004;176:101–110. doi: 10.1016/j.atherosclerosis.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 71.Rohm I, Atiskova Y, Drobnik S, et al. Decreased regulatory T cells in vulnerable atherosclerotic lesions: imbalance between pro- and anti-inflammatory cells in atherosclerosis. Mediators Inflamm. 2015;2015:364710. doi: 10.1155/2015/364710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krzyzak L, Seitz C, Urbat A, et al. CD83 Modulates B Cell Activation and Germinal Center Responses. J Immunol. 2016;196:3581–3594. doi: 10.4049/jimmunol.1502163. [DOI] [PubMed] [Google Scholar]
- 73.Fujimoto Y, Tu L, Miller AS, et al. CD83 expression influences CD4+ T cell development in the thymus. Cell. 2002;108:755–767. doi: 10.1016/s0092-8674(02)00673-6. [DOI] [PubMed] [Google Scholar]
- 74.von Rohrscheidt J, Petrozziello E, Nedjic J, et al. Thymic CD4 T cell selection requires attenuation of March8-mediated MHCII turnover in cortical epithelial cells through CD83. J Exp Med. 2016;213:1685–1694. doi: 10.1084/jem.20160316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kretschmer B, Luthje K, Ehrlich S, et al. CD83 on murine APC does not function as a costimulatory receptor for T cells. Immunol Lett. 2008;120:87–95. doi: 10.1016/j.imlet.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Chen L, Zhu Y, Zhang G, et al. CD83-stimulated monocytes suppress T-cell immune responses through production of prostaglandin E2. Proc Natl Acad Sci U S A. 2011;108:18778–18783. doi: 10.1073/pnas.1018994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foy TM, Aruffo A, Bajorath J, et al. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 78.Jansen MF, Hollander MR, van Royen N, et al. CD40 in coronary artery disease: a matter of macrophages? Basic Res Cardiol. 2016;111:38. doi: 10.1007/s00395-016-0554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chatzigeorgiou A, Lyberi M, Chatzilymperis G, et al. CD40/CD40L signaling and its implication in health and disease. Biofactors. 2009;35:474–483. doi: 10.1002/biof.62. [DOI] [PubMed] [Google Scholar]
- 80.Fuleihan R, Ramesh N, Geha RS. Role of CD40-CD40-ligand interaction in Ig-isotype switching. Curr Opin Immunol. 1993;5:963–967. doi: 10.1016/0952-7915(93)90113-7. [DOI] [PubMed] [Google Scholar]
- 81.Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 82.Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cella M, Scheidegger D, Palmer-Lehmann K, et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suttles J, Stout RD. Macrophage CD40 signaling: A pivotal regulator of disease protection and pathogenesis. Semin Immunol. 2009;21:257–264. doi: 10.1016/j.smim.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 85.Kiener PA, Morandavis P, Rankin BM, et al. Stimulation of Cd40 with Purified Soluble Gp39 Induces Proinflammatory Responses in Human Monocytes. Journal of Immunology. 1995;155:4917–4925. [PubMed] [Google Scholar]
- 86.Ferrari-Lacraz S, Nicod LP, Chicheportiche R, et al. Human lung tissue macrophages, but not alveolar macrophages, express matrix metalloproteinases after direct contact with activated T lymphocytes. Am J Respir Cell Mol Biol. 2001;24:442–451. doi: 10.1165/ajrcmb.24.4.4008. [DOI] [PubMed] [Google Scholar]
- 87.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 88.Munroe ME, Bishop GA. A costimulatory function for T cell CD40. J Immunol. 2007;178:671–682. doi: 10.4049/jimmunol.178.2.671. [DOI] [PubMed] [Google Scholar]
- 89.Bullock TNJ, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8(+) T cell responses in the absence of CD4(+) T cells. Journal of Immunology. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 90.Wang M, Li Y, Li W, et al. The CD40 gene polymorphism rs1883832 is associated with risk of acute coronary syndrome in a Chinese case-control study. DNA Cell Biol. 2011;30:173–178. doi: 10.1089/dna.2010.1129. [DOI] [PubMed] [Google Scholar]
- 91.Ma Y, Wang SX, Liu Y, et al. Single nucleotide polymorphism of CD40 in the 5’-untranslated region is associated with ischemic stroke. Gene. 2013;529:257–261. doi: 10.1016/j.gene.2013.07.086. [DOI] [PubMed] [Google Scholar]
- 92.Zhou L, Xie L, Zheng D, et al. Genetic Variants of CD40 Gene Are Associated with Coronary Artery Disease and Blood Lipid Levels. Biomed Res Int. 2016;2016:1693619. doi: 10.1155/2016/1693619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lutgens E, Gorelik L, Daemen MJ, et al. Requirement for CD154 in the progression of atherosclerosis. Nat Med. 1999;5:1313–1316. doi: 10.1038/15271. [DOI] [PubMed] [Google Scholar]
- 94.Lutgens E, Cleutjens KB, Heeneman S, et al. Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype. Proc Natl Acad Sci U S A. 2000;97:7464–7469. doi: 10.1073/pnas.97.13.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mach F, Schonbeck U, Sukhova GK, et al. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 96.Schonbeck U, Sukhova GK, Shimizu K, et al. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci U S A. 2000;97:7458–7463. doi: 10.1073/pnas.97.13.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 98.Lievens D, Zernecke A, Seijkens T, et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116:4317–4327. doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gerdes N, Seijkens T, Lievens D, et al. Platelet CD40 Exacerbates Atherosclerosis by Transcellular Activation of Endothelial Cells and Leukocytes. Arterioscler Thromb Vasc Biol. 2016;36:482–490. doi: 10.1161/ATVBAHA.115.307074. [DOI] [PubMed] [Google Scholar]
- 100.Bavendiek U, Zirlik A, LaClair S, et al. Atherogenesis in mice does not require CD40 ligand from bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2005;25:1244–1249. doi: 10.1161/01.ATV.0000161420.55482.ef. [DOI] [PubMed] [Google Scholar]
- 101.Smook ML, Heeringa P, Damoiseaux JG, et al. Leukocyte CD40L deficiency affects the CD25(+) CD4 T cell population but does not affect atherosclerosis. Atherosclerosis. 2005;183:275–282. doi: 10.1016/j.atherosclerosis.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 102.Zirlik A, Maier C, Gerdes N, et al. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 2007;115:1571–1580. doi: 10.1161/CIRCULATIONAHA.106.683201. [DOI] [PubMed] [Google Scholar]
- 103.Lutgens E, Lievens D, Beckers L, et al. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010;207:391–404. doi: 10.1084/jem.20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Getz GS, Reardon CA. Do the Apoe−/− and Ldlr−/− Mice Yield the Same Insight on Atherogenesis? Arterioscler Thromb Vasc Biol. 2016;36:1734–1741. doi: 10.1161/ATVBAHA.116.306874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.von Scheidt M, Zhao Y, Kurt Z, et al. Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. Cell Metab. 2017;25:248–261. doi: 10.1016/j.cmet.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17:275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Nolte MA, van Olffen RW, van Gisbergen KP, et al. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev. 2009;229:216–231. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 108.Hendriks J, Xiao Y, Rossen JW, et al. During viral infection of the respiratory tract, CD27, 4–1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 109.Tesselaar K, Xiao Y, Arens R, et al. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 110.Coquet JM, Ribot JC, Babala N, et al. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27-CD70 pathway. J Exp Med. 2013;210:715–728. doi: 10.1084/jem.20112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Laouar A, Haridas V, Vargas D, et al. CD70(+) antigen-presenting cells control the proliferation and differentiation of T cells in the intestinal mucosa. Nat Immunol. 2005;6:698–706. doi: 10.1038/ni1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hendriks J, Gravestein LA, Tesselaar K, et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 113.Kobata T, Jacquot S, Kozlowski S, et al. CD27-CD70 interactions regulate B-cell activation by T cells. Proc Natl Acad Sci U S A. 1995;92:11249–11253. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xiao Y, Hendriks J, Langerak P, et al. CD27 is acquired by primed B cells at the centroblast stage and promotes germinal center formation. J Immunol. 2004;172:7432–7441. doi: 10.4049/jimmunol.172.12.7432. [DOI] [PubMed] [Google Scholar]
- 115.Jacquot S, Kobata T, Iwata S, et al. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J Immunol. 1997;159:2652–2657. [PubMed] [Google Scholar]
- 116.Agematsu K, Kobata T, Yang FC, et al. CD27/CD70 interaction directly drives B cell IgG and IgM synthesis. Eur J Immunol. 1995;25:2825–2829. doi: 10.1002/eji.1830251017. [DOI] [PubMed] [Google Scholar]
- 117.Agematsu K, Nagumo H, Oguchi Y, et al. Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction. Blood. 1998;91:173–180. [PubMed] [Google Scholar]
- 118.van Montfrans JM, Hoepelman AI, Otto S, et al. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J Allergy Clin Immunol. 2012;129:787–793. e786. doi: 10.1016/j.jaci.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salzer E, Daschkey S, Choo S, et al. Combined immunodeficiency with life-threatening EBV-associated lymphoproliferative disorder in patients lacking functional CD27. Haematologica. 2013;98:473–478. doi: 10.3324/haematol.2012.068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alkhairy OK, Perez-Becker R, Driessen GJ, et al. Novel mutations in TNFRSF7/CD27: Clinical, immunologic, and genetic characterization of human CD27 deficiency. J Allergy Clin Immunol. 2015;136:703–712. e710. doi: 10.1016/j.jaci.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 121.Arens R, Nolte MA, Tesselaar K, et al. Signaling through CD70 regulates B cell activation and IgG production. J Immunol. 2004;173:3901–3908. doi: 10.4049/jimmunol.173.6.3901. [DOI] [PubMed] [Google Scholar]
- 122.Dang LV, Nilsson A, Ingelman-Sundberg H, et al. Soluble CD27 induces IgG production through activation of antigen-primed B cells. J Intern Med. 2012;271:282–293. doi: 10.1111/j.1365-2796.2011.02444.x. [DOI] [PubMed] [Google Scholar]
- 123.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Oosterwijk MF, Juwana H, Arens R, et al. CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int Immunol. 2007;19:713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- 125.Peperzak V, Xiao Y, Veraar EA, et al. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J Clin Invest. 2010;120:168–178. doi: 10.1172/JCI40178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dolfi DV, Boesteanu AC, Petrovas C, et al. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8(+) T cells. Journal of Immunology. 2008;180:2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van Olffen RW, de Bruin AM, Vos M, et al. CD70-driven chronic immune activation is protective against atherosclerosis. J Innate Immun. 2010;2:344–352. doi: 10.1159/000314772. [DOI] [PubMed] [Google Scholar]
- 128.Arens R, Tesselaar K, Baars PA, et al. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 129.Winkels H, Meiler S, Smeets E, et al. CD70 limits atherosclerosis and promotes macrophage function. Thromb Haemost. 2016 doi: 10.1160/TH16-04-0318. [DOI] [PubMed] [Google Scholar]
- 130.Itani HA, Xiao L, Saleh MA, et al. CD70 Exacerbates Blood Pressure Elevation and Renal Damage in Response to Repeated Hypertensive Stimuli. Circ Res. 2016;118:1233–1243. doi: 10.1161/CIRCRESAHA.115.308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shimizu J, Yamazaki S, Takahashi T, et al. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 132.Kanamaru F, Youngnak P, Hashiguchi M, et al. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 133.Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 134.Placke T, Kopp HG, Salih HR. Glucocorticoid-induced TNFR-related (GITR) protein and its ligand in antitumor immunity: functional role and therapeutic modulation. Clin Dev Immunol. 2010;2010:239083. doi: 10.1155/2010/239083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gurney AL, Marsters SA, Huang RM, et al. Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Curr Biol. 1999;9:215–218. doi: 10.1016/s0960-9822(99)80093-1. [DOI] [PubMed] [Google Scholar]
- 136.Kwon B, Yu KY, Ni J, et al. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J Biol Chem. 1999;274:6056–6061. doi: 10.1074/jbc.274.10.6056. [DOI] [PubMed] [Google Scholar]
- 137.Kim WJ, Bae EM, Kang YJ, et al. Glucocorticoid-induced tumour necrosis factor receptor family related protein (GITR) mediates inflammatory activation of macrophages that can destabilize atherosclerotic plaques. Immunology. 2006;119:421–429. doi: 10.1111/j.1365-2567.2006.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meiler S, Smeets E, Winkels H, et al. Constitutive GITR Activation Reduces Atherosclerosis by Promoting Regulatory CD4+ T-Cell Responses-Brief Report. Arterioscler Thromb Vasc Biol. 2016;36:1748–1752. doi: 10.1161/ATVBAHA.116.307354. [DOI] [PubMed] [Google Scholar]
- 139.Clouthier DL, Watts TH. Cell-specific and context-dependent effects of GITR in cancer, autoimmunity, and infection. Cytokine Growth Factor Rev. 2014;25:91–106. doi: 10.1016/j.cytogfr.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 140.Croft M, So T, Duan W, et al. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song J, So T, Cheng M, et al. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 142.Chen AI, McAdam AJ, Buhlmann JE, et al. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 143.Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stuber E, Strober W. The T cell-B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J Exp Med. 1996;183:979–989. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Karulf M, Kelly A, Weinberg AD, et al. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J Immunol. 2010;185:4856–4862. doi: 10.4049/jimmunol.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang X, Ria M, Kelmenson PM, et al. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat Genet. 2005;37:365–372. doi: 10.1038/ng1524. [DOI] [PubMed] [Google Scholar]
- 147.Olofsson PS, Soderstrom LA, Jern C, et al. Genetic variants of TNFSF4 and risk for carotid artery disease and stroke. J Mol Med (Berl) 2009;87:337–346. doi: 10.1007/s00109-008-0412-5. [DOI] [PubMed] [Google Scholar]
- 148.Ria M, Eriksson P, Boquist S, et al. Human genetic evidence that OX40 is implicated in myocardial infarction. Biochem Biophys Res Commun. 2006;339:1001–1006. doi: 10.1016/j.bbrc.2005.11.092. [DOI] [PubMed] [Google Scholar]
- 149.van Wanrooij EJ, van Puijvelde GH, de Vos P, et al. Interruption of the Tnfrsf4/Tnfsf4 (OX40/OX40L) pathway attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:204–210. doi: 10.1161/01.ATV.0000251007.07648.81. [DOI] [PubMed] [Google Scholar]
- 150.Foks AC, van Puijvelde GH, Bot I, et al. Interruption of the OX40-OX40 ligand pathway in LDL receptor-deficient mice causes regression of atherosclerosis. J Immunol. 2013;191:4573–4580. doi: 10.4049/jimmunol.1200708. [DOI] [PubMed] [Google Scholar]
- 151.Kennedy MK, Willis CR, Armitage RJ. Deciphering CD30 ligand biology and its role in humoral immunity. Immunology. 2006;118:143–152. doi: 10.1111/j.1365-2567.2006.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee SY, Kandala G, Liou ML, et al. CD30/TNF receptor-associated factor interaction: NF-kappa B activation and binding specificity. Proc Natl Acad Sci U S A. 1996;93:9699–9703. doi: 10.1073/pnas.93.18.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gaspal FM, Kim MY, McConnell FM, et al. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol. 2005;174:3891–3896. doi: 10.4049/jimmunol.174.7.3891. [DOI] [PubMed] [Google Scholar]
- 154.Shanebeck KD, Maliszewski CR, Kennedy MK, et al. Regulation of murine B cell growth and differentiation by CD30 ligand. Eur J Immunol. 1995;25:2147–2153. doi: 10.1002/eji.1830250805. [DOI] [PubMed] [Google Scholar]
- 155.Foks AC, Bot I, Frodermann V, et al. Interference of the CD30-CD30L pathway reduces atherosclerosis development. Arterioscler Thromb Vasc Biol. 2012;32:2862–2868. doi: 10.1161/ATVBAHA.112.300509. [DOI] [PubMed] [Google Scholar]
- 156.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kwon BS, Hurtado JC, Lee ZH, et al. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002;168:5483–5490. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 158.Vinay DS, Choi BK, Bae JS, et al. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J Immunol. 2004;173:4218–4229. doi: 10.4049/jimmunol.173.6.4218. [DOI] [PubMed] [Google Scholar]
- 159.Lee SW, Park Y, So T, et al. Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells. Nat Immunol. 2008;9:917–926. doi: 10.1038/ni.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Choi BK, Kim YH, Kwon PM, et al. 4-1BB functions as a survival factor in dendritic cells. J Immunol. 2009;182:4107–4115. doi: 10.4049/jimmunol.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baessler T, Charton JE, Schmiedel BJ, et al. CD137 ligand mediates opposite effects in human and mouse NK cells and impairs NK-cell reactivity against human acute myeloid leukemia cells. Blood. 2010;115:3058–3069. doi: 10.1182/blood-2009-06-227934. [DOI] [PubMed] [Google Scholar]
- 162.Lee SW, Vella AT, Kwon BS, et al. Enhanced CD4 T cell responsiveness in the absence of 4–1BB. J Immunol. 2005;174:6803–6808. doi: 10.4049/jimmunol.174.11.6803. [DOI] [PubMed] [Google Scholar]
- 163.Olofsson PS, Soderstrom LA, Wagsater D, et al. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008;117:1292–1301. doi: 10.1161/CIRCULATIONAHA.107.699173. [DOI] [PubMed] [Google Scholar]
- 164.Jeon HJ, Choi JH, Jung IH, et al. CD137 (4-1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010;121:1124–1133. doi: 10.1161/CIRCULATIONAHA.109.882704. [DOI] [PubMed] [Google Scholar]
- 165.Jung IH, Choi JH, Jin J, et al. CD137-inducing factors from T cells and macrophages accelerate the destabilization of atherosclerotic plaques in hyperlipidemic mice. FASEB J. 2014;28:4779–4791. doi: 10.1096/fj.14-253732. [DOI] [PubMed] [Google Scholar]
- 166.Tu TH, Kim CS, Nam-Goong IS, et al. 4-1BBL signaling promotes cell proliferation through reprogramming of glucose metabolism in monocytes/macrophages. FEBS J. 2015;282:1468–1480. doi: 10.1111/febs.13236. [DOI] [PubMed] [Google Scholar]
- 167.Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 168.Morel Y, Schiano de Colella JM, Harrop J, et al. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol. 2000;165:4397–4404. doi: 10.4049/jimmunol.165.8.4397. [DOI] [PubMed] [Google Scholar]
- 169.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 170.Sandberg WJ, Halvorsen B, Yndestad A, et al. Inflammatory interaction between LIGHT and proteinase-activated receptor-2 in endothelial cells: potential role in atherogenesis. Circ Res. 2009;104:60–68. doi: 10.1161/CIRCRESAHA.108.188078. [DOI] [PubMed] [Google Scholar]
- 171.Lee WH, Kim SH, Lee Y, et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001;21:2004–2010. doi: 10.1161/hq1201.098945. [DOI] [PubMed] [Google Scholar]
- 172.Celik S, Langer H, Stellos K, et al. Platelet-associated LIGHT (TNFSF14) mediates adhesion of platelets to human vascular endothelium. Thromb Haemost. 2007;98:798–805. [PubMed] [Google Scholar]
- 173.von Hundelshausen P, Weber KS, Huo Y, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 174.Otterdal K, Smith C, Oie E, et al. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood. 2006;108:928–935. doi: 10.1182/blood-2005-09-010629. [DOI] [PubMed] [Google Scholar]
- 175.Lo JC, Wang Y, Tumanov AV, et al. Lymphotoxin beta receptor-dependent control of lipid homeostasis. Science. 2007;316:285–288. doi: 10.1126/science.1137221. [DOI] [PubMed] [Google Scholar]
- 176.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Koga N, Suzuki J, Kosuge H, et al. Blockade of the interaction between PD-1 and PD-L1 accelerates graft arterial disease in cardiac allografts. Arterioscler Thromb Vasc Biol. 2004;24:2057–2062. doi: 10.1161/01.ATV.0000145015.23656.e4. [DOI] [PubMed] [Google Scholar]
- 178.Rodig N, Ryan T, Allen JA, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 179.Grabie N, Gotsman I, DaCosta R, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116:2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 180.Chen WJ, Hu XF, Yan M, et al. Human umbilical vein endothelial cells promote the inhibitory activation of CD4(+)CD25(+)Foxp3(+) regulatory T cells via PD-L1. Atherosclerosis. 2016;244:108–112. doi: 10.1016/j.atherosclerosis.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 181.Gotsman I, Grabie N, Dacosta R, et al. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee J, Zhuang Y, Wei X, et al. Contributions of PD-1/PD-L1 pathway to interactions of myeloid DCs with T cells in atherosclerosis. J Mol Cell Cardiol. 2009;46:169–176. doi: 10.1016/j.yjmcc.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 183.Bu DX, Tarrio M, Maganto-Garcia E, et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1100–1107. doi: 10.1161/ATVBAHA.111.224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cochain C, Chaudhari SM, Koch M, et al. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS One. 2014;9:e93280. doi: 10.1371/journal.pone.0093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Qiu MK, Wang SC, Dai YX, et al. PD-1 and Tim-3 Pathways Regulate CD8+ T Cells Function in Atherosclerosis. PLoS One. 2015;10:e0128523. doi: 10.1371/journal.pone.0128523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hughes PE, Caenepeel S, Wu LC. Targeted Therapy and Checkpoint Immunotherapy Combinations for the Treatment of Cancer. Trends Immunol. 2016;37:462–476. doi: 10.1016/j.it.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 187.Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 188.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 189.Ewing MM, Karper JC, Abdul S, et al. T-cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int J Cardiol. 2013;168:1965–1974. doi: 10.1016/j.ijcard.2012.12.085. [DOI] [PubMed] [Google Scholar]
- 190.Ma K, Lv S, Liu B, et al. CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T-cell overactivation in apoE(−/−) mice. Cardiovasc Res. 2013;97:349–359. doi: 10.1093/cvr/cvs330. [DOI] [PubMed] [Google Scholar]
- 191.Matsumoto T, Sasaki N, Yamashita T, et al. Overexpression of Cytotoxic T-Lymphocyte-Associated Antigen-4 Prevents Atherosclerosis in Mice. Arterioscler Thromb Vasc Biol. 2016;36:1141–1151. doi: 10.1161/ATVBAHA.115.306848. [DOI] [PubMed] [Google Scholar]
- 192.Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48:719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 193.Andre P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 194.Fayad L, Ansell SM, Advani R, et al. Dacetuzumab plus rituximab, ifosfamide, carboplatin and etoposide as salvage therapy for patients with diffuse large B-cell lymphoma relapsing after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone: a randomized, double-blind, placebo-controlled phase 2b trial. Leuk Lymphoma. 2015;56:2569–2578. doi: 10.3109/10428194.2015.1007504. [DOI] [PubMed] [Google Scholar]
- 195.Advani R, Forero-Torres A, Furman RR, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 196.Fanale M, Assouline S, Kuruvilla J, et al. Phase IA/II, multicentre, open-label study of the CD40 antagonistic monoclonal antibody lucatumumab in adult patients with advanced non-Hodgkin or Hodgkin lymphoma. Br J Haematol. 2014;164:258–265. doi: 10.1111/bjh.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rosetti F, Mayadas TN. The many faces of Mac-1 in autoimmune disease. Immunol Rev. 2016;269:175–193. doi: 10.1111/imr.12373. [DOI] [PubMed] [Google Scholar]
- 198.Wolf D, Hohmann JD, Wiedemann A, et al. Binding of CD40L to Mac-1’s I-domain involves the EQLKKSKTL motif and mediates leukocyte recruitment and atherosclerosis--but does not affect immunity and thrombosis in mice. Circ Res. 2011;109:1269–1279. doi: 10.1161/CIRCRESAHA.111.247684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zarzycka B, Seijkens T, Nabuurs SB, et al. Discovery of small molecule CD40-TRAF6 inhibitors. J Chem Inf Model. 2015;55:294–307. doi: 10.1021/ci500631e. [DOI] [PubMed] [Google Scholar]
- 200.Chatzigeorgiou A, Seijkens T, Zarzycka B, et al. Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance. Proc Natl Acad Sci U S A. 2014;111:2686–2691. doi: 10.1073/pnas.1400419111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Diegmann J, Junker K, Gerstmayer B, et al. Identification of CD70 as a diagnostic biomarker for clear cell renal cell carcinoma by gene expression profiling, real-time RT-PCR and immunohistochemistry. Eur J Cancer. 2005;41:1794–1801. doi: 10.1016/j.ejca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 202.Junker K, Hindermann W, von Eggeling F, et al. CD70: a new tumor specific biomarker for renal cell carcinoma. J Urol. 2005;173:2150–2153. doi: 10.1097/01.ju.0000158121.49085.ba. [DOI] [PubMed] [Google Scholar]
- 203.Ryan MC, Kostner H, Gordon KA, et al. Targeting pancreatic and ovarian carcinomas using the auristatin-based anti-CD70 antibody-drug conjugate SGN-75. Br J Cancer. 2010;103:676–684. doi: 10.1038/sj.bjc.6605816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wischhusen J, Jung G, Radovanovic I, et al. Identification of CD70-mediated apoptosis of immune effector cells as a novel immune escape pathway of human glioblastoma. Cancer Res. 2002;62:2592–2599. [PubMed] [Google Scholar]
- 205.Trebing J, El-Mesery M, Schafer V, et al. CD70-restricted specific activation of TRAILR1 or TRAILR2 using scFv-targeted TRAIL mutants. Cell Death Dis. 2014;5:e1035. doi: 10.1038/cddis.2013.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang ZZ, Grote DM, Xiu B, et al. TGF-beta upregulates CD70 expression and induces exhaustion of effector memory T cells in B-cell non-Hodgkin’s lymphoma. Leukemia. 2014;28:1872–1884. doi: 10.1038/leu.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Grewal IS. CD70 as a therapeutic target in human malignancies. Expert Opin Ther Targets. 2008;12:341–351. doi: 10.1517/14728222.12.3.341. [DOI] [PubMed] [Google Scholar]
- 208.https://clinicaltrials.gov/ct2/results?term=cd70+tumor&Search=Search.
- 209.van de Ven K, Borst J. Targeting the T-cell co-stimulatory CD27/CD70 pathway in cancer immunotherapy: rationale and potential. Immunotherapy. 2015;7:655–667. doi: 10.2217/imt.15.32. [DOI] [PubMed] [Google Scholar]
- 210.https://clinicaltrials.gov/ct2/results?term=varlilumab&Search=Search.
- 211.He LZ, Prostak N, Thomas LJ, et al. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191:4174–4183. doi: 10.4049/jimmunol.1300409. [DOI] [PubMed] [Google Scholar]
- 212.Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 213.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]


