Abstract
Cardiac arrest (CA) is one of the leading causes of mortality and morbidity in the world. Fast, reversible and controllable pharmaceutical-induced hypothermia (PIH) is strongly desired to treat ischemia-reperfusion brain injury. Dihydrocapsaicin (DHC), an agonist of transient receptor potential vanilloid type 1 cation channel (TRPV1), is an emerging candidate for PIH. Its capability to lower body temperature has been validated in both healthy and stroke animal models. However, DHC has shown cardiovascular effects and its safety and feasibility in a CA model has not been tested. Additionally, activated TRPV1 has multiple functions in addition to regulating body temperature and its effect on neurological recovery needs to be evaluated. In this study, we compared two methods of DHC administration, bolus injection and infusion via the femoral vein. We found that cardiovascular effects were only seen with a large dose DHC bolus injection. Then, we applied DHC-induced hypothermia in an asphyxial-CA rat model. We showed that DHC-treated rats were viable. Four-hour infusion of DHC at a rate of 0.75 mg/kg/h after CA maintained a body temperature of about 34 °C for at least 8 hours. DHC-treated rats had higher electrical activity during the first 4 hours after CA and had better neurological recovery during the 3 days after CA compared with normothermia rats. Additional pathway investigation of DHC administration following CA will further uncover the benefits of DHC-induced hypothermia.
I. Introduction
Cardiac arrest (CA) is one of the leading causes of mortality and morbidity in the world. Therapeutic hyperthermia (TH) is the most effective treatment for CA patients [1]. At present, clinical TH protocols involve methods of physical cooling, of which one of the shortcomings is the shivering response that must be countered by sedatives and mechanical ventilation, which complicate the prognosis and recovery [2]. Therefore, fast, reversible and controllable pharmaceutical-induced hypothermia (PIH) would provide a good alternative methodology.
Several candidates targeting distinct signaling pathways have been tested for PIH including a neurotensin receptor agonist HPI201/ABS201 in stroke and TBI models [3, 4], a cannabinoid receptor agonist WIN55, 212-2 in a ventricular defibrillation CA model [5], the A1 adenosine receptor agonist 8-SPT in an asphyxial-CA model [6], the muscarinic cholinergic partial agonist U-80816E in the gerbil brief bilateral carotid occlusion ischemia model [7], a dopaminergic agonist Talipexole in stroke model [8], a serotonergic agonist S14671 in a stroke model [9] and the transient receptor potential cation channel vanilloid type 1 (TRPV1) agonist dihydrocapsaicin (DHC) in a stroke model [10].
Body temperature is often dysregulated after CA, embodied by either fever or spontaneous hypothermia. TRPV1 has a role in body temperature sensation and regulation [11]. TRPV1 is widely expressed in all tissues and activated by heat (> 43 °C), low pH and some chemicals such as capsaicin and ethanol. DHC, a capsaicin analog, is roughly 65% more effective than capsaicin in producing hypothermia [12], and is consequently a promising candidate for PIH. The capability of DHC to lower body temperature has been validated in healthy mice, rats, cows and monkeys [13, 14] and in stroke mice [10]. However, DHC has displayed cardiovascular effects [15], and whether CA victims could tolerate DHC has not been determined. Since activating TRPV1 may be protective or detrimental in neuronal injury [10, 16, 17], it is unclear whether or not CA victims could benefit from DHC administration.
In this study, we tested the applicability of DHC-induced hypothermia in an asphyxial-CA rat model by comparing the electrical activity during the first 4 hours after CA and the neurological deficit score (NDS) during the 3 days after CA, between DHC-treated rats and the vehicle-treated rats.
II. Methods
The experimental protocols were approved by the University of Maryland Animal Care and Use Committee. A total of nine adult Wistar rats (300–350 g) were used. Three rats were used to test the DHC administration methods. Six rats were randomly allocated into two groups (DHC-treated CA vs normothermia CA vehicle control, n=3 per group) for the asphyxial-CA model.
A. Asphyxial-CA model
The experimental procedure to induce CA and resuscitation was modified from previously published papers [18–20]. Briefly, rats were implanted with cortical electrodes 3 days before CA. On the day of CA, rats were anesthetized by 1.5% isoflurane and intubated. The femoral artery and vein were cannulated to administer drugs and monitor mean arterial blood pressure (MAP). Before CA, there was a 2-min anesthesia washout period with 100% oxygen, then the rats were paralyzed via an i.v. bolus injection of vecuronium (2 mg/kg) followed by 3 min of room air ventilation. CA was induced by clamping the breathing circuit and disconnecting the ventilator for 9 min. The time of CA was recorded when pulse pressure <10 mmHg. Cardiopulmonary resuscitation (CPR) was initiated immediately after 9 min of asphyxia with oxygenation, sternal chest compressions, epinephrine and NaHCO3 until MAP > 60 mmHg, which we defined as return of spontaneous circulation (ROSC). The femoral arterial blood gases (ABG) were measured before CA and 20 min after ROSC. Electroencephalogram (EEG) and electrocardiogram (ECG) were monitored by electrodes connected to a TDT System3 data acquisition system (Tucker-Davis Technologies, Alachua, FL). Core temperature was measured with a rectal probe and recorded periodically.
B. DHC-induced hypothermia
DHC (Cayman, Ann Arbor, MI) was dissolved in dimethylsulfoxide (DMSO) to 33 mg/ml as stock solution and stored at −20 °C. On the day of CA, the DHC solution was diluted in 2% Tween-20 saline to a final concentration of 0.75 mg/ml [14]. First, two methods were employed to deliver DHC via the femoral vein. The bolus injection method was only applied to healthy isoflurane-anaesthetized rats. Either 750 μg/kg or 100 μg/kg was administered per injection for a maximum dose of 3 mg/kg to test the cardiovascular effect of DHC. In the syringe pump method, the DHC solution was infused from 10 min to 4 h after ROSC at a rate of 0.75 mg/kg/h. Normothermia CA control rats were infused the same way with the vehicle solution.
Before CA, the rectal temperature of rats was maintained at 37 ± 0.5 °C by a heating lamp. After CA, for the normothermia CA control rats, the environmental temperature was controlled at 28 ± 0.5 °C with a heating lamp. For DHC rats, the environmental temperature was maintained at 28 ± 0.5 °C with the heating lamp after the rectal temperature of rats reached 34 °C. Eight hours after ROSC, rats were put in a neonatal incubator at 28 °C to prevent spontaneous hypothermia overnight.
C. EEG quantification and NDS evaluation
The EEG signals were examined for noise, both in the time domain using TDT software and in the frequency domain using MATLAB (Mathworks, Natick, MA). Artifacts were excluded in the final analysis. Entropy-based quantitative EEG informational quantity (qEEG-IQ) was used to objectively track the cerebral electrical activity recovery after CA [21]. The qEEG-IQ was computed every 30 min after ROSC and normalized to baseline. An IQ of 0 was assigned to dead animals and a higher IQ indicated better recovery.
The NDS of each animal ranged from 0–80 based on a series of behavioral tests [18] at 6 h, 24 h, 48 h and 72 h after ROSC. The NDS examination was performed by a trained examiner blinded to groups and the functional outcome was defined by the 72-hr NDS score.
D. Statistical analysis
The data was analyzed by SPSS (version 22, Armonk, NY). Group values that are parametric (i.e. body temperature and qEEG-IQ) are reported as mean ± SEM. Nonparametric variables (i.e., NDS) are reported as median (25th–75th, percentile). Univariate analysis was used to compare aggregated qEEG-IQ during the 4 hours after ROSC and the averaged qEEG-IQ at the indicated time intervals.
III. Results
A. Comparison of bolus injection and infusion: the two methods of DHC administration
Bolus administration of the drug was preferred for convenience. We first tested whether bolus injection of DHC was feasible in two healthy anesthetized rats. A 750 μg/kg/bolus i.v. injection of DHC caused breathing and the heart to stop, which was reversed by CPR in a timely manner. Rats responded to 100 μg/kg/bolus injections of DHC with relatively mild cardiovascular and blood pressure effects. Specifically, there was a transient increase of MAP for about 1 min, and an episode of cardiac arrhythmia lasting tens of seconds (Fig 1). Infusion of DHC in a healthy anesthetized rat at a rate of 750 μg/kg/h had no noticeable cardiovascular effect. Thus, we decided to administer DHC via infusion in the subsequent CA rats.
Fig 1.
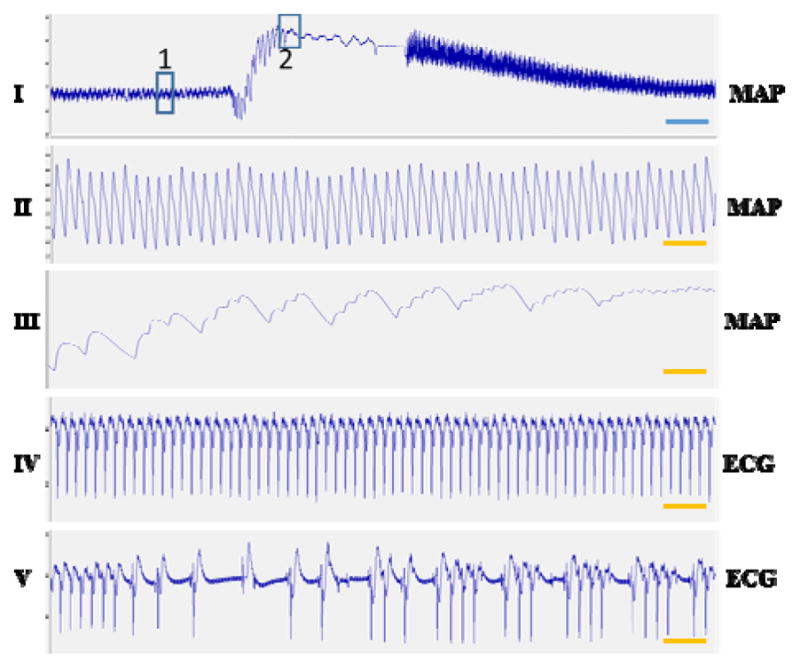
Cardiovascular effect of DHC by bolus intravenous injection at the dose of 100 μg/kg in healthy rat. I: a 3 min recording of MAP. II: Enlarged MAP of box 1 in I. III: Enlarged MAP of box 2 in I. IV: ECG at the same time interval of box 1. V: ECG at the same time of box 2. Box1 is before and box 2 is after the injection of DHC. Blue bar indicates 20 sec, orange bar indicates 2 sec. There is a transient increase of MAP (III compare with II) and arrhythmia (V compare with IV)
B. Body temperature management with DHC
Next, we tested the hypothermia effect of DHC on a rat CA model. DHC was infused from 10 min to 4 h after ROSC. The target temperature of 32–34 °C was reached in 80 ± 15 min. After the infusion was completed, hypothermia was continuously maintained for at least 4 more hours. The normothermia CA vehicle-control rats had a normal body temperature throughout the experiment (37 ± 0.5 °C) (Fig. 2).
Fig 2.
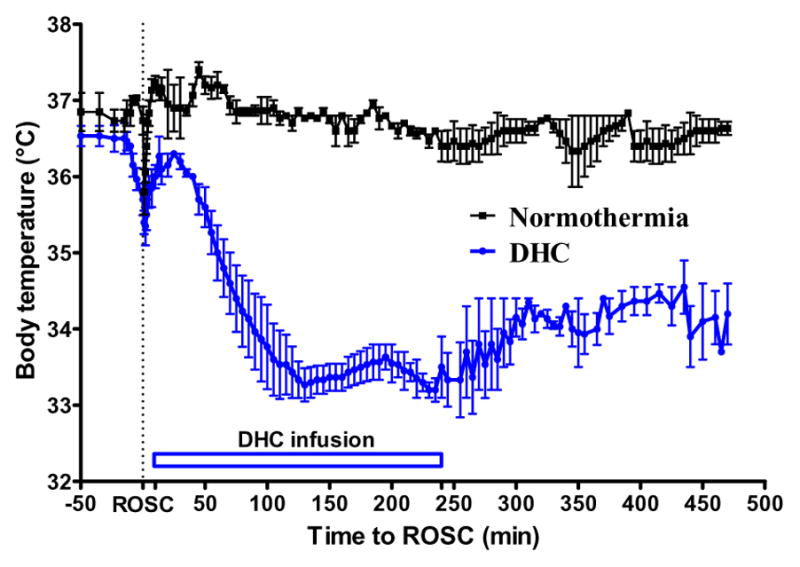
DHC-induced hypothermia in CA model. DHC was infused from 10 min until 4 h after ROSC.
C. Effect of DHC-induced hypothermia on qEEG-IQ after CA
The aggregated qEEG-IQ of DHC-treated rats were higher than that of normothermia CA control rats (0.73 ± 0.04 vs 0.60 ± 0.04, p = 0.04) during the first 4 hours after ROSC. The averaged qEEG-IQ of DHC-treated rats were significantly higher than that of normothermia CA control rats (0.64 ± 0.05 vs 0.43 ± 0.05, p = 0.033) at the early time interval of 30–60 min after ROSC. The qEEG-IQ was similarly higher in DHC-treated rats than control rats at 60–90 min (p = 0.052) and 90–120 min (p = 0.065) after ROSC, but the differences were not significant with the current cohort (Fig. 3).
Fig. 3.
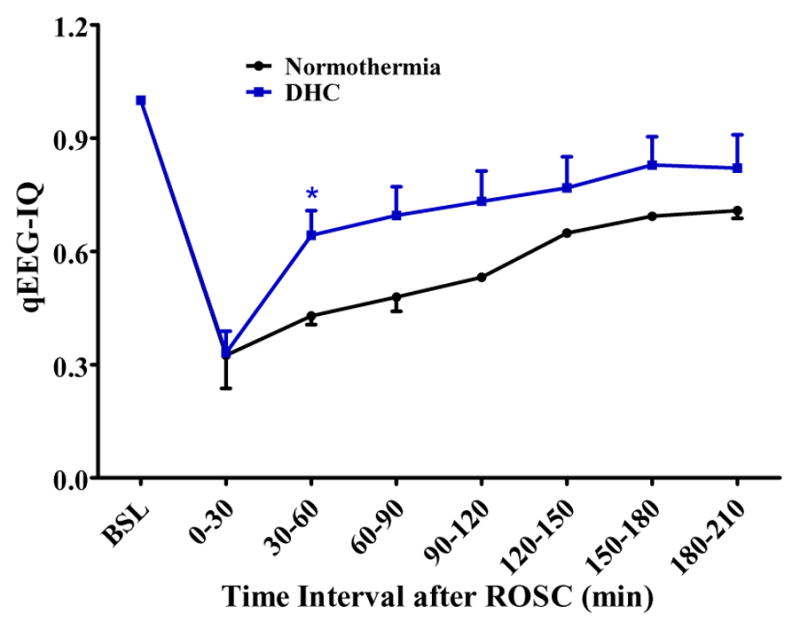
Comparison of qEEG-IQ between normothermia CA control rats and DHC-treated CA rats.
D. Effect of DHC-induced hypothermia on the neurological recovery after CA
At 72 h, the mortality rate of DHC-treated rats was the same as the normothermia CA control rats (1 out of 3 in each group). NDS was compared between normothemia CA control and DHC-treated CA rats at 6 h, 24 h, 48 h and 72 h after ROSC (Fig. 4). Due to the small number of experimental animals in this pilot study, statistical analysis of NDS between groups was not the current focus. Overall, the median NDS was better in DHC-treated CA rats (67) than normothermia CA control rats (47).
Fig 4.
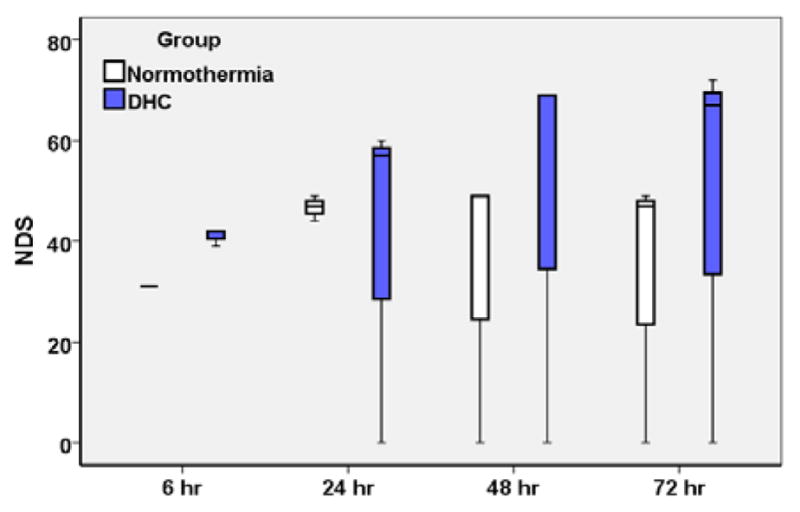
Comparison of NDS between normothermia CA control rats and DHC-treated CA rats. Box-plot of median and interquartile range (25th and 75th percentile,).
IV. Discussion
This is the first study to test the feasibility of DHC-induced hypothermia in treating post-resuscitation rats in an asphyxial-CA model. We showed that DHC-treated rats were viable. DHC steadily decreased the body temperature below 34 °C within 80 ± 15 min, and after DHC infusion, the body temperature was maintained around 34 °C for at least 4 more hours (Fig 2). There were no notable complications with DHC administration in post-resuscitation animals. The NDS of DHC-treated rats tended to be better, with a higher median NDS than that of normothermia rats (Fig 4) and comparable with surface cooling controlled hypothermia (data not shown). Therefore, DHC is a promising PIH candidate to treat CA patients.
It was a concern that DHC might induce the Bezold-Jarisch reflex and airway reflex that cause tachycardia, hypotension, cough and bronchoconstriction [15]. However, we showed that the negative effect of DHC was related to the administration method. The large bolus injection (0.75 mg/kg) induced respiratory and heart stoppage, and the small bolus injection (100 μg/kg) caused mild tachycardia and hypotension, however, infusion at a rate of 0.75 mg/kg/h had no cardiovascular effects. Fosgerau et al reported that DHC infusion at the rate of 0.65 mg/kg/h induced several episodes of transient tachycardia and hypotension, which is contradictory to our findings. This may be related to the composition of infusion solution, strain of rats or the recovery status of CA rats. In our case, 0.75 mg/kg/h infusion rate was safe for Wistar rats.
In addition to thermal sensation and regulation [22], TRPV1 is also involved in sensation of pain, susceptibility to infection [23] and vascular-related pathologies including hypertension and ischemia-reperfusion injury [24]. These many functions must be considered to effectively evaluate the overall effect of DHC on TRPV1. In stroke mice, DHC-activated TRPV1 decreased infarct volume and improved neuro-function [10]. In our pilot study, DHC-treated CA rats showed an early increase in cerebral electrical activity in the early recovery period and improved median NDS at 72 h after CA. However, it is reported that capsaicin activated TRPV1 caused a blood-brain barrier disruption in the rat [25]. Stroke activated TRPV1 exacerbates neurological and motor deficits and infarction [16]. These conflicting effects of TRPV1 on neuronal function may depend on the method of activation, degree of activation and severity of brain injury.
The mechanisms of TRPV1 regulation after CA and TH are unknown. Impaired thermoregulation (mainly spontaneous hypothermia) is common after CA [26], and high incidence of fever (41–89%) was reported after TH in post-CA patients [27]. We have observed occasional fever from 24 h to 72 h in post-CA rats under normothermia, surface cooling hypothermia, and DHC-induced hypothermia (data not shown). In future work, DHC involved pathway, which contributes to the post-resuscitation recovery, will be of our primary interest.
V. Conclusion
In this pilot study, we showed that DHC-induced hypothermia was applicable to CA and showed its benefits to neurological recovery. Further studies on the alteration of TRPV1 after CA, at both the expression and activity levels, are needed to elucidate the mechanism of DHC’s protective role in brain ischemia-reperfusion injury.
Acknowledgments
Research supported by R01HL118084 from NIH (to XJ). Dr. Jia was supported in partial by Maryland Stem Cell Research Fund (2013-MSCRFE-146-00) (to XJ).
Contributor Information
Junyun He, Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, MD, 21201, USA
Leanne Young, Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD 21205
Xiaofeng Jia, Department of Neurosurgery, Orthopaedics, University of Maryland School of Medicine, Baltimore, MD, 21201, USA, and Department of Biomedical Engineering, Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, 21205, USA.
References
- 1.Palmers PJ, Hiltrop N, Ameloot K, Timmermans P, Ferdinande B, Sinnaeve P, et al. From therapeutic hypothermia towards targeted temperature management: a decade of evolution. Anaesthesiol Intensive Ther. 2015;47:156–61. doi: 10.5603/AIT.a2014.0066. [DOI] [PubMed] [Google Scholar]
- 2.Feketa VV, Balasubramanian A, Flores CM, Player MR, Marrelli SP. Shivering and tachycardic responses to external cooling in mice are substantially suppressed by TRPV1 activation but not by TRPM8 inhibition. Am J Physiol Regul Integr Comp Physiol. 2013 Nov 1;305:R1040–50. doi: 10.1152/ajpregu.00296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu X, Wei ZZ, Espinera A, Lee JH, Ji X, Wei L, et al. Pharmacologically induced hypothermia attenuates traumatic brain injury in neonatal rats. Exp Neurol. 2015 May;267:135–42. doi: 10.1016/j.expneurol.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi KE, Hall CL, Sun JM, Wei L, Mohamad O, Dix TA, et al. A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. Faseb j. 2012 Jul;26:2799–810. doi: 10.1096/fj.11-201822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weng Y, Sun S, Park J, Ye S, Weil MH, Tang W. Cannabinoid 1 (CB1) receptor mediates WIN55, 212–2 induced hypothermia and improved survival in a rat post-cardiac arrest model. Resuscitation. 2012 Sep;83:1145–51. doi: 10.1016/j.resuscitation.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Jinka TR, Combs VM, Drew KL. Translating drug-induced hibernation to therapeutic hypothermia. ACS Chem Neurosci. 2015 Jun 17;6:899–904. doi: 10.1021/acschemneuro.5b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall ED, Andrus PK, Pazara KE. Protective efficacy of a hypothermic pharmacological agent in gerbil forebrain ischemia. Stroke. 1993 May;24:711–5. doi: 10.1161/01.str.24.5.711. [DOI] [PubMed] [Google Scholar]
- 8.Johansen FF, Jorgensen HS, Reith J. Prolonged drug-induced hypothermia in experimental stroke. J Stroke Cerebrovasc Dis. 2003 Mar-Apr;12:97–102. doi: 10.1053/jscd.2003.14. [DOI] [PubMed] [Google Scholar]
- 9.Johansen FF, Hasseldam H, Nybro Smith M, Rasmussen RS. Drug-induced hypothermia by 5HT1A agonists provide neuroprotection in experimental stroke: new perspectives for acute patient treatment. J Stroke Cerebrovasc Dis. 2014 Nov-Dec;23:2879–87. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Cao Z, Balasubramanian A, Marrelli SP. Pharmacologically induced hypothermia via TRPV1 channel agonism provides neuroprotection following ischemic stroke when initiated 90 min after reperfusion. Am J Physiol Regul Integr Comp Physiol. 2014 Jan 15;306:R149–56. doi: 10.1152/ajpregu.00329.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M. Attenuated fever response in mice lacking TRPV1. Neurosci Lett. 2005 Apr 11;378:28–33. doi: 10.1016/j.neulet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Miller MS, Brendel K, Buck SH, Burks TF. Dihydrocapsaicin-induced hypothermia and substance P depletion. Eur J Pharmacol. 1982 Sep 24;83:289–92. doi: 10.1016/0014-2999(82)90263-1. [DOI] [PubMed] [Google Scholar]
- 13.Feketa VV, Zhang Y, Cao Z, Balasubramanian A, Flores CM, Player MR, et al. Transient receptor potential melastatin 8 channel inhibition potentiates the hypothermic response to transient receptor potential vanilloid 1 activation in the conscious mouse. Crit Care Med. 2014 May;42:e355–63. doi: 10.1097/CCM.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fosgerau K, Weber UJ, Gotfredsen JW, Jayatissa M, Buus C, Kristensen NB, et al. Drug-induced mild therapeutic hypothermia obtained by administration of a transient receptor potential vanilloid type 1 agonist. BMC Cardiovasc Disord. 2010;10:51. doi: 10.1186/1471-2261-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fosgerau K, Ristagno G, Jayatissa M, Axelsen M, Gotfredsen JW, Weber UJ, et al. Increased susceptibility to cardiovascular effects of dihydrocapcaicin in resuscitated rats. Cardiovascular effects of dihydrocapsaicin. BMC Cardiovasc Disord. 2010;10:39. doi: 10.1186/1471-2261-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyanohara J, Shirakawa H, Sanpei K, Nakagawa T, Kaneko S. A pathophysiological role of TRPV1 in ischemic injury after transient focal cerebral ischemia in mice. Biochem Biophys Res Commun. 2015 Oct 8; doi: 10.1016/j.bbrc.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto K, Kuroki T, Okuno Y, Sekiya H, Watanabe A, Sagawa T, et al. Activation of the TRPV1 channel attenuates N-methyl-D-aspartic acid-induced neuronal injury in the rat retina. Eur J Pharmacol. 2014 Jun 15;733:13–22. doi: 10.1016/j.ejphar.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Jia X, Koenig MA, Shin HC, Zhen G, Yamashita S, Thakor NV, et al. Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats. Brain Res. 2006 Sep 21;1111:166–75. doi: 10.1016/j.brainres.2006.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia X, Koenig MA, Shin HC, Zhen G, Pardo CA, Hanley DF, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008 Mar;76:431–42. doi: 10.1016/j.resuscitation.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J, Lu H, Deng R, Young L, Tong S, Jia X. Real-time monitoring of cerebral blood flow by laser speckle contrast imaging after cardiac arrest in rat. Conf Proc IEEE Eng Med Biol Soc. 2015 Aug;:6971–4. doi: 10.1109/EMBC.2015.7319996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia X, Koenig MA, Nickl R, Zhen G, Thakor NV, Geocadin RG. Early electrophysiologic markers predict functional outcome associated with temperature manipulation after cardiac arrest in rats. Crit Care Med. 2008 Jun;36:1909–16. doi: 10.1097/CCM.0b013e3181760eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292:R64–76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 23.Billeter AT, Hellmann JL, Bhatnagar A, Polk HC., Jr Transient receptor potential ion channels: powerful regulators of cell function. Ann Surg. 2014 Feb;259:229–35. doi: 10.1097/SLA.0b013e3182a6359c. [DOI] [PubMed] [Google Scholar]
- 24.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev. 2015 Apr;95:645–90. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu DE, Easton AS, Fraser PA. TRPV1 activation results in disruption of the blood-brain barrier in the rat. Br J Pharmacol. 2005 Oct;146:576–84. doi: 10.1038/sj.bjp.0706350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benz-Woerner J, Delodder F, Benz R, Cueni-Villoz N, Feihl F, Rossetti AO, et al. Body temperature regulation and outcome after cardiac arrest and therapeutic hypothermia. Resuscitation. 2012 Mar;83:338–42. doi: 10.1016/j.resuscitation.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Vanhengel K, De Deyne C, Dens J. Post-cooling controlled normothermia and pyrexia after therapeutic hypothermia. Resuscitation. 2013 Aug;84:e87. doi: 10.1016/j.resuscitation.2012.12.030. [DOI] [PubMed] [Google Scholar]


