Abstract
Endocrine therapy is the mainstay of treatment of estrogen-receptor-positive (ER+) breast cancer with an overall survival benefit. However, some adaptive mechanisms in the tumor emerge leading to the development of a resistance to this therapy. A better characterization of this process is needed to overcome this resistance and to develop new tailored therapies. Mechanisms of resistance to hormone therapy result in activation of transduction signal pathways, including the cell cycle regulation with cyclin D/CDK4/6/Rb pathway. The strategy of combined hormone therapy with targeted agents has shown an improvement of progression-free survival (PFS) in several phase II or III trials, including three different classes of drugs: mTOR inhibitors, PI3K and CDK4/6 inhibitors. A recent phase III trial has shown that fulvestrant combined with a CDK 4/6 inhibitor doubles PFS in aromatase inhibitor-pretreated postmenopausal ER+ breast cancer. Other combinations are ongoing to disrupt the interaction between PI3K/AKT/mTOR and cyclin D/CDK4/6/Rb pathways. Despite these successful strategies, reliable and reproducible biomarkers are needed. Tumor genomics are dynamic over time, and blood-based biomarkers such as circulating tumor DNA represent a major hope to elucidate the adaptive mechanisms of endocrine resistance. The optimal combinations and biomarkers to guide this strategy need to be determined.
Keywords: adaptive mechanism, CDK4/6 inhibitor, hormonoresistance, PI3K/mTOR inhibitor
Introduction
The estrogen receptor (ER) pathway is directly or indirectly activated by estrogen leading to proliferation, differentiation, invasion and cell survival. This pathway is considered an addictive oncogenic pathway in breast cancer cells. In frontline therapy, response rates to anti-estrogenic agents range from 20 to 40%, with a median duration of response of 14 months. In second-line treatment, resistance to anti-estrogenic agents is relatively high as the response rates are less than 10%, with a median progression-free survival of around 4 months.
There is no clear definition of the resistance to hormone therapy (HT). ESO–ESMO guidelines define primary endocrine resistance as: relapse while on the first 2 years of adjuvant endocrine therapy (ET) or progression of disease (PD) within the first 6 months of first-line ET for metastatic breast cancer. Secondary (acquired) resistance is defined as relapse while on adjuvant ET but after the first 2 years, or relapse within 12 months of completing adjuvant ET, or PD 6 months after initiating ET for metastatic breast cancer (MBC).1
The expression of ER is not sufficient to identify patients who will respond to anti-estrogenic therapy. Several adaptive mechanisms of escape to anti-estrogenic therapy have been identified: an increase in concentration of estrogen in the tumor environment, post-translational modifications, ligand-independent activation of the receptor via ESR1 gene mutation, amplification of the feedback loops mediated by transmembrane growth factor receptors [human epidermal growth factor receptor (HER), fibroblast growth factor receptor (FGF-R), insulin growth factor receptor (IGF-R)], by the RAS/rapidly accelerated fibrosarcoma (RAF)/mitogen-activated protein kinase (MAPKinase) pathway and the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway.2 Downstream, hormone therapy resistance could be characterized by deregulation of the cell cycle involving the cyclin D/CDK4/6/Rb pathway.3 Several combination strategies with HT have failed in advanced breast cancer such as that combining EGFR inhibitors (gefitinib) and IGFR 1 inhibitors (ganitumumab).4,5 Other combinations with a SRC (rous sarcoma protein) inhibitor (dasatinib), an antiapoptotic inhibitor (bortezomib) or with histone deacetylase inhibitors (HDAC inhibitors) have shown discordant or interesting responses and need more investigations (Table 1).6–8
Table 1.
Trials with endocrine therapy resistance (without PI3K/Akt/mTOR or CDK4/6/Rb pathways inhibition).
| Reference | Target/pathway | Results | PFS |
|---|---|---|---|
| Paul et al., 2013 | Dasatinib | Phase IIR First line | Let: 9.9 months |
| (Inhibitor of SRC) | n = 120 | Let + dasa: 20.1 months | |
| Yardley et al., 2013 | Entinostat | Phase IIR Second line | Exe 2.3 months |
| (Inhibitor of HDAC) | n = 130 | Exe + enti: 4.3 months | |
| Trifonidiseur et al., 2016 | Gefinitib | Phase IIR First line | Ana + gef: 35% (at 1 year) |
| Anti HER1 | n = 71 | Ana: 32% (at 1 year) | |
| Robertson et al., 2013 | Ganitumab | Phase IIR | Ful ou exe + gan: 3.9 months |
| Anti IGF1 | n = 156 | Ful ou exe: 5.7 months | |
| Kaufmann et al., 2009 | HER 2 | Phase III, First line | Ana + trast: 4.8 months versus |
| Trastuzumab | n = 207 | Ana 2.4 months | |
| Jonhston et al., 2009 | HER2 | Phase III, First line | Lapa + let: 8.3 months |
| Lapatinib | n = 1286 | Let: 3 months |
Dasa, dasatinib; let, letrozole; exe, exemestane; enti, entinostat; gan, ganitumab; gef, gefitinib; ana, anastrozole; ful, fulvestrant; trast, trastuzumab; lapa, lapatinib; SRC, sarcoma; HDAC, histone deacetylase inhibitors; PFS, progression-free survival.
We hypothesize that addition is more efficient than a substitution strategy for the treatment of endocrine-resistant MBC, as shown in the HER2+ MBC context.9 We will review randomized phase II and III clinical trials exploiting this strategy.
The proof of concept of adaptive mechanisms with PI3K/Akt/mTOR inhibitors
The PI3K/Akt/mTOR pathway plays a key role in cell signaling, regulating proliferation, survival and differentiation.10 The PI3K proteins are kinases divided into three classes (I-III) according to their structure and substrate specificity.
Aberrant activation of the PI3K/Akt/mTOR pathway plays a major role in the mechanisms of resistance to HT. It is a prime target for the treatment of ER-positive breast cancers, the aim being to prevent and revert resistance to HT.11–13 The IGF/IGF-1-IRS pathway (insulin receptor substrate 1) induces activation of PI3K and activates mTORC1 with an indirect correlation between PI3K and the mTOR1 effector. Inactivation of PI3K induces inhibition of S6K1 and 4E-BP, and PI3K activation is negatively regulated by the tumor suppressor gene PTEN (phosphatase and tensin counterpart deleted one chromosome ten). The main effector of PI3K is AKT.14,15 This serine/threonine kinase belongs to the family of AGC kinases and exists in three isoforms, Akt-1, 2, 3, encoded by three different genes. Activated AKT induces the activation of the mTOR pathway promoting cell proliferation and inducing inhibition of proapoptotic proteins. MTOR was identified in the yeast Saccharomyces cerevisiae as a therapeutic target of the macrolide antibiotic, rapamycin.16 It plays a key role in the regulation of critical cell processes such as growth, proliferation, cytoskeleton organization, transcription, protein synthesis, ribosome biogenesis and autophagy17,18 (Figure 1).
Figure 1.
PI3K/Akt/mTOR signaling pathway.18
Hyperactivation of the PI3K/Akt/mTOR pathway will induce tumor adaptation to anti-estrogenic therapy and can be defined by a mutation of PI3K (catalytic domain, or helical), AKT mutation, loss of PTEN function (deletion or loss of expression, epigenetics) or by the regulatory function of proteins TSC1/TSC2 (tuberous sclerosis complex) (deletion–mutation) (Figure 2).
Figure 2.
Pathway hyperactivation defined by alterations of the PI3K/AKT/mTOR pathway.
Randomized clinical trials with mTOR inhibitors
Several clinical studies have been conducted in patients with ER + (endocrine receptor) HER2- MBC by combining anti-estrogenic [selective estrogen receptor modulators (SERM), selective estrogen down regulators (SERD)], antiaromatase inhibitors (AI) with agents targeting PI3K/Akt/mTOR such as PI3K inhibitors (panspecific or specific to the subunit 110 α or δ), AKT inhibitors, mTOR inhibitors or inhibitors of both mTOR and PI3K (dual inhibitor).
The Horizon study, a randomized phase III study, compared the combination letrozole 2.5 mg daily/temsirolimus 30 mg daily (5 days every 2 weeks) to letrozole/placebo as first-line therapy in 1112 AI-naïve patients with ER+ MBC. This study didn’t show any difference in terms of PFS between the two arms: median PFS 9 months; [hazard ratio 0.90; 95% confidence interval (CI), 0.76 to 1.07; p = 0.25].19
TAMRAD was the first open-label, phase II study randomizing postmenopausal women with ER+/HER2–, AI-resistant MBC to tamoxifen 20 mg/day combined with everolimus 10 mg/day (n = 54) or tamoxifen 20 mg/day alone (n = 57). Randomization was stratified, based on primary and secondary hormone resistance. Everolimus improved the clinical benefit rate (CBR) (the primary endpoint), and time to progression (TTP).20 In retrospective biomarker analyses, a correlation was observed between late effectors of mTORC1 activation and Akt-independent mTORC1 activation, and an inverse correlation was observed between the canonical PI3K/Akt/mTOR pathway and everolimus efficacy. These exploratory analyses need to be validated in a larger prospective study.21
The BOLERO-2 study was the first phase III study demonstrating a benefit in terms of PFS with inhibition of one of the activated adaptive pathways mediating resistance to HT. This randomized, double-blind phase III trial evaluated everolimus (10 mg/day) with exemestane (25 mg/day) versus placebo with exemestane 25 mg/day in 764 postmenopausal women with ER+ advanced breast cancer with prior exposure to nonsteroidal AI. The median number of previous treatments was 3. 84% of the patients had previous sensitivity to endocrine therapy. With a median follow up of 18 months, the experimental arm significantly reduced the risk of relapse with an hazard ratio of 0.45 (95% CI 0.38–0.54, p < 0.0001) and a PFS of 7.8 months versus 3.2 months in the placebo arm.22 The benefit was consistent regardless of the presence of visceral metastases and hormone sensitivity.23 Despite clinically and statistically significant extension of PFS, this combination did not confer any overall survival benefit.24 Preserving quality of life is an essential component of palliative care in the advanced cancer setting; quality of life provides essential information on disease burden and guides treatment decisions. The most common grade 3 or 4 adverse events (AEs) were stomatitis (8% in the everolimus arm versus 1% in the exemestane plus placebo group), anemia (6% versus <1%), dyspnea (4% versus 1%), hyperglycemia (4% versus <1%), fatigue (4% versus 1%), and pneumonitis (3% versus 0%). A post hoc analysis confirmed the clinical benefit with no adverse impact on health-related quality of life.25
What may explain the differences between Horizon, TAMRAD and BOLERO-2?
Unlike BOLERO 2 and TAMRAD, the Horizon study is a first-line setting MBC with only 40% of the patients having received previous adjuvant endocrine therapy. In Horizon, patients didn’t receive any aromatase inhibitor as adjuvant therapy, and likely didn’t have any hyperactivation of the PI3K/Akt/mTOR. MTOR inhibitor might be less effective without an adaptive mechanism as hyperactivation of the PI3K/AKt/mTOR, induced by hormone-therapy resistance.
There is a real heterogeneity of responses to these drugs and patient outcomes, and biomarker analysis may help identify patients who will derive the greatest benefit from everolimus. In an exploratory analysis of BOLERO-2, the benefit of everolimus was maintained regardless of the presence or absence of an alteration in PIK3CA, FGFR1, CCND1 or their respective pathways. However, when the patients were assigned to the subgroups on the basis of mutations in PI3KCA exon 20 or 9, PFS benefit of everolimus appeared to be greater in those with exon 9 mutation [hazard ratio 0.26, 95% CI (0.12–0.54)] than in those with exon 20 mutation [hazard ratio 0.56, CI (0.31–1)]. Moreover, these data suggest that tumor with low chromosomal instability (CIN) might benefit from the addition of everolimus to exemestane, with a median PFS gain of 5.5 months for patients with CIN score below the 75th percentiles [hazard ratio 0.39, CI (0.28–0.54)].26
Recently, recurrent mutations have been identified in the estrogen receptor. Chandarlapaty and colleagues evaluated blood samples from 541 of the 724 patients enrolled in BOLERO-2. They detected a D538G ESR1 mutation in samples from 114 (21.1%) patients, a Y537S ESR1 mutation in samples from 72 (13.3%) patients, and double mutations in samples from 30 patients. Median overall survival was 32.1 months for patients with neither a D538G nor Y537S ESR1 mutation, 26 months for those with only a D538G mutation, 20 months for those with only a Y537S mutation, and 15.2 months for those with double mutations. Exploratory analyses showed that adding everolimus to exemestane doubled PFS for patients with any ESR1 mutation and for those with a D538G mutation, and didn’t increase PFS for patients with a Y537S mutation. However, further studies are needed before the validation of these biomarkers.27
The mechanism of action of everolimus could lead to incomplete inhibition of mTORC1-dependent protein synthesis, limiting its efficacy.28 Everolimus sets off a negative feedback mechanism leading to increased Akt signaling and treatment resistance. AZD2014, a dual inhibitor of mTORC1 (rapamycin-sensitive) and mTORC2 (rapamycin insensitive) has shown superior activity to everolimus both in hormone-sensitive and -resistant models.29 A randomized phase II trial (MANTA) [ClinicalTrials.gov identifier: NCT02216786] is ongoing for postmenopausal women with ER+/HER2-negative ABC, hormone refractory, comparing AZD2014 and fulvestrant to fulvestrant alone and to fulvestrant and everolimus.
Randomized clinical trials with PI3K inhibitors
One phase II trial and one phase III trial evaluated a PI3K inhibitor in ER+/HER2-MBC.
Recently, the FERGI trial assessed the addition of PI3K inhibition to HT in the second-line setting. In this phase II trial, investigators randomized pictilisib (GDC-0941), a potent oral inhibitor of multiple class I PI3K kinase isoforms in combination with fulvestrant (n = 89) versus fulvestrant with placebo (n = 79) in patients with MBC resistant to AI, with or without PIK3CA mutation. This study demonstrated a nonsignificant benefit in PFS in the pictilisib arm compared with the placebo arm (6.2 months versus 3.8 months; hazard ratio, 0.77; 95% CI, 0.50–1.19) in the overall population. This improvement was independent of PIK3CA mutation status in the tumor. Exploratory subgroup analyses suggested that patients with centrally confirmed oestrogen receptor+/progesterone receptor+ tumors are more likely to benefit from the addition of pictilisib to fulvestrant (7.2 months versus 3.7 months, hazard ratio, 0.46; 95% CI, 0.27–0.78), even if the subgroup analyses were limited by the sample size.30 The absence of efficacy could be explained by the toxicity of pictilisib, with 22% of patients discontinuing experimental treatment, and the numerous dose reductions. Alternative strategies with specific inhibition of subunit α PI3K (alpelisib) or mutated PI3K (taselisib) are ongoing with a putative improved therapeutic index. Another explanation of this failure was put forward by Bachelot and colleagues. There are no direct correlations between PI3K AKT activation and late effectors of mTORC1 activation. The PI3K mutation in HER2-negative breast cancer is associated with low histological grade, high hormone sensitivity, good prognosis, and low mTORC1 activity. And high-grade tumors are associated with mTORC1 activation and few PI3KCA mutations, a potential target of PI3K inhibitor.31–33 The oncogenic action of PI3K mutation–PI3K inhibition combined with endocrine therapy might be a target only in a subgroup of patients with MBC with endocrine resistance.
BELLE-2 is a prospective randomized phase III study in 1147 patients with ABC whose disease progressed on an AI. This study compared buparlisib [a specific oral inhibitor of the pan-class I phosphatidylinositol 3-kinase (PI3K)] with fulvestrant with fulvestrant and placebo. The study was stratified according to the presence of visceral metastasis and activation of the PI3K pathway (PI3K mutation, PTEN loss of function) in the primary tumor. The primary endpoint was PFS in the overall population and in patients with activation of PI3K. PIK3CA mutation status in blood samples was also analyzed in a subset of 587 patients. In the overall study population, PFS was 6.9 months in the combination therapy group versus 5.0 months in the control group (hazard ratio 0.78, 0.67–0.89). As in the FERGI study, patients with activation of the PI3K pathway in their primary tumor had no improvement in PFS with buparlisib added to fulvestrant (hazard ratio = 0.76; 0.60–0.97). However, this combination improved the median PFS in patients with a PI3K mutation detected by circulating DNA, with a 44% reduction in risk for progression (7 months versus 3.2 months; hazard ratio = 0.56; 0.39–0.80). The combination of fulvestrant and buparlisib was associated with serious side effects leading to discontinuation of therapy in 13.2%. The most common grade 3 and 4 AEs were liver dysfunction (ALT: 26 versus 1%, AST: 18 versus 3%), rash (8 versus 0%), hyperglycemia (15 versus 0.2%), and mood disorders (4.4% versus 0.4%).34
The discrepant results regarding the PIK3CA status and the benefit of PI3K inhibitors can probably be explained by the fact that the primary tumor is not suitable for measuring PI3K activation which should probably be analyzed in the metastatic lesions or in circulating DNA at the time of metastatic relapse. In a study of 104 patients, synchronous genetic heterogeneity and changing PI3K mutational status were demonstrated between primary and metastatic breast tumors.35 The analysis of PI3K mutation status on the circulating tumor DNA (ctDNA) is more feasible, less invasive and more reliable as shown by the unpublished results of the BELLE-2 study. However, prospective well conducted studies are still needed to confirm whether the presence of a PIK3CA mutation in circulating tumor DNA can predict response to this treatment.
CDK 4/6 inhibition in association to AI in a first- or second-line setting?
The mechanisms of resistance to HT often include upregulation with or without activation of signal transduction pathways that involve cell cycle regulation. CDK4/6 phosphorylates and inactivates Rb (retinoblastoma) tumor suppressor proteins, leading to dissociation of E2F transcription factors and transcriptional regulation of genes for G1/S transition and cell cycle progression. Mitogenic signals or growth factor receptor signaling pathways converge on the cyclin D/CDK4 or CDK6 pathways, like ER and PI3K/Akt/mTOR. The activation leading to Rb phosphorylation is associated with resistance to endocrine therapy.3,36 Rb dysfunction is associated with luminal B-type breast cancer and is predictive of a poor response to endocrine therapies. CDK 4/6 inhibitors reverse the endocrine resistance in preclinical studies.37
PALOMA-1, a phase II trial randomized letrozole combined with palbociclib (a highly selective, orally administered CDK4/6 inhibitor) versus letrozole alone in postmenopausal patients with ER-positive MBC in the frontline setting. Palbociclib was administered at a dose of 125 mg/day for 3 weeks out of 4. In cohort 1 (n = 66), patients were enrolled on the basis of their ER+/Her2- biomarker status alone, whereas in cohort 2 (n = 99), they were also required to have cancers with amplification of cyclin D1 (CCND1), loss of p16 (INK4A or CDKN2A), or both. A third of the patients in each group had received previous endocrine therapy and half of these individuals had previously received AI. The final analysis showed a doubling of PFS in the overall population from 10.2 months with letrozole alone to 20.2 months with the letrozole–palbociclib combination [risk ratio (RR) of 0.488] and a decrease in risk of progression of 51% in favor of the combination (p = 0.0004). This improvement was observed in all subgroups, independent of cyclin D1 amplification or loss of p16. The number of death events was small at the time of publication, but was not statistically different between the palbociclib plus letrozole arm and the letrozole alone arm (37.5 versus 33.3; p = 0.42). The safety profile of palbociclib is favorable, including primarily hematological toxicity with 50% of patients presenting with Grade 3 or 4 neutropenia. However, no febrile neutropenia was observed. Approximately 40% of patients required a dose reduction or a recovery period.38 A pivotal phase III study, PALOMA-2, with the same design as the PALOMA-1 study was presented at ASCO 2016 and confirmed the safety and the clinical benefit of palbociclib with median PFS at 24.8 months (P+L) versus 14.5 months (PLB+L) [hazard ratio = 0.58 (0.46–0.72), p < 0.000001]. Despite immature overall survival (OS) data, the FDA has approved palbociclib for ABC patients who had not received prior systemic therapy for their advanced disease.39
However, fulvestrant could be a new option in a first-line setting. Fulvestrant is a selective estrogen-receptor degrader that targets the function of the hormone receptor currently used in second-line after AI.40 A randomized open label phase II trial, the FIRST trial, compared fulvestrant (500 mg) with anastrozole in the first-line setting and suggested that fulvestrant was superior in TTP.41 The extension phase III trial with the same design FALCON in 462 postmenopausal patients confirms statistically significant improvement in PFS with fulvestrant versus anastrozole [hazard ratio 0.797 (95% confidence interval 0.637, 0.999); p = 0.0486; median PFS, 16.6 versus 13.8 months, respectively]. However, a subgroup analysis showed an even greater impact on PFS in patients whose disease had not spread to the liver or lungs at baseline (22.3 versus 13.8 months).42 Quality of life was similar between the two arms and the most common AEs were arthralgia (joint pain) (16.7% versus 10.3%) and hot flushes (11.4% versus 10.3%) for fulvestrant and anastrozole, respectively. Fulvestrant could be a first-line option for patients requiring a low-toxicity approach such as older patients, or those with low-volume disease, or those for whom adherence to oral treatment is complicated. It should be noted that in the FALCON trial, none of the patients had prior HT, even for the treatment of early breast cancer.
PALOMA-3 is a phase III trial randomizing fulvestrant-placebo versus fulvestrant-palbociclib among 521 patients with ER+/HER2-metastatic breast cancer, previously treated with hormone therapy. Premenopausal women could be included if they had received treatment with an LHRH agonist. The randomization was 2:1, with stratification based on the presence of visceral metastases, prior sensitivity on HT and menopausal status. With a median follow-up of 8.9 months, the median PFS was 9.5 months for the fulvestrant–palbociclib combination versus 4.6 months for fulvestrant alone (hazard ratio: 0.46, 95% CI 0.36–0.59, p < 0.0001). The OS follow-up is in progress. The safety profile was similar to that observed in the PALOMA-1 study, with neutropenia and maintained quality of life being common to both arms. Neither PI3KCA status in ctDNA nor the level of ER level expression predicts the response to palbociclib.43,44 These results confirm remarkable efficacy of this new therapeutic class, and standard therapy in the metastatic setting is changing. Other CDK4/6 inhibitors are under development such as ribociclib or abemaciclib.
Ribociclib has been evaluated in the MONALEESA-2 trial. This phase III study randomized 668 postmenopausal women with ER+ HER2 ABC, who had not received any prior systemic treatment. Patients received ribociclib (600 mg/day, 3 weeks on/1 week off) and letrozole (2.5 mg/day, continuous), or letrozole plus placebo. In the ribociclib arm, there was a 44% improvement in PFS compared with the placebo arm (hazard ratio: 0.556, p = 0.00000329). Median PFS was 14.7 months in the placebo arm, but was not reached in the ribociclib arm at data cut off. AEs were similar to those when using palbociclib, with 59.3% of neutropenia.45
The MONARCH 1 study is the only phase II study that evaluated the single-agent activity and safety of a CDK 4/6 inhibitor, abemaciclib, in patients with refractory metastatic breast cancer whose disease had progressed following multiple prior treatments, including chemotherapy. With a median of three lines of prior therapy for advanced disease and at the 8-month interim analysis, the confirmed odds risk ratio was 17.4%, the clinical benefit rate (complete response + partial response + stable disease ⩾6 months) was 42.4%, and median PFS was 5.7 months. The treatment was well tolerated with only 6.8% discontinuations for AEs.46
MONARCH 2, a phase III trial, compared abemaciclib plus fulvestrant versus placebo with fulvestrant in women with ER+ HER2- ABC. Patients had experienced disease progression on or within 12 months of receiving endocrine treatment in the neoadjuvant or adjuvant setting or while receiving first-line endocrine therapy for metastatic disease. The primary endpoint is PFS. MONARCH 3 is a phase III trial of abemaciclib in combination with AI in patients with HR+, HER2- ABC.
Regardless the CDK 4/6 inhibitor, biomarkers are needed to optimize endocrine therapy and the levels of estrogen (ER) and progesterone receptor (PgR) could be one. Low proliferation with a low Ki67 level and high eostrogen and progesterone receptor expression (progesterone receptor) are probably predictive of response to endocrine therapy in neoadjuvant BC.47 Low PgR and higher Ki67 expression seem to be associated with poor prognosis and help to strengthen HT. Indeed, in the TEXT and SOFT trials, Regan and colleagues showed that the combination of exemestane + ovarian function suppression (OFS) is more beneficial than tamoxifen (tam) alone or tam + OFS in adjuvant endocrine therapy for women with poor prognostic features.48 In patients with newly metastatic disease or disease recurring after adjuvant tamoxifen, negative or low-level ER could be a predictive factor of response to gefitinib (EGFR inhibitor) and tamoxifen.49 No biomarkers have been found to predict the response of CDK4/6 inhibitors. In the PALOMA-2 trial, biomarker analyses on cell-cycle-related genes using immunohistochemistry for ER, Rb, p16, cyclin D1, and Ki-67 revealed no additional markers with sensitivity to palbociclib + letrozole beyond ER+.50 A recent phase II study evaluated if short-term preoperative palbociclib treatment is associated with decreased proliferation and early biomarker changes in patients with early breast cancer. Palbociclib decreases Ki67 and is dependent on molecular subtypes; it is not effective on HER2+ and triple-negative breast cancer, and is correlated with changes in pRB. Additional analyses are ongoing (CCND1 amplification, pAKT, pER, PIK3CA, AKT1).51
Given these remarkable results, acquired resistance to CDK4/6 inhibitors will be an emerging clinical challenge.
Novel combinations
The interaction between the PI3K/AKT/mTOR and cyclin D–CDK4/6–INK4–Rb pathways is thought to play a critical role in ER-driven breast cancer and preclinical ER+ breast cancer models. Hyperactivation of PI3K/Akt/mTOR might be an adaptive mechanism leading to endocrine therapy and CDK4/6 inhibitor resistance, and combination strategies are widely evaluated. The combination of ribociclib, a CDK4/6 inhibitor (LEE011; LEE), alpelisib, an alpha isoform of class I PI3K inhibitor (BYL719; BYL), and letrozole (LET) has recently shown enhanced activity versus each agent alone.52 A phase Ib combination of LET, LEE and BYL has shown an acceptable safety profile and demonstrates preliminary clinical activity in heavily pretreated patients with ER+/HER2- ABC. A total of 15 patients discontinued treatment: 7 (19%) due to progression of disease (PD) and 8 (22%) due to AEs. The most frequent study drug-related AEs (all grades >35%) were: nausea (all grades, 44%; G3/4, 6%), hyperglycemia (44%; 17%), neutropenia (42%; 22%), and fatigue (36%; 11%). Among 27 evaluable patients, 2 (7%) had a partial response (PR), 4 (15%) had unconfirmed partial response, 6 (22%) had stable disease (SD), 6 (22%) had non-CR (complete response), non-PD, and 5 (19%) had PD as best overall response. Inhibition of the three pathways provides sustained downregulation of Ki67, potentially preventing a feedback mechanism and hence delaying progression through therapy. Future randomized studies will compare LET + LEE or BYL with LET + LEE + BYL.53
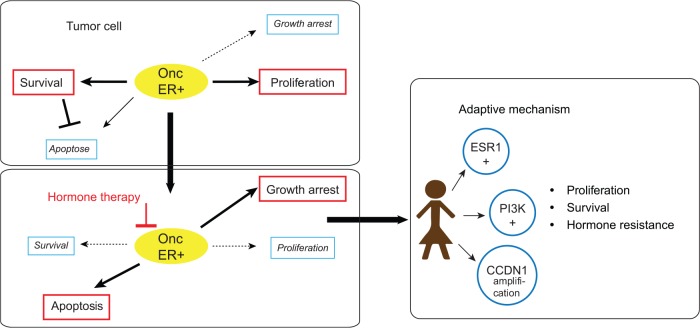
In summary, practice-changing trials have been successfully conducted combining HT with targeted therapies, disrupting the adaptive mechanisms of resistance to HT. This illustrated the successful concept of adding therapies. Fulvestrant as monotherapy is also an option, but recent trials have shown that PFS benefits can be achieved from it being combined with an inhibitor of the cyclin D–CDK4/6–INK4–Rb pathways.42,43 Fulvestrant, in combination with a PI3K inhibitor could be another option, but trials are still ongoing. Everolimus + exemestane is also an option in a second-line setting in postmenopausal women with ER+ cancer with prior exposure to nonsteroidal anti-inflammatories (letrozole or anastrozole), despite the absence of OS improvement (Figure 3).22 New and robust biomarkers are needed to define what is the best combination and the best sequence of treatment for hormone refractory MBC. To monitor the dynamics of tumor genomics over time, reliable and reproducible biomarkers for given patients are urgently needed. And ctDNA seems to be a useful one. There is a real interest in integrating liquid biopsies in prospective combination trials, but the sensibility and reproducibility of these tests are still being evaluated.
Figure 3.
Resistance to endocrine therapy and adaptive mechanism in advanced breast cancer.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Paule Augereau, Department of Medical Oncology, Institut cancerologie de l’ouest site Paul Papin, 15 rue Andre Bocquel 49055 Angers Cedex 02, France.
Anne Patsouris, Department of Medical Oncology, Institut cancerologie de l’ouest site Paul Papin, France.
Emmanuelle Bourbouloux, Department of Medical Oncology, Institut cancerologie de l’ouest site René Gauducheau, Saint Herblain, France.
Carole Gourmelon, Department of Medical Oncology, Institut cancerologie de l’ouest site René Gauducheau, Saint Herblain, France.
Sophie Abadie Lacourtoisie, Department of Medical Oncology, Institut cancerologie de l’ouest site Paul Papin, France.
Dominique Berton Rigaud, Department of Medical Oncology, Institut cancerologie de l’ouest site René Gauducheau, Saint Herblain, France.
Patrick Soulié, Department of Medical Oncology, Institut cancerologie de l’ouest site Paul Papin, France.
Jean Sebastien Frenel, Department of Medical Oncology, Institut cancerologie de l’ouest site René Gauducheau, Saint Herblain, France.
Mario Campone, Department of Medical Oncology, Institut cancerologie de l’ouest site Paul Papin, France Department of Medical Oncology, Institut cancerologie de l’ouest site René Gauducheau, Saint Herblain, France.
References
- 1. Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014; 23: 489–502. [DOI] [PubMed] [Google Scholar]
- 2. Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res 2010; 16: 1979–1987. [DOI] [PubMed] [Google Scholar]
- 3. Thangavel C, Dean JL, Ertel A, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer 2011; 18: 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tryfonidis K, Basaran G, Bogaerts J, et al. A European Organisation for Research and Treatment of Cancer randomized, double-blind, placebo-controlled, multicentre phase II trial of anastrozole in combination with gefitinib or placebo in hormone receptor-positive advanced breast cancer [ClinicalTrials.gov identifier: NCT00066378]. Eur J Cancer 2016; 53:144–154. [DOI] [PubMed] [Google Scholar]
- 5. Robertson JF, Ferrero JM, Bourgeois H, et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol 2013;14: 228–235. [DOI] [PubMed] [Google Scholar]
- 6. Paul D, Vukelja SJ, Holmes FA, et al. Letrozole plus dasatinib improves progression-free survival (PFS) in hormone receptor-positive, HER2-negative postmenopausal metastatic breast cancer (MBC) patients receiving first-line aromatase inhibitor therapy. Paper presented at 2013 San Antonio Breast Cancer Symposium, December 10–14, 2013; San Antonio, TX. Abstract S3–07. [Google Scholar]
- 7. Adelson KB, Raptis G, Sparano J, et al. Randomized phase II study of fulvestrant versus fulvestrant plus bortezomib in postmenopausal women with estrogen receptor (ER) positive, aromatase-inhibitor (AI) resistant metastatic breast cancer (MBC). New York Cancer Consortium trial P8457. Cancer Res 2014; 71: OT3 01 01. [Google Scholar]
- 8. Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 2013; 31: 2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campone M, Juin P, Andre F, et al. Resistance to HER2 inhibitors: is addition better than substitution? Rationale for the hypothetical concept of drug sedimentation. Crit Rev Oncol Hematol 2011; 78: 195–205. [DOI] [PubMed] [Google Scholar]
- 10. Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 2009; 8: 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cavazzoni A, Bonelli MA, Fumarola C, et al. Overcoming acquired resistance to letrozole by targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones. Cancer Lett 2012; 323: 77–87. [DOI] [PubMed] [Google Scholar]
- 12. Miller TW, Rexer BN, Garrett JT, et al. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 2011;13: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barone I, Cui Y, Herynk MH, et al. Expression of the K303R estrogen receptor-alpha breast cancer mutation induces resistance to an aromatase inhibitor via addiction to the PI3K/Akt kinase pathway. Cancer Res 2009; 69: 4724–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franke TF. PI3K/Akt: getting it right matters. Oncogene 2008; 27: 6473–6488. [DOI] [PubMed] [Google Scholar]
- 15. Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008; 27: 5497–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991; 253: 905–909. [DOI] [PubMed] [Google Scholar]
- 17. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007; 12: 9–22. [DOI] [PubMed] [Google Scholar]
- 18. Efeyan A, Sabatini DM. mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol 2010; 22: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolff AC, Lazar AA, Bondarenko I, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 2013; 31: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 2012; 30: 2718–2724. [DOI] [PubMed] [Google Scholar]
- 21. Treilleux I, Arnedos M, Cropet C, et al. Translational studies within the TAMRAD randomized GINECO trial: evidence for mTORC1 activation marker as a predictive factor for everolimus efficacy in advanced breast cancer. Ann Oncol 2015; 26: 120–125. [DOI] [PubMed] [Google Scholar]
- 22. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012; 366: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campone M, Bachelot T, Gnant M, et al. Effect of visceral metastases on the efficacy and safety of everolimus in postmenopausal women with advanced breast cancer: subgroup analysis from the BOLERO-2 study. Eur J Cancer 2013; 49: 2621–2632. [DOI] [PubMed] [Google Scholar]
- 24. Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann Oncol 2014; 25: 2357–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campone M, Beck JT, Gnant M, et al. Health-related quality of life and disease symptoms in postmenopausal women with HR(+), HER2(–) advanced breast cancer treated with everolimus plus exemestane versus exemestane monotherapy. Curr Med Res Opin 2013; 29: 1463–1473. [DOI] [PubMed] [Google Scholar]
- 26. Hortobagyi GN, Chen D, Piccart M, et al. Correlative Analysis of genetic alterations and everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from BOLERO-2. J Clin Oncol 2016; 34: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chandarlapaty S, Chen D, He W, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol 2016; 2: 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol 2010; 28: 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jordan NJ, Dutkowski CM, Barrow D, et al. Impact of dual mTORC1/2 mTOR kinase inhibitor AZD8055 on acquired endocrine resistance in breast cancer in vitro. Breast Cancer Res 2014; 16: R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krop IE, Mayer IA, Ganju V, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016; 17:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sabine VS, Crozier C, Brookes CL, et al. Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. J Clin Oncol 2014; 32: 2951–2958. [DOI] [PubMed] [Google Scholar]
- 32. Loi S, Haibe-Kains B, Majjaj S, et al. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Natl Acad Sci U S A 2010; 107: 10208–10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bachelot T, Campone M, Tredan O. PI3K targeting in breast cancer: the end of the beginning? Lancet Oncol 2016; 17: 696–697. [DOI] [PubMed] [Google Scholar]
- 34. Baselga J, Im S, Iwata H, et al. PIK3CA status in circulating tumor DNA predicts efficacy of buparlisib plus fulvestrant in postmenopausal women with endocrine-resistant HR+/HER2– advanced breast cancer: first results from the randomized, phase III BELLE-2 trial. Paper presented at San Antonio Breast Cancer Symposium, Texas, December 8–12, 2015 Abstract: S6–01. 2015. [Google Scholar]
- 35. Dupont Jensen J, Laenkholm AV, Knoop A, et al. PIK3CA mutations may be discordant between primary and corresponding metastatic disease in breast cancer. Clin Cancer Res 2011; 17: 667–677. [DOI] [PubMed] [Google Scholar]
- 36. Lange CA, Yee D. Killing the second messenger: targeting loss of cell cycle control in endocrine-resistant breast cancer. Endocr Relat Cancer 2011; 18: C19–C24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16: 25–35. [DOI] [PubMed] [Google Scholar]
- 39. Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. The New England journal of medicine. 17 November 2016;375(20):1925–36. [DOI] [PubMed] [Google Scholar]
- 40. Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol 2009; 27: 4530–4535. [DOI] [PubMed] [Google Scholar]
- 41. Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST Study. J Clin Oncol 2015; 33: 3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ellis MJ, Bondarenko I, Trishkin E, et al. FALCON: a phase III randomised trial of fulvestrant 500 mg versus anastrozole for hormone receptor-positive advanced breast cancer. Paper Presented at ESMO 2016 meeting 2016: Abstract LBA14_PR. [Google Scholar]
- 43. Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015; 373: 209–219. [DOI] [PubMed] [Google Scholar]
- 44. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17: 425–439. [DOI] [PubMed] [Google Scholar]
- 45. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 46. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH1: results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. J Clin Oncol 2016; 34(Suppl.): ASCO 2016, Abstract 510. [Google Scholar]
- 47. Colleoni M, Montagna E. Neoadjuvant therapy for ER-positive breast cancers. Ann Oncol 2012; 23(Suppl 10): x243–x248. [DOI] [PubMed] [Google Scholar]
- 48. Regan MM, Pagani O, Francis PA, et al. Predictive value and clinical utility of centrally assessed ER, PgR, and Ki-67 to select adjuvant endocrine therapy for premenopausal women with hormone receptor-positive, HER2-negative early breast cancer: TEXT and SOFT trials. Breast Cancer Res Treat 2015; 154: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Osborne CK, Neven P, Dirix LY, et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res 2011; 17: 1147–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Finn R, Jiang Y, Rugo H, et al. Biomarker analyses from the phase 3 PALOMA-2 trial of palbociclib (P) with letrozole (L) compared with placebo (PLB) plus L in postmenopausal women. Ann Oncol 2016; 27(6): 1–36. [Google Scholar]
- 51. Arnedos M, Cheaib B, Bayar MA, et al. Anti-proliferative response and predictive biomarkers to palbociclib in early breast cancer. The Preoperative Palbociclib (POP) randomized trial. Oral presentation at AACR Annual Meeting 2016; 16–20 April 2016; New Orleans, LA, 2016. [Google Scholar]
- 52. Kim S, Loo A, Chopra R, et al. LEE011: an orally bioavailable, selectivesmall molecule inhibitor of CDK4/6—reactivating Rb in cancer. Paper presented at the AACR-NCI-EORTC International Conference: Molecular Targets and CancerTherapeutics, 19–23 October 2013, Boston, MA, USA 2013. [Google Scholar]
- 53. Juric D, Ismail-Khan R, Campone M, et al. Phase Ib/II study of ribociclib and alpelisib and letrozole in ER+, HER2– breast cancer: safety, preliminary efficacy and molecular analysis. Paper presented at the 2015 San Antonio Breast Cancer Symposium Publication Number: P3–14–01 2015. [Google Scholar]