Abstract
Background:
Mucosal healing (MH), the proposed treat to target in Crohn’s disease (CD), is associated with improved disease outcomes. There are still scant data on factors associated with achieving MH in clinical practice. We evaluated the probability of achieving MH and identified factors predictive of subsequent MH in patients with CD.
Methods:
This was a retrospective, observational cohort study. A total of 272 patients with CD with serial endoscopy assessment and subsequent therapeutic management were reviewed. The primary outcome was MH. The cumulative incidence of MH and endoscopic improvement was estimated using the Kaplan–Meier method. Factors independently associated with MH were identified using the Cox proportional hazards model.
Results:
Of the 272 patients, 126 (46.32%) achieved MH after a median follow-up period of 33 months (interquartile range: 27–38 months). Factors independently associated with MH by multivariate analysis were time between endoscopic procedures within 26 weeks (hazard ratio [HR]: 1.56; 95% confidence interval [CI]: 1.05–3.39), adjustment of medical therapy when MH was not achieved (HR: 2.07; 95% CI: 1.26–2.33), prior enteric fistula (HR: 0.22; 95% CI: 0.06–0.91), perianal disease at CD diagnosis (HR: 0.58; 95% CI: 0.35–0.95), and C-reactive protein normalization within 12 weeks (HR: 3.23; 95% CI: 1.82–5.88). Similar factors have also been identified for endoscopic improvement.
Conclusions:
Performing serial endoscopic procedures at a 26-week interval and subsequent adjustment in medical treatment are helpful in achieving MH. Endoscopic monitoring plays an important role in the treating to target of CD.
Keywords: Crohn’s disease, endoscopic, mucosal healing, treat to target
Introduction
Setting mucosal healing (MH) as a therapeutic goal has gained increasing attention, stemming from observations that treatment aimed solely at resolution of clinical symptoms does not eliminate long-term bowel damage in patients with Crohn’s disease (CD). Evolving evidence indicates that an intensive care strategy aiming at abrogating intestinal inflammation and endoscopic MH might improve long-term outcome of CD with diminished rates of relapse, hospitalizations, and the need for surgery.1–3 A post hoc analysis of the ACCENT-1 trial demonstrated that using MH as an end point for decision-making strategy was cost-effective.4
Therefore, MH as the treatment goal and outcome measure is being increasingly considered in patients with CD. However, little is known about the factors associated with the achievement of MH. Early introduction of tumor necrosis factor (TNF) antagonists in the course of the disease, particularly in combination with immunosuppressives, is one strategy for improving MH.5,6 Similarly, a subgroup analysis of the recent EXTEND trial showed a higher rate of MH among patients who received adalimumab and had a CD duration shorter than 2 years.7 This is further confirmed by a recent study which demonstrated that longer duration of CD, previous surgery, and previous exposure to immunosuppressives were associated with poor rates of MH. However, none of these factors were independently predictive of MH in multivariate models.8
Identifying predictors of MH is of great clinical significance in guiding treatment strategy to treat to target of CD.9 The aim of our study was to evaluate the probability of achieving MH and identify factors independently predictive of subsequent MH in patients with CD.
Methods
Patients and design
This was a retrospective, observational cohort study of all consecutive patients with CD who underwent colonoscopy at the Inflammatory Bowel Disease Center, The First Affiliated Hospital of Sun Yat-sen University between 2008 and 2013. Diagnoses of CD were determined according to the criteria of Lennard-Jones,10 based on clinical, endoscopic, histopathological, and radiological findings. Disease phenotype was established according to the Montreal Classification.11
The inclusion criteria for patients enrolled were: (a) age between 18 years and 80 years; (b) ulcers detected by (ileo) colonoscopy at the initial endoscopy procedure; (c) at least one repeated colonoscopy after the initial colonoscopy. The exclusion criteria were: (a) patients with incomplete endoscopic procedures; (b) age under 18 years; (c) patients with isolated proximal small bowel involvement at the time of diagnosis.11
The study protocol was approved by the Clinical Research Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University and all patients signed an informed consent.
Endoscopic documentation
All endoscopic procedures were performed by skilled endoscopists with the standard protocol. The static endoscopic picture was recorded in the patients’ pro forma questionnaire sheet and also saved as a digital version in the endoscopy registry. The score was assessed retrospectively by a central endoscopic reading according to the saved endoscopy images. Of note, the second and subsequent endoscopic assessments were usually planned within 6 months by the treating physician to assess the response to therapy (i.e. the scheduled endoscopic follow-up evaluation to assess MH).
Clinical follow up
The clinical follow up and additional pertinent data in the medical files of the patients, the Inflammatory Bowel Disease Center register, and the endoscopy register were reassessed by two experienced gastroenterologists. A pre-determined structured data-sheet was used to collect data from the medical files at the time of each endoscopic procedure, including the indication for procedure, type and findings of the procedure, medical therapies being used at the beginning of the study period, any treatment adjustment after endoscopic procedures, general wellbeing, and symptoms at the time of each endoscopic procedure and within 3–6 months after procedure.
Definitions and outcomes
The primary outcome of the study was MH, and the secondary outcome was endoscopic improvement. The endoscopic score system was adopted from that of af Björkesten and colleagues.12 Briefly, the endoscopy reports were scored according to mucosal activity in the most affected area as: 0 (remission); 1–2 (mild inflammatory activity: light mucosal erythema or granularity or aphthous inflammation, without ulcerations); 3–4 (moderate activity: superficial ulcerations); 5–6 (severe activity: deep ulcerations, with a diameter of under or over 2 cm). The criterion for endoscopic improvement was a decrease in the endoscopic score of at least two. MH was determined as a mucosal activity score of 0–2. The duration of follow up was calculated from the time of the index endoscopy up to the time of MH or endoscopic improvement, loss of follow up, or end of study (30 December 2013). The definition of medical treatment adjustment after endoscopic procedure was adopted from Bouguen and colleagues8 as follows: the introduction or switch of immunosuppressives; the introduction, optimization, or switch within the class or out of the class of biologics; or change in both immunosuppressive agents and biologics.
Statistical analysis
Demographic and clinical parameters were compiled and summary statistics were calculated. Data were described using medians with interquartile range (IQR) for continuous data and percentages for discrete data. Fisher’s exact test or chi-square tests were used to compare the nonparametric categorical data between groups. The cumulative probabilities of MH and endoscopic improvement were estimated using the Kaplan–Meier method. For computing the cumulative probability of achieving MH, the Kaplan–Meyer analysis commences at the first endoscopy and is terminated at the time point of the first procedure during which MH/improvement was observed or at the last known follow-up endoscopy.8 Univariate analyses using the log-rank test were performed to identify factors predictive of each event. Factors analyzed by univariate analysis with p < 0.1 were integrated in multivariate Cox regression. The SPSS 15.0 software (SPSS, Chicago, IL, USA) was used to perform all appropriate statistical analyses. Statistical significance was set at p < 0.05.
Results
Demographic characteristics
A total of 272 (75.3%) patients (169 males, 103 females; median age 33 years; IQR: 24–41 years) who had ulcers detected by initial endoscopic procedure underwent at least two endoscopic procedures during the study period at our center. Of these 272 patients, 154 patients were in clinical remission at the first following endoscopic procedure, which comprised the study population for the final analysis. Baseline demographic characteristics are shown in Table 1. During follow up, 154 (56.6%) patients were treated with immunosuppressives. TNF-α antagonists were introduced only in 49 (18%) patients, and in 28 (10.3%) of these patients were combined with thiopurines.
Table 1.
Baseline characteristics.
| Variable | n = 272 |
|---|---|
| Sex, M:F | 169:103 |
| Median disease duration, months (IQR) | 13.2 (4.0–37.5) |
| Median age at referral, years (IQR) | 33 (24–41) |
| Montreal classification at Crohn’s disease diagnosis, n (%) | |
| L1: Ileal | 62 (22.8) |
| L2: Colonic | 45 (16.5) |
| L3: Ileocolonic | 165 (60.7) |
| Perianal lesion | 66 (24.3) |
| B1: Nonpenetrating nonstricturing | 153 (56.3) |
| B2: Stricturing | 86 (31.6) |
| B3: Penetrating | 33 (12.1) |
| Previous treatment, n (%) | |
| Prior surgery | 60 (23) |
| Prior medical treatment | |
| Steroid | 20 (7.4) |
| Immunosuppressives (azathioprine/6-mercaptopurine or methotrexate) | 14 (5.1) |
| Median erythrocyte sedimentation rate (IQR), mm/h | 35 (18–53) |
| Median C-reactive protein (IQR), mg/L | 11.03 (3.65–13.09) |
| Median Crohn’s Disease Activity Index (IQR) | 178 (114–250) |
IQR, interquartile range.
Endoscopic assessment
Initial endoscopy of the 272 patients showed deep ulcers in 91 patients (33%) and superficial ulcers in 181 patients (67%). An additional 535 endoscopic procedures were performed subsequently following the index procedure in the study patients (median follow-up period of 33 months, IQR: 27–38 months). In our study, the endoscopic procedures were usually planned a priori at the time of the previous endoscopic procedure for the purpose of assessing MH rather than in response to clinical symptoms, only 27 of 535 (5.04%) endoscopic procedures were performed because of a disease flare. The overall median interval between two consecutive endoscopic procedures was 24 weeks (IQR: 17–38 weeks). MH was achieved in 126 patients (46.3%) during follow up (Figure 1). The cumulative probabilities of MH were 10%, 22%, 46%, 63%, 72%, and 77.6% at 6, 12, 24, 36, 48, and 60 months from the time of the initial endoscopic procedure, respectively (Figure 2). The cumulative probabilities of endoscopic improvement were 13%, 29%, 53.5%, 72%, 80%, and 83.6% at 6, 12, 24, 36, 48, and 60 months, respectively (Figure 2).
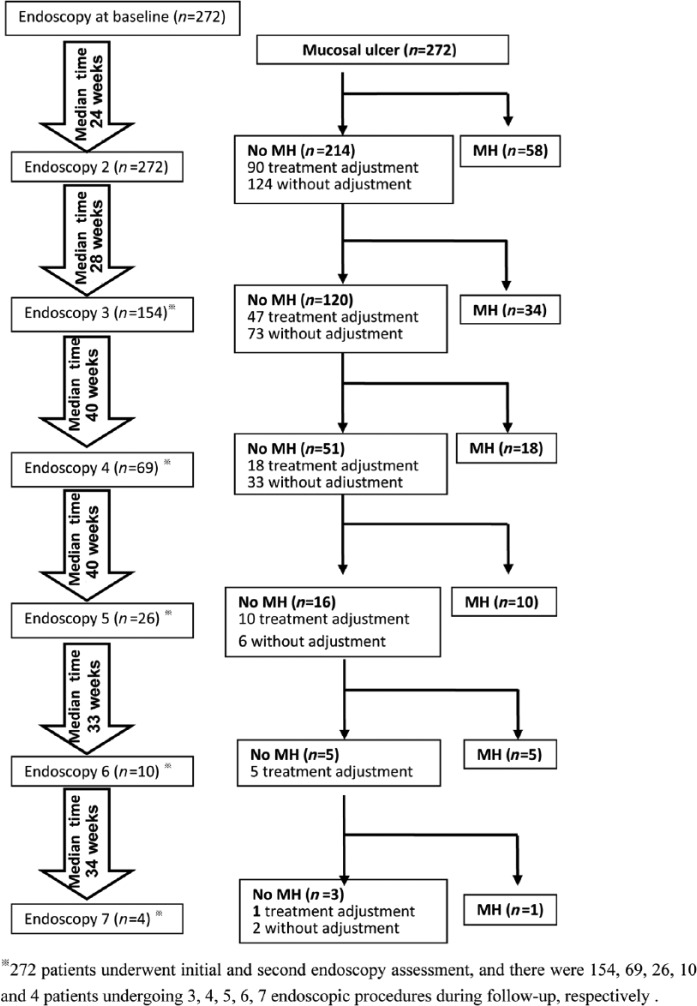
Figure 1.
Flow chart of adjustments in medical therapy according to endoscopic findings during the study period. MH, mucosal healing.
Figure 2.
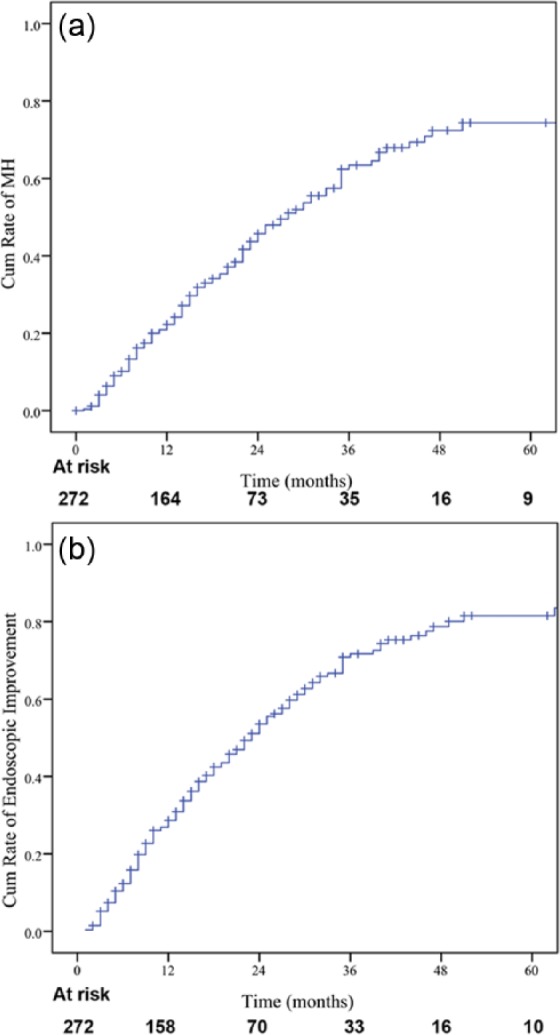
Cumulative probability of achieving (a) mucosal healing; (b) endoscopic improvement.
Medical therapy adjustment after endoscopic assessment
After endoscopic procedures, a total of 171 patients received adjustments in medical therapy in treating to target of MH (Figure 1). The medical adjustments were made based on comprehensive assessments of patients’ symptoms, (ileo)colonoscopic findings, therapies already tried, response to prior therapies, treatment durations, and patients’ willingness. As shown in Figure 1, after endoscopic procedures, a total of 237 adjustments in medical therapy were performed as a result of finding ulcers at endoscopy, whereas 430 endoscopic procedures were not followed by adjustments in medical therapy despite the presence of ulcers. TNF antagonists were introduced in 42 naïve patients, optimized with dose escalation or interval shortening in four patients, and three patients switched to adalimumab. Immunosuppressive monotherapy (azathioprine [AZA]/6-mercaptopurine [6MP] or methotrexate [MTX] or thalidomide) was initiated in 122 patients; 28 out of the above 49 patients receiving anti-TNF treatment were on the combined therapy (AZA/6MP combined with anti-TNFs).
Overall, MH was achieved in 78/237 treatment adjustments. Regarding no MH under thiopurines, 30 medical treatment adjustments were performed based on endoscopic findings and 17/30 (57%) patients achieved MH. Similarly, 24/42 (57.1%) patients achieved MH on the introduction or optimization of biologics.
Predictors of MH and endoscopy improvement
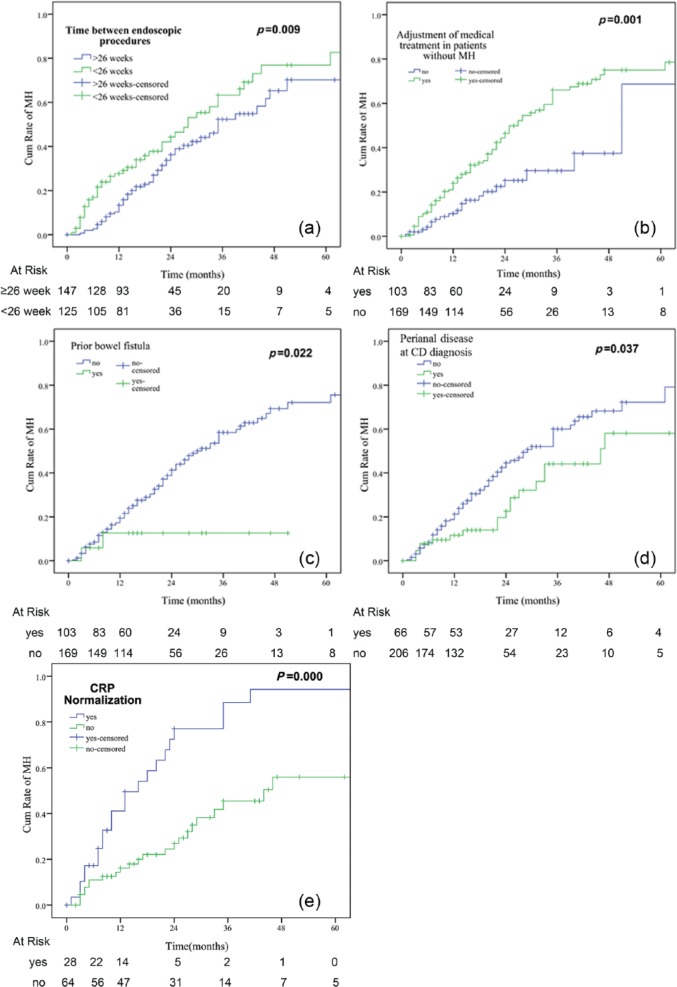
On univariate analysis,disease duration of 18 months or less at the initial endoscopy (hazard ratio [HR]: 1.62; 95% confidence interval [CI]: 1.07–2.47), C-reactive protein (CRP) normalization within 12 weeks (HR: 1.72; 95% CI: 1.05–2.86), repeated endoscopic procedures within 26 weeks of the previous one (HR: 1.69; 95% CI: 1.14–2.5), medical treatment adjustment when MH was not achieved (HR: 2.29; 95% CI: 1.4–3.74), and immunosuppressive use at follow up (HR: 1.61; 95% CI: 1.05–2.48) were positively associated with MH. In contrast, perianal disease at CD diagnosis (HR: 0.58; 95% CI: 0.35–0.95), prior enteric fistula (HR: 0.23; 95% CI: 0.06–0.91), and prior bowel stricture (HR: 0.65; 95% CI: 0.42–0.99) were negatively associated with MH (Table 2 and Figure 3). By multivariate analysis,time between endoscopic procedures of less than 26 weeks (HR: 1.56; 95% CI: 1.05–3.39), adjustment of medical therapy (HR: 2.07; 95% CI: 1.26–2.33), prior enteric fistula (HR: 0.22; 95% CI: 0.06–0.91), perianal disease at CD diagnosis (HR: 0.58; 95% CI: 0.35–0.95), and CRP normalization within 12 weeks (HR: 3.23; 95% CI: 1.82–5.88) were independently associated with MH (Table 2).
Table 2.
Predictors of mucosal healing by univariate and multivariate analysis.
| Baseline factors | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| p | HR (95% CI) | p | HR (95% CI) | |
| Gender, female | 0.14 | 1.34 (0.91–1.98) | ||
| Active smoker | 0.08 | 1.67 (0.81–3.45) | ||
| Age, < 40 years | 0.18 | 1.37 (0.87–2.15) | ||
| Disease duration, < 18 months | 0.02 | 1.62 (1.07–2.47) | 0.51 | 1.58 (0.97–2.56) |
| Montreal L at CD diagnosis | 0.19 | 0.86 (0.69–1.08) | ||
| Montreal B at CD diagnosis | 0.80 | 0.96 (0.73–1.28) | ||
| Perianal disease at CD diagnosis | 0.04 | 0.58 (0.35–0.95) | 0.04 | 0.58 (0.35–0.95) |
| Extraintestinal manifestation | 0.59 | 1.13 (0.72–1.77) | ||
| Prior abdominal surgery | 0.43 | 0.81 (0.48–1.36) | ||
| Prior enteric fistula | 0.04 | 0.23 (0.06–0.91) | 0.04 | 0.22 (0.06–0.91) |
| Prior anal fistula | 0.84 | 1.08 (0.5–2.34) | ||
| Prior bowel stricture | 0.046 | 0.65 (0.42–0.99) | 0.07 | 0.45 (0.19–1.08) |
| Prior steroids use | 0.99 | 1.01 (0.49–2.07) | ||
| Prior immunosuppressives use (AZA/6MP or MTX) | 0.41 | 0.65 (0.24–1.78) | ||
| Crohn’s Disease Activity Index at referral | 0.19 | 1.00 (0.99–1.00) | ||
| CRP | 0.75 | 0.99 (0.95–1.04) | ||
| Erythrocyte sedimentation rate | 0.62 | 1.00 (0.99–1.00) | ||
| Deep endoscopic ulcer | 0.89 | 1.03 (0.68–1.57) | ||
| Time between endoscopic procedures, < 26 weeks | 0.01 | 1.69 (1.14–2.5) | 0.03 | 1.56 (1.05–3.39) |
| Adjustment of medical therapy when there was no mucosal healing | 0.00 | 2.29 (1.4–3.74) | 0.00 | 2.07 (1.26–2.33) |
| Treatment during follow up | ||||
| AZA/6MP/MTX/thalidomide | 0.03 | 1.61 (1.05–2.48) | 0.31 | 1.36 (0.67–2.7) |
| Tumor necrosis factor-α antagonists monotherapy | 0.07 | 1.53 (0.96–2.43) | 0.81 | 1.09 (0.55–1.42) |
| Combined therapy* | 0.35 | 1.26 (0.77–2.05) | ||
| CRP normalization < 12 weeks | 0.00 | 1.72 (1.05–2.86) | 0.00 | 3.23 (1.82–5.88) |
Numbers in bold indicate statistical significance.
Infliximab plus thiopurine. 6MP, 6-mercaptopurine; AZA, azathioprine; CD, Crohn’s disease; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; MTX, methotrexate.
Figure 3.
Kaplan–Meier analysis of achieving mucosal healing according to factors identified by multivariate analysis. CD, Crohn’s disease; CRP, C-reactive protein; MH, mucosal healing.
A similar result was found regarding endoscopic improvement. Age younger than 40 years (HR: 0.98; 95% CI: 0.96–0.99), endoscopic procedures within 26 weeks (HR: 1.64; 95% CI: 1.14–2.34), adjustment of medical therapy (HR: 2.21; 95% CI: 1.39–3.53), use of immunosuppressives at follow up (HR: 1.98; 95% CI: 1.25–3.15), and CRP normalization within 12 weeks (HR: 2.17; 95% CI: 1.28–3.70) independently predicted endoscopic improvement (Supplementary Table 1).
Long-term follow up
Sustained MH
The median time of endoscopic recurrence in patients who achieved MH was 24.97 months (95% CI: 9.51–40.4 months). As depicted in Figure 4(a), the cumulative probability of maintaining MH was 78.6%, 57.9%, 40.9%, and 40.9% at 1, 2, 3, and 4 years, respectively.
Figure 4.
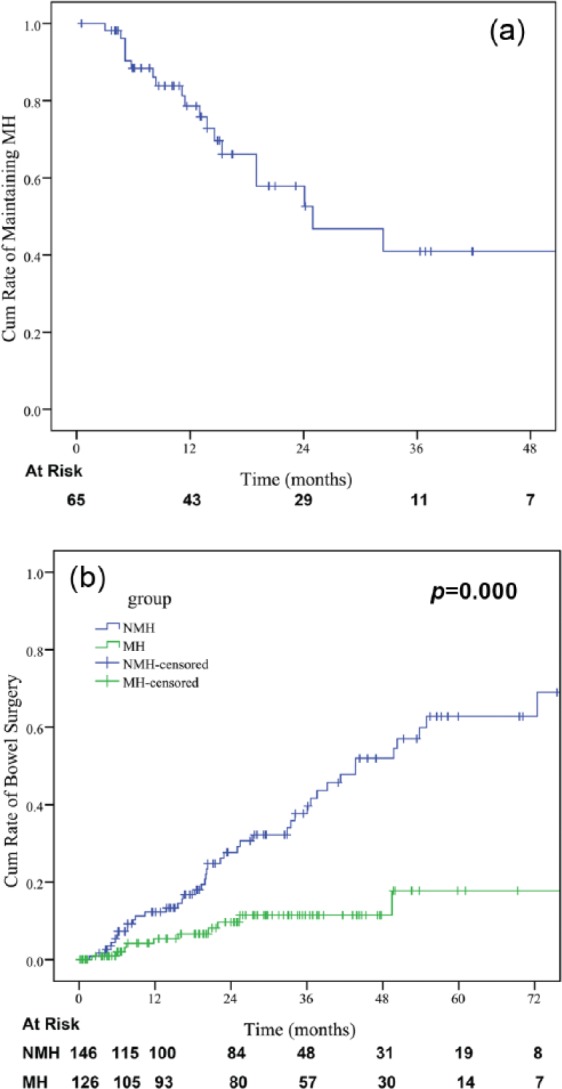
The cumulative probability of (a) maintaining mucosal healing (MH); (b) cumulative probability of bowel surgery for patients who achieved MH; (c) no mucosal healing (NMH).
Bowel surgery
Patients who achieved MH had a decreased rate of surgery when compared with patients with sustained ulceration with a median bowel surgery-free survival of 97.8 + 5.2 months versus 52.4 + 5.2 months (p = 0.000) (Figure 4(b)). Similarly, patients who achieved endoscopic improvement had fewer bowel surgeries (p = 0.000).
Discussion
As demonstrated in the present study, MH was associated with improved clinical outcomes, i.e. decreased rates of bowel surgery when compared with patients with sustained endoscopic ulceration. MH is increasingly considered as the treatment target for CD, but the feasibility and the rate of achievement of MH in clinical practice remains unclear. Data from 1-year trials, i.e. the EXTEND trial and ACCENT I trial, indicate rates of MH ranging from 24% to 31%.13,14 In the SONIC trial, MH occurred in 43.9% of the patients who received combination therapy at 6 months.6 Bouguen and colleagues8 found that the cumulative probabilities of MH were 12.7% and 45.0% at 24 weeks and 52 weeks after adjusting medical treatment based on a treat-to-target strategy, respectively. However, data regarding the MH rate in the long term are limited. Colombel and colleagues6 reported that 73.1% of patients receiving early combination therapy achieved MH at week 104. In the present study, the cumulative probabilities of MH at 26 weeks and 52 weeks were 10% and 22%, respectively, while MH rates rose to 46%, 63%, and 72% at 2, 3, and 4 years, respectively. The reason for the relatively low MH rate may partly be due to the limited use of biologics in our study. TNF-α antagonists were introduced only in 49 (18%) patients. Moreover, a large number of patients included in our study had experienced complications and perianal lesions, which may also explain the relatively lower MH rate, as MH is more achievable for patients with early CD.7
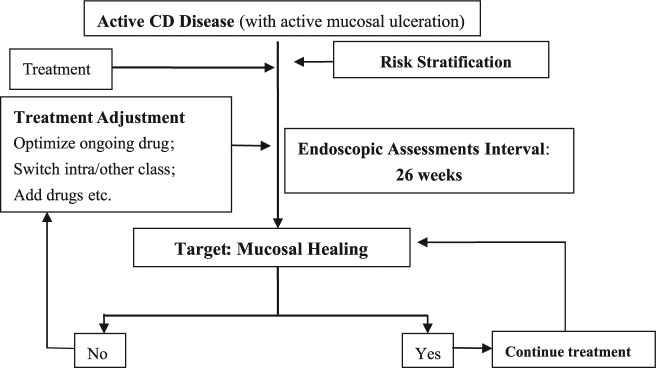
Little is known about the predictors of MH. Until now no controlled prospective trials have been designed to identify predictors of MH. Some studies reported that shorter disease duration was associated with higher MH rate.7 In contrast, perianal disease at CD diagnosis and prior enteric fistula and stricture were associated negatively with MH.15–24 Our study showed that risk factors for lower probability of MH were prior enteric fistula and perianal disease at CD diagnosis. The incidence of internal fistula, perianal disease, and stenosis implies a type B2/3 disease behavior with a more aggressive disease progression, which is consistent with previous studies.20 Moreover, our study demonstrated that a shorter time between endoscopic procedures and subsequent medical treatment adjustment was associated with a higher rate of subsequent MH. These findings may be interpreted as patients who are more closely followed endoscopically also have higher prospects for attaining MH thereafter. While the reason for this association cannot be definitively ascertained from the present study, it is possible that the result of a higher chance of active inflammation being disclosed by repeated endoscopies and prompt medical interventions in these patients, and/or that this subgroup of patients undergoing more frequent endoscopies was cared for by more proactive physicians. Recently, the concept of treat-to-target strategy, which employs the principles of systematic follow up of patients and optimization of all available treatments to reach the target in the treatment of CD, has been increasingly advocated.9 Bouguen and colleagues reported that treat to target is achievable by appropriate endoscopic assessment and treatment adjustment in patients with both CD8 and ulcerative colitis25. This demonstrated the potential of adjusting therapy on the basis of an objective treatment target according to a predefined time frame. However, the follow up of these studies was only 62 weeks and 76 weeks, respectively. Our present study evaluated the role of endoscopic assessment in treat to target of CD with long follow up. Based on our result, we formulated an algorithm for the treat to target of the MH approach in patients with CD based on endoscopy assessment and subsequent treatment adjustment (Figure 5).
Figure 5.
Role of endoscopic monitoring in treat to target of mucosal healing in Crohn’s disease (CD) (adapted from Bouguen and colleagues9).
Introduction of biologics early in the course of the disease, particularly in combination with immunosuppressive agents, is one strategy for obtaining higher rates of MH, as demonstrated in the SONIC and step-up/top-down trials.5,6 In our study, the use of biologics was significantly associated with an increased rate of endoscopic improvement (p = 0.01), but only showed a trend for higher MH that did not reach statistical significance (p = 0.07). This may possibly be due to the relatively small number of anti-TNF-treated patients and to the fact that biologics were mainly used in a step-up fashion in our center, which is known to be associated with a less favorable response to biologics.
Kiss and colleagues26 reported CRP at week 12 after adalimumab treatment significantly correlated with MH. Similarly, our results demonstrated the CRP normalization at week 12 independently predicted MH and endoscopic improvement. However, our study failed to demonstrate a positive association between baseline CRP and subsequent MH. This may be explained by a discrepancy between clinical symptoms and objective findings of inflammation.27 In our study, 10.4% of patients experienced clinical symptoms despite MH, whereas 33.5% of patients with significant endoscopic lesions presented no clinical symptoms.
At present, there is still some dispute about the definition of MH. Schnitzler and colleagues2 reported that patients with CD who experienced endoscopic improvement had similar long-term clinical outcomes compared with patients who experienced complete MH. In patients with CD, endoscopic response could serve as a reliable predictor of clinical outcome based on a post hoc analysis of data from the SONIC trial.28 Our result showed that the cumulative probabilities of endoscopic improvement were 13%, 29%, 53.5%, 72%, 80%, and 83.6% at 6, 12, 24, 36, 48, and 60 months, respectively. Bouguen and colleagues8 reported that the rates of endoscopic improvement were 22.4%, 49.2%, and 61.1% at 24, 52, and 62 weeks, respectively. Results from the MUSIC trial demonstrated endoscopic response rates in patients with active CD treated with certolizumab pegol of 74.4% at week 54.29 Regarding the risk factors, our study demonstrated that age younger than 40 years, endoscopic procedures within 26 weeks, adjustment of medical therapy, and the use of immunosuppressives (AZA/6MP or MTX) during follow up were independent predictors of endoscopic improvement, which were consistent with previous studies.8,30
Our study has certain limitations. Firstly, for the retrospective nature of our study, one may argue that the lack of standard endoscopic evaluation could induce bias towards more endoscopic procedures in patients with clinical symptoms. To minimize bias, the endoscopic procedures were usually planned a priori at the time of the previous endoscopic procedure for the purpose of assessing MH rather than being planned at the time of the subsequent endoscopic procedure in response to clinical symptoms. Secondly, the lack of a control group (patients not undergoing fixed endoscopies, or asymptomatic patients undergoing endoscopies but without treatment adaptation in the case of endoscopic lesions) is another limitation. Further randomized clinical trials with control groups are needed to confirm our preliminary result. Last but not least, due to the nature of the retrospective, observational design of the present study, not all of the treatment adjustments were followed with endoscopic examinations, so we did not calculate the exact rate of MH for each treatment adjustment. Further prospective clinical trials are warranted in this setting.
In conclusion, our results suggest that more frequent endoscopic monitoring, adjustments of medical treatment after colonoscopy, and CRP normalization within 12 weeks were associated with a higher rate of subsequent MH, while prior enteric fistula and perianal disease at CD diagnosis were associated with lower probability of MH. For patients with identified risk factors, close endoscopic monitoring and subsequent adjustment of medical treatment are desirable for the achievement of treat to target of MH in patients with CD.
Supplementary Material
Acknowledgments
RM and YQ contributed equally: study design, data collection, statistical analysis, interpretation, and manuscript drafting/revision. MC: study concept, design, and critical revision of the manuscript for important intellectual content; SZ, RF, BC, YH, ZZ, and SB-H: study concept, design, and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Footnotes
Funding: This study was financially supported in part by the National Key Clinical Department in the Ministry of Public Health, China (No.303004269002) and Scientific Project of Guangzhou, China (No.2011YZ-00004), the National Natural Science Foundation of China (NSFC grant No.81470821 and No.81270473).
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Contributor Information
Ren Mao, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China.
Yun Qiu, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China.
Bai-Li Chen, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China.
Sheng-Hong Zhang, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China.
Rui Feng, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China.
Yao He, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China.
Zhi- Rong Zeng, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China.
Shomron Ben-Horin, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China, and IBD Service, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine, Tel-Aviv University, Tel HaShomer, Israel.
Min-Hu Chen, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, 58 Zhongshan II Road, Guangzhou 510080, People’s Republic of China.
References
- 1. Froslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007; 133: 412–422. [DOI] [PubMed] [Google Scholar]
- 2. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009; 15: 1295–1301. [DOI] [PubMed] [Google Scholar]
- 3. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010; 138: 463–468; quiz e10–e11. [DOI] [PubMed] [Google Scholar]
- 4. Ananthakrishnan AN, Korzenik JR, Hur C. Can mucosal healing be a cost-effective endpoint for biologic therapy in Crohn’s disease? A decision analysis. Inflamm Bowel Dis 2013; 19: 37–44. [DOI] [PubMed] [Google Scholar]
- 5. D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008; 371: 660–667. [DOI] [PubMed] [Google Scholar]
- 6. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 7. Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12: 414–422. e5. [DOI] [PubMed] [Google Scholar]
- 8. Bouguen G, Levesque BG, Pola S, et al. Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12: 978–985. [DOI] [PubMed] [Google Scholar]
- 9. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13: 1042–1050. e2. [DOI] [PubMed] [Google Scholar]
- 10. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl 1989; 170: 2–6. [DOI] [PubMed] [Google Scholar]
- 11. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Suppl. A): 5A–36A. [DOI] [PubMed] [Google Scholar]
- 12. af Björkesten CG, Nieminen U, Turunen U, et al. Endoscopic monitoring of infliximab therapy in Crohn’s disease. Inflamm Bowel Dis 2011; 17: 947–953. [DOI] [PubMed] [Google Scholar]
- 13. Rutgeerts P, Diamond RH, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc 2006; 63: 433–442. [DOI] [PubMed] [Google Scholar]
- 14. Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012; 142: 1102–1111. e2. [DOI] [PubMed] [Google Scholar]
- 15. Cosnes J, Beaugerie L, Carbonnel F, et al. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology 2001; 120: 1093–1099. [DOI] [PubMed] [Google Scholar]
- 16. Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol 2007; 5: 1430–1438. [DOI] [PubMed] [Google Scholar]
- 17. Sands BE, Arsenault JE, Rosen MJ, et al. Risk of early surgery for Crohn’s disease: implications for early treatment strategies. Am J Gastroenterol 2003; 98: 2712–2718. [DOI] [PubMed] [Google Scholar]
- 18. Louis E, Michel V, Hugot JP, et al. Early development of stricturing or penetrating pattern in Crohn’s disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut 2003; 52: 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allez M, Lemann M, Bonnet J, et al. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol 2002; 97: 947–953. [DOI] [PubMed] [Google Scholar]
- 20. Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn’s disease. Gastroenterology 2006; 130: 650–656. [DOI] [PubMed] [Google Scholar]
- 21. Wolters FL, Russel MG, Sijbrandij J, et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut 2006; 55: 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chow DK, Sung JJ, Wu JC, et al. Upper gastrointestinal tract phenotype of Crohn’s disease is associated with early surgery and further hospitalization. Inflamm Bowel Dis 2009; 15: 551–557. [DOI] [PubMed] [Google Scholar]
- 23. Beaugerie L, Sokol H. Clinical, serological and genetic predictors of inflammatory bowel disease course. World J Gastroenterol 2012; 18: 3806–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Romberg-Camps MJ, Dagnelie PC, Kester AD, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol 2009; 104: 371–383. [DOI] [PubMed] [Google Scholar]
- 25. Bouguen G, Levesque BG, Pola S, et al. Feasibility of endoscopic assessment and treating to target to achieve mucosal healing in ulcerative colitis. Inflamm Bowel Dis 2014; 20: 231–239. [DOI] [PubMed] [Google Scholar]
- 26. Kiss LS, Szamosi T, Molnar T, et al. Early clinical remission and normalisation of CRP are the strongest predictors of efficacy, mucosal healing and dose escalation during the first year of adalimumab therapy in Crohn’s disease. Aliment Pharmacol Ther 2011; 34: 911–922. [DOI] [PubMed] [Google Scholar]
- 27. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014; 63: 88–95. [DOI] [PubMed] [Google Scholar]
- 28. Ferrante M, Colombel JF, Sandborn WJ, et al. Validation of endoscopic activity scores in patients with Crohn’s disease based on a post hoc analysis of data from SONIC. Gastroenterology 2013; 145: 978–986. e5. [DOI] [PubMed] [Google Scholar]
- 29. Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12: 423–431. e1. [DOI] [PubMed] [Google Scholar]
- 30. Lakatos PL, Kiss LS. Is the disease course predictable in inflammatory bowel diseases? World J Gastroenterol 2010; 16: 2591–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.