Abstract
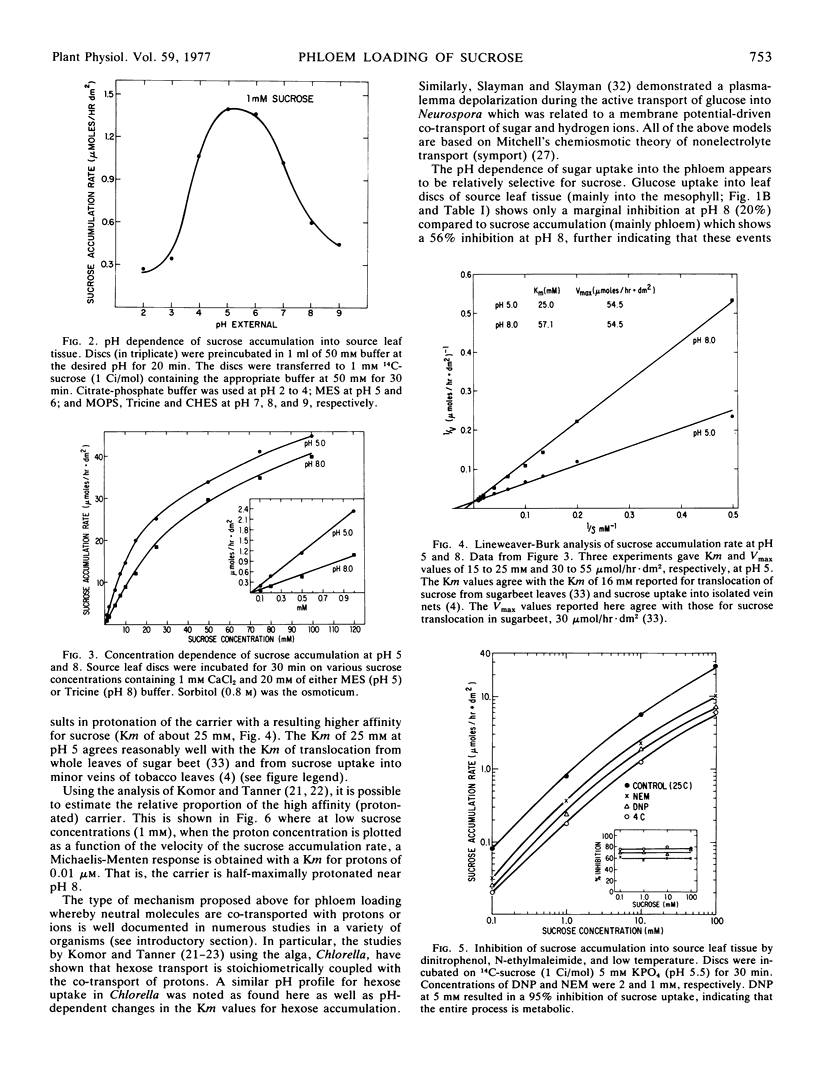
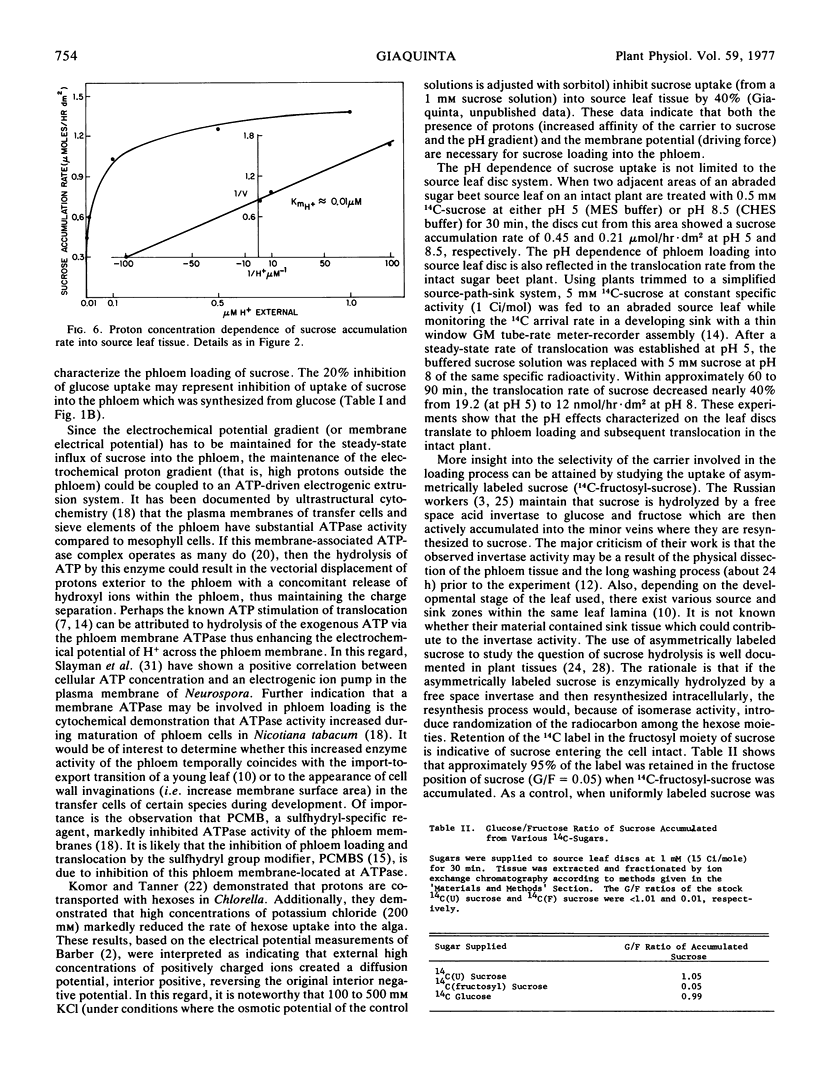
Autoradiographic, plasmolysis, and 14C-metabolite distribution studies indicate that the majority of exogenously supplied 14C-sucrose enters the phloem directly from the apoplast in source leaf discs of Beta vulgaris. Phloem loading of sucrose is pH-dependent, being markedly inhibited at an apoplast pH of 8 compared to pH 5. Kinetic analyses indicate that the apparent Km of the loading process increases at the alkaline pH while the maximum velocity, Vmax, is pH-independent. The pH dependence of sucrose loading into source leaf discs translates to phloem loading in and translocation of sucrose from intact source leaves. Studies using asymmetrically labeled sucrose 14C-fructosyl-sucrose, show that sucrose is accumulated intact from the apoplast and not hydrolyzed to its hexose moieties by invertase prior to uptake. The results are discussed in terms of sucrose loading being coupled to the co-transport of protons (and membrane potential) in a manner consistent with the chemiosmotic hypothesis of nonelectrolyte transport.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber J. Measurement of the membrane potential and evidence for active transport of ions in Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Jun 11;150(4):618–625. doi: 10.1016/0005-2736(68)90051-5. [DOI] [PubMed] [Google Scholar]
- Cataldo D. A. Vein Loading: The Role of the Symplast in Intercellular Transport of Carbohydrate between the Mesophyll and Minor Veins of Tobacco Leaves. Plant Physiol. 1974 Jun;53(6):912–917. doi: 10.1104/pp.53.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley R. A., Rothstein A. Chloroplast membrane characteristics. Biochim Biophys Acta. 1967 Jul 3;135(3):427–443. doi: 10.1016/0005-2736(67)90032-6. [DOI] [PubMed] [Google Scholar]
- Eddy A. A., Nowacki J. A. Stoicheiometrical proton and potassium ion movements accompanying the absorption of amino acids by the yeast Saccharomyces carlsbergensis. Biochem J. 1971 May;122(5):701–711. doi: 10.1042/bj1220701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows R. J., Geiger D. R. Structural and Physiological Changes in Sugar Beet Leaves during Sink to Source Conversion. Plant Physiol. 1974 Dec;54(6):877–885. doi: 10.1104/pp.54.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Giaquinta R. T., Sovonick S. A., Fellows R. J. Solute distribution in sugar beet leaves in relation to Phloem loading and translocation. Plant Physiol. 1973 Dec;52(6):585–589. doi: 10.1104/pp.52.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Sovonick S. A., Shock T. L., Fellows R. J. Role of free space in translocation in sugar beet. Plant Physiol. 1974 Dec;54(6):892–898. doi: 10.1104/pp.54.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. T., Geiger D. R. Mechanism of inhibition of translocation by localized chilling. Plant Physiol. 1973 Feb;51(2):372–377. doi: 10.1104/pp.51.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Mechanism of cyanide inhibition of Phloem translocation. Plant Physiol. 1977 Feb;59(2):178–180. doi: 10.1104/pp.59.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder J., Cronshaw J. A biochemical and cytochemical study of adenosine triphosphatase activity in the phloem of Nicotiana tabacum. J Cell Biol. 1974 Jan;60(1):221–235. doi: 10.1083/jcb.60.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton cotransport system of chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J Gen Physiol. 1974 Nov;64(5):568–581. doi: 10.1085/jgp.64.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton symport system of Chlorella vulgaris. Specificity, stoichiometry and energetics of sugar-induced proton uptake. Eur J Biochem. 1974 May 2;44(1):219–223. doi: 10.1111/j.1432-1033.1974.tb03476.x. [DOI] [PubMed] [Google Scholar]
- Kriedemann P., Beevers H. Sugar Uptake and Translocation in the Castor Bean Seedling II. Sugar Transformations During Uptake. Plant Physiol. 1967 Feb;42(2):174–180. doi: 10.1104/pp.42.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A., Hatch M. D., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. III. Physical & Metabolic Aspects of Cycle in Immature Storage Tissues. Plant Physiol. 1963 May;38(3):348–354. doi: 10.1104/pp.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaston A., Inkson C., Eddy A. A. The absorption of protons with specific amino acids and carbohydrates by yeast. Biochem J. 1973 Aug;134(4):1031–1043. doi: 10.1042/bj1341031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L., Long W. S., Lu C. Y. The relationship between ATP and an electrogenic pump in the plasma membrane of Neurospora crassa. J Membr Biol. 1973;14(4):305–338. doi: 10.1007/BF01868083. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Slayman C. W. Depolarization of the plasma membrane of Neurospora during active transport of glucose: evidence for a proton-dependent cotransport system. Proc Natl Acad Sci U S A. 1974 May;71(5):1935–1939. doi: 10.1073/pnas.71.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovonick S. A., Geiger D. R., Fellows R. J. Evidence for active Phloem loading in the minor veins of sugar beet. Plant Physiol. 1974 Dec;54(6):886–891. doi: 10.1104/pp.54.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I., Mitchell P. Proton-coupled beta-galactoside translocation in non-metabolizing Escherichia coli. J Bioenerg. 1972 Aug;3(5):445–462. doi: 10.1007/BF01516082. [DOI] [PubMed] [Google Scholar]



