Abstract
Remarkable advancements in techniques of genomic profiling and bioinformatics have led to the accumulation of vast amounts of knowledge on the genomic profiles of biliary tract cancer (BTC). Recent largescale molecular profiling studies have not only highlighted genomic differences characterizing tumors of the intrahepatic and extrahepatic bile ducts and gallbladder, but have also revealed differences in genomic profiles pertaining to associated risk factors. Novel genomic alterations such as FGFR2 fusions and IDH1/2 mutations in intrahepatic cholangiocarcinoma (ICC) and ERBB2 alterations in gallbladder cancer (GBCA) are emerging as targeted therapy options capable of advancing precision medicine for the care of these patients. Moreover, variable genomic alterations also appear to impact prognosis and overall disease outcome independent from their therapy selection value. High mutational burden and increased expression of immune checkpoint-related proteins observed in a subset of BTC also show a potential for guidance of immunotherapy. Thus, comprehensive genomic profiling (CGP) is rapidly achieving status as an integral component of precision medicine and is starting to become invaluable in guiding the management of patients with BTC, a rare disease with dismal outcome.
Keywords: biliary tract cancer, cholangiocarcinoma, comprehensive genomic profiling, gallbladder cancer, genomic alteration, next-generation sequencing, precision medicine, targeted therapy
Introduction
Biliary tract cancer (BTC), also known as cholangiocarcinoma, can be defined as an adenocarcinoma arising from epithelium of the intrahepatic and extrahepatic biliary tree, and gallbladder [Nakanuma et al. 2010; Malhi and Gores, 2006]. While the International Classification of Diseases for Oncology by the World Health Organization classifies hilar cholangiocarcinoma (Klatskin tumor) as extrahepatic [Welzel et al. 2006], the European Network for the Study of Cholangiocarcinoma classifies BTC into intrahepatic, perihilar and distal [Banales et al. 2016]. About 50–60% of BTC is located in the liver hilum with or without direct extension to the hepatic parenchyma [Malhi and Gores, 2006]. The incidence of extrahepatic BTC may be decreasing [Global Burden of Disease Cancer Collaboration, 2015], while the incidence of intrahepatic BTC appears to be increasing worldwide [Welzel et al. 2006].
BTC comprises about 3% of all gastrointestinal tract neoplasms [Augustine and Fong, 2014; Lee et al. 2016]. The rarity of BTC and overall poor prognosis make it challenging to design robust clinical trials designed to optimize treatment. Therefore, it would be of great benefit for patients with BTC to be able to identify targetable genomic alterations and offer individualized treatments. Recent advancement in comprehensive genomic profiling (CGP) technologies and bioinformatics have allowed us to have a better understanding of the pathobiology of BTC and have recently led to the discovery of numerous genomic alterations and mechanistic pathways that may be targetable. Herein, we review the recent developments and impact of CGP of BTC and how these discoveries are shaping modern treatment regimens employing targeted therapies and immunotherapies for this disease.
The literature was searched via PubMed using the combination of keywords including ‘cholangiocarcinoma’, ‘bile duct cancer’, ‘biliary cancer’, ‘targeted therapy’, ‘next-generation sequencing’ and ‘genomic profiling’, and reviewed by reading the articles and related articles. Foundation Medicine Inc. [(FMI) Cambridge, Massachusetts, USA] cases are the cornerstone of the manuscript and the updated data, including previously published and new cases, are integrated throughout the manuscript.
Epidemiology and risk factors
BTC is the second most common primary malignancy of the liver [Global Burden of Disease Cancer Collaboration, 2015]. Notably, there is a marked geographic variation for this cancer due to certain risk factors prevalent in some geographic areas and genetic predisposition of the population. For example, liver fluke including Clonorchis sinensis and Opisthorchis viverrini, and hepatolithiasis is endemic in Asia, contributing to increased incidence of BTC in this region [Shin et al. 2010]. In Northeast Thailand, BTC constitutes approximately 85% of primary liver malignancies [Poomphakwaen et al. 2009]. Additional well established risk factors of BTC include primary sclerosing cholangitis (PSC), bile duct cysts and exposure to thorotrast [Augustine and Fong, 2014]. Gallbladder cancer (GBCA) is frequent in the Andean region, in Native Americans, in Alaskan Natives, and in Mexican Americans, possibly in association with genetic predisposition and living conditions [Andia et al. 2008; Augustine and Fong, 2014; Jain et al. 2016]. Relatively well established risk factors of GBCA include cholelithiasis, infection, anomalous pancreaticobiliary duct junction and gallbladder polyps [Augustine and Fong, 2014].
Other postulated risk factors for BTC include viral hepatitis, human immunodeficiency viral infection, idiopathic inflammatory bowel disease independent of PSC, cirrhosis, alcohol intake, smoking, fatty liver disease, obesity and choledocholithiasis. Obesity, diabetes and genetic predisposition have been postulated to be additional risk factors of GBCA [Khan et al. 2008; Kongpetch et al. 2015; Zhou et al. 2012; Augustine and Fong, 2014; Jain et al. 2016].
Conventional treatment and prognosis
Complete surgical resection or liver transplantation is potentially curative for resectable tumor, and conventional classifications of BTC according to anatomical location are relevant to surgical planning when the patients present with a localized resectable disease [Nathan et al. 2007; Rosen et al. 2010]. Unfortunately, only 13–55% of BTC patients are surgical candidates and most BTC is detected at an advanced, inoperable stage due to lack of specific symptoms and effective screening. Even after the resection, local recurrence rate is high and overall 5-year survival is in the range of 11–44% [Banales et al. 2016; Chong and Zhu, 2016; Skipworth et al. 2011]. Therefore, many patients receive palliative systemic chemotherapy for the disease management [Chong and Zhu, 2016; Banales et al. 2016]. To date, there is no site-specific or widely adopted standardized chemotherapy regimen for BTC, as high-quality data derived from clinical trials are scarce. Gemcitabine-based regimens have been extracted from pancreatic cancer management protocols, and used with or without combination with platinum agents or 5-fluorouracil, regardless of the site [Valle et al. 2010; Yang et al. 2013]. The prognosis of inoperable BTC remains dismal with <12 months of overall survival [Chong and Zhu, 2016; Lee et al. 2016].
Advancement in genomic profiling of biliary tract cancer
Variable methodologies have been employed to identify common molecular alterations of BTC in the past decade, which have contributed to continuous growth in understanding of the pathogenesis of this disease [Lee et al. 2016]. In 2012, Ong and colleagues carried out whole-exome sequencing of eight liver-fluke-related BTC. Using a hotspot mutation panel, the authors selected 15 major genes with somatic mutations, and validated the mutations in further 46 cases. In addition to common mutations that had been previously known, novel somatic mutations in MLL3, ROBO2, RNF43, PEG3 and GNAS were identified [Ong et al. 2012]. Subsequently, further technical evolution of next-generation sequencing (NGS) techniques and the increased utilization of NGS clinical tests have led to an accumulation of a vast amount of genomic profiling data of BTC in a relatively short period of time [Borger et al. 2012; Chan-On et al. 2013; Jiao et al. 2013; Simbolo et al. 2014; Zou et al. 2014; Ross et al. 2014; Li et al. 2014; Lee et al. 2016; Churi et al. 2014; Jain et al. 2016]. These more recent studies have revealed differences in genomic profiling per anatomical location of the BTC as well as shedding light on the underlying risk factors for the disease and leading to the potential for the development of personalized targeted therapies.
Borger and colleagues showed that isocitrate dehydrogenase (IDH1/2) mutation is almost exclusively identified in intrahepatic cholangiocarcinomas (ICCs) (9 of 40 cases, 23%), but not in 22 extrahepatic cholangiocarcinomas (ECCs) and 25 GBCA [Borger et al. 2012]. Ong and colleagues’ study was expanded to compare 108 ICC caused by liver-fluke infection and 101 cases without the infection by exome sequencing. This study showed that certain mutations were more frequent in liver-fluke-associated tumors compared with the tumors with other risk factors [Chan-On et al. 2013]. Jiao and colleagues reported frequent inactivating mutations of chromatin-remodeling genes in 32 ICC and frequent TP53 mutation in 9 GBCA [Jiao et al. 2013]. Simbolo and colleagues carried out a mutational survey of 56 cancer-related genes in 70 ICC, 57 ECC and 26 GBCA. The molecular profiles of the tumors differed based on anatomical site, and 68% of tumors harbored targetable pathway alterations [Simbolo et al. 2014]. Zou and colleagues performed an exome sequencing of 102 ICC from Chinese patients, and reported the associations between HBsAg serology and gene mutation [Zou et al. 2014]. Site-specific (intrahepatic, extrahepatic and gallbladder) CGP studies by a deeper sequencing with broader coverage followed. These studies confirmed previous findings and identified additional clinically relevant genomic alterations [Ross et al. 2014; Lee et al. 2016; Li et al. 2014].
Differential genomic profile based on anatomical location and risk factors
A small series of genomic alterations are significantly enriched, based on the anatomical location of the BTC. Genomic alterations in IDH1/2 (19%; 5–36%) and fibroblast growth factor receptor (FGFR) 2 fusion (6%; 4–20%) are almost exclusively identified in ICC [Jain and Javle, 2016; Jain et al. 2016; Javle et al. 2016; Ross et al. 2014; Chong and Zhu, 2016]. BAP1 alteration is also common in ICC (12%; 1–38%) compared with ECC and GBCA [Jain and Javle, 2016; Jain et al. 2016; Javle et al. 2016; Ross et al. 2014; Chong and Zhu, 2016]. In contrast, ERBB2 mutations are rare in ICC, while regularly identified in ECC (10%; 9– 25%) and GBCA (11%; 0–17%) [Jain and Javle, 2016; Jain et al. 2016; Javle et al. 2016]. PRKACA or PRKACB fusion was identified only in ECC, while EGFR, ERBB3 and PTEN mutations preferentially occurred in GBCA [Nakamura et al. 2015]. Inactivating TP53 mutations are more common in ECC (38%; 14–45%) and GBCA (57%; 46–59%) than in ICC (11%; 0–17%) [Jain and Javle, 2016; Jain et al. 2016; Javle et al. 2016; Ross et al. 2014; Chong and Zhu, 2016] (Figures 1–4).
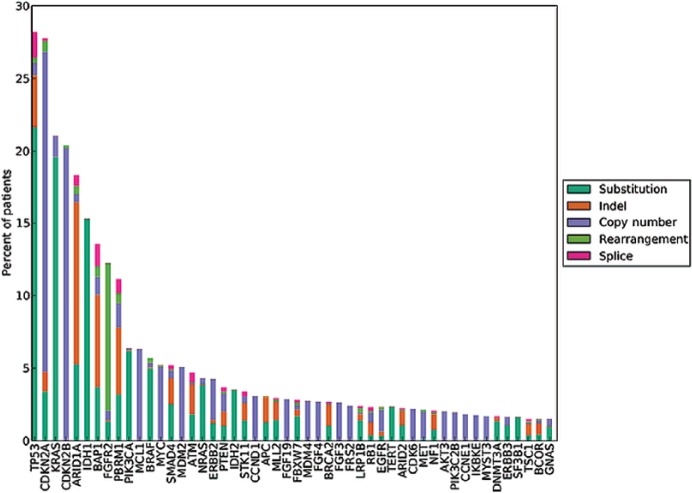
Figure 1.
Long tail plot of the distribution of genomic alterations in 1682 cases of ICC (Provided by Foundation Medicine, Inc., Cambridge, MA, USA).
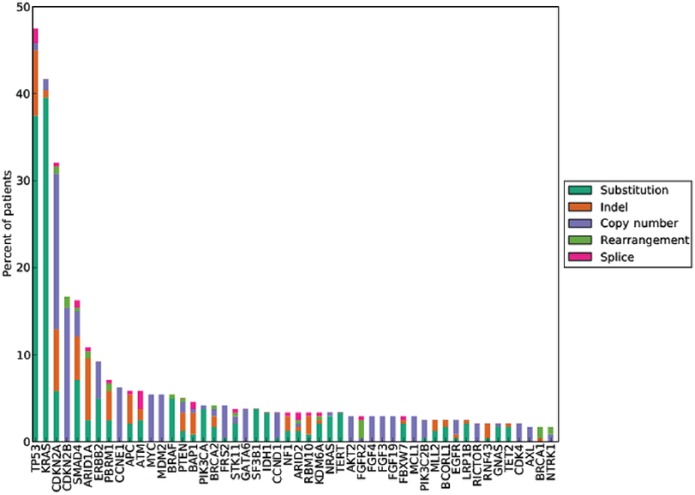
Figure 2.
Long tail plot of the distribution of genomic alterations in 251 cases of ECC (Provided by Foundation Medicine, Inc., Cambridge, MA, USA).
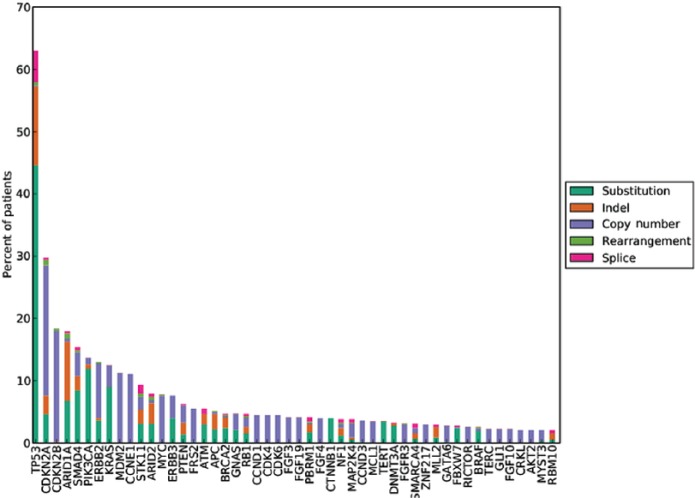
Figure 3.
Long tail plot of the distribution of genomic alterations in 593 cases of GBCA (Provided by Foundation Medicine, Inc., Cambridge, MA, USA).
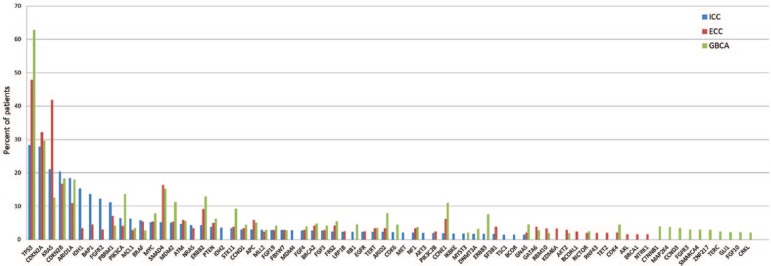
Figure 4.
Combined long tail plot (combination of Figures 1–3) of ICC (intrahepatic cholangiocarcinoma), ECC (extrahepatic cholangiocarcinoma) and GBCA (gallbladder cancer).
Certain genomic alterations in BTC are associated with the pathogenesis of the disease (see Table 1). Liver-fluke-associated ICC shows higher somatic mutation burden compared with nonparasite-associated BTC [Chan-On et al. 2013].
Table 1.
Genomic alterations pertaining to associated risk factors (grey). Oncovirus-hepatitis B and C, human papilloma virus and human T-lymphotrophic virus 1. Ong et al. [2012]; Chan-On et al. [2013]; Jang et al. [2014]; Zou et al. [2014]; Nakamura et al. [2015]; Jain and Javle [2016].
| Liver fluke |
Chronic liver disease |
Serum HBsAg |
Oncovirus | ||||
|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | ||
| TP53 |
ESPNL BCOR FAM40B DNAH6 KDM6B ARID2 |
||||||
| KRAS | |||||||
| CDKN2A | |||||||
| SMAD4 | |||||||
| IDH1/2 | |||||||
| PIK3CA | |||||||
| FGFR | |||||||
| EGFR | |||||||
| MLH1 | |||||||
| GNAS | |||||||
| BAP1 | |||||||
| MLL3 | |||||||
| PTEN | |||||||
HBsAg, hepatitis B virus surface antigen.
Genomic profiling and prognostic relevance
Wang and colleagues reported that IDH1/2 mutation showed a better prognosis with a longer time-to-tumor-recurrence in ICC [Wang et al. 2013]. On the contrary, Jiao and colleagues reported reduced 3-year survival in ICC with these mutations, though they had only six cases with the mutations [Jiao et al. 2013]. Churi and colleagues performed NGS-based genomic profiling of 50 ICC and 25 ECC and correlated clinical outcome with genomic alterations. In ICC, KRAS, TP53 or mitogen-activated protein kinase/mammalian target of rapamycin (MAPK/mTOR), alterations were associated with worse prognosis, whereas FGFR alterations (including amplification and fusion) were associated with a relatively indolent disease course. IDH1 mutation did not confer a prognostic relevance in this study. In ECC, BAP1 and PBRM1 alterations were associated with worse prognosis with aggressive clinical course [Churi et al. 2014]. Subsequent case series study of 22 BTC (20 ICC and 2 ECC) with BAP1 alteration reported that 59% of patients showed aggressive clinical course [Al-Shamsi et al. 2016]. Nakamura and colleagues showed that TP53, KRAS and ARID2 mutation were associated with poor prognosis in BTC by univariate analysis [Nakamura et al. 2015]. A recent multi-institutional study of BTC confirmed the correlation of genomic profiles with clinical outcome. Javle and colleagues performed genomic profiling for 554 cases of BTC (412 ICC, 57 ECC and 85 GBCA) and correlated the mutational profiles with clinical outcome for 321 patients. In keeping with previous studies, alterations in TP53, KRAS, CDKN2A/B and the MAPK/extracellular signal-regulated kinase (MAPK/ERK) pathway correlated with poor overall survival, whereas FGFR2 mutations were associated with improved overall survival. Twenty ICC patients with FGFR mutations received FGFR-specific targeted therapy, and showed superior overall survival compared with patients treated with conventional chemotherapy. IDH1 again did not show prognostic relevance [Javle et al. 2016].
Clinically relevant genomic alterations
When ‘clinically relevant’ genomic alterations are defined as alterations for which targetable treatment or registered clinical trials are available, up to 83% of BTC feature clinically relevant and potentially actionable alterations [Simbolo et al. 2014; Lee et al. 2016; Ross et al. 2014].
-
(1) FGFR2 fusions
Fibroblast growth factor receptor (FGFR 1–4) is a transmembrane receptor tyrosine kinase family that regulates cell proliferation, migration, differentiation and angiogenesis via binding to the ligands (fibroblast growth factors, FGFs) and affecting subsequent signaling responses through multiple pathways [Theelen et al. 2016]. Variable FGFR2 fusion gene products paired with -BICC1, -KIAA1598, -TACC3, -PARK2, -AHCYL1, -MGEA5, -KCTD1, -PPHLN1, -CCDC6 and -TXLNA have been identified in ICC [Banales et al. 2016; Jain et al. 2016].The FGFR2 fusion is diagnostically useful with its exclusive association with ICC [Arai et al. 2014]. In addition, ICCs with FGFR2 fusion were associated with female predilection, younger age at onset, improved survival and relatively indolent disease course [Javle et al. 2016; Graham et al. 2014]. This fusion is also of therapeutic significance given that nonselective FGFR inhibitors such as brivanib, nintedanib, lenvatinib, pazopanib, regorafenib, dovitinib, lucitanib and ponatinib are on the market as well as under consideration in mechanism-driven clinical trials for expansion of original approvals [Ang, 2015; Abou-Alfa et al. 2016]. Also, selective novel FGFR-targeted tyrosine kinase inhibitors such as BGJ398, AZD4547, and JNJ42756493 are under investigation. Especially, BGJ398 is being evaluated in a phase II clinical trial, with promising response in patients with FGFR2-altered advanced or metastatic ICC [Jain et al. 2016]. A study using mouse xenograft model with FGFR2-CCDC6 fusion protein showed that BGJ398 may outperform nonselective FGFR inhibitors ponatinib and dovitinib [Wang et al. 2016]. -
(2) IDH1/2 mutations
IDH1 and IDH2 encode enzymes involved in conversion of isocitrate to alpha-ketoglutarate (α-KG) while reducing NADP to NADPH. IDH1/2 mutations lead to production of oncometabolite D-2-hydroxyglutarate (D-2HG) instead of α-KG. This metabolite inhibits α-KG-dependent dioxygenases and contributes to epigenetic alterations such as hypermethylation of histone and DNA, and inhibits cell differentiation. Moreover, D-2HG may be used as surrogate biomarker to predict IDH mutations in cancer [Mondesir et al. 2016].Mutations in IDH1/2 have been identified in gliomas, hematologic malignancies, chondrosarcoma, and 30–40% of ICC [Jain and Javle, 2016; Jain et al. 2016; Javle et al. 2016; Ross et al. 2014; Chong and Zhu, 2016]. Currently, selective IDH1/2 inhibitors, AG-120 and AG-221 are in phase I clinical trials using IDH1/2 genomic alterations as a trial entry requirement for ICC patients. For example, a clinical trial is recruiting patients with IDH1-mutated ICC to evaluate firstly, the safety and tolerability, and secondly, the efficacy of AG-120, which is showing encouraging preliminary results [ClinicalTrials.gov identifier: NCT02073994]. One of twenty patients with ICC showed partial response and eleven of them showed stable disease [Mondesir et al. 2016; Chong and Zhu, 2016].Alternatively, therapeutic options targeting hypermethylation induced by downstream product D-2HG are also of interest. In an IDH1-mutant glioma-xenograft mouse model study, tumor regression was observed only in the xenograft group that was treated with hypomethylating agent, 5-azacytidine. There was no tumor regression in the group without the treatment [Borodovsky et al. 2013]. Furthermore, IDH1 mutation may be a potential target for immunotherapy given its ubiquitous expression and potent antigenicity [Schumacher et al. 2014]. The prognostic significance of IDH1/2 mutations in ICC has not been established [Wang et al. 2013; Javle et al. 2016]. -
(3) ERBB2(HER2) mutation
Anti-HER2-targeted therapies have been the mainstay of treatment for breast cancer since 1998. Routine HER2 overexpression and amplification testing achieved standard of care for management of both early- and late-stage breast cancer. In addition, anti-HER2-targeted therapy has successfully been adopted for gastric and gastroesophageal cancer management [Bang et al. 2010]. ERBB2 alterations, either gene amplifications or sequence mutations, are seen in approximately 10–11% of ECC and GBCA, while they are rare in ICC [Jain and Javle, 2016; Jain et al. 2016; Javle et al. 2016; Chong and Zhu, 2016]. In a study of 187 GBCA, 13% of the GBCAs demonstrated HER2 overexpression by immunohistochemistry when commonly accepted scoring criteria were used, and these patients showed overall worse survival [Roa et al. 2014].Although slightly less frequent than seen in breast and upper gastrointestinal cancers, ERBB2 gene amplifications may emerge as an attractive target in BTC, especially GBCA. However, the efficacy of anti-HER2 targeted therapies in BTC currently remains unclear [Lee et al. 2016; Jain et al. 2016]. Javle and colleagues retrospectively reviewed the clinical responses of anti-HER2 antibody (trastuzumab)-directed therapy in 9 GBCA (8 with ERBB2 amplification or overexpression) and 5 ICC (3 with ERBB2 amplification) patients. Although tumors with ERBB2 gene amplification showed promising responses (Figure 5), those with ERBB2 sequence mutations showed mixed responses or no radiological responses [Javle et al. 2015]. Therefore, while anti-HER2-directed therapy is promising in BTC with ERBB2 amplification, further investigation of small molecule anti-ERBB2 tyrosine kinase inhibitors such as lapatinib, neratinib and canertinib may be worthwhile for BTC with ERBB2 mutations in the kinase domain [Lee et al. 2016; Bose et al. 2013; Jain et al. 2016]. -
(4) EGFR (HER1) and its signaling pathway
Alterations in EGFR and its downstream signaling pathways including KRAS and PIK3CA, have been a subject of extensive research. EGFR overexpression and amplification is seen in 20–30% of BTC, and its overexpression may portend a poor prognosis in ICC [Yoshikawa et al. 2008; Yang et al. 2014].In a multicenter randomized phase III study of BTC, no significant difference in progression-free survival (PFS) was noted between the group with and without the addition of the anti-EGFR tyrosine kinase inhibitor erlotinib to gemcitabine and oxaliplatin. However, the addition of erlotinib showed an increased rate of objective tumor response in this group [Lee et al. 2012]. Subsequent molecular subgroup analysis of the same cohort revealed that tumors with wild-type KRAS showed improved response rate toward the addition of erlotinib compared with tumors harboring a KRAS mutation. Tumors with wild-type PIK3CA also showed a favorable trend for overall response [Kim et al. 2015]. These studies indicate that similar to other types of cancer, response to anti-EGFR therapy is associated with a complex interplay between various genomic alterations that await further investigation. -
(5) VEGF
Overexpression of VEGF was reported in 55–60% of BTC [Chong and Zhu, 2016]. Several clinical trials are underway to evaluate the efficacy of variable VEGF inhibitors such as bevacizumab, sorafenib, sunitinib and cediranib in combination with conventional gemcitabine-based regimens on BTC but have not, as yet, demonstrated benefit in overall survival [Chong and Zhu, 2016; Lee et al. 2013]. Notably, Valle and colleagues showed that the addition of cediranib in combination with conventional regimen improved overall survival when the baseline PDGFbb concentration level was high [Valle et al. 2015]. PDGFbb has been shown to induce VEGF secretion in ovarian cancer [Matei et al. 2007]. -
(6) MET
Alteration of the proto-oncogene MET promotes survival of neoplastic cells by increasing cell motility and angiogenesis in variable solid tumors. MET overexpression is known to confer a poor prognosis, and may contribute to resistance to anti-EGFR treatment [Chong and Zhu, 2016]. However, when MET expression detected by immunohistochemistry was used as a requirement for clinical trial entry in non-small cell lung cancer, anti-MET targeted therapies did not achieve their endpoints and regulatory approval for these agents was not granted [Scagliotti et al. 2015; Spigel et al. 2013]. MET amplification was detected in 2–7% of ICC using CGP (Figure 6) [Ross et al. 2014; Churi et al. 2014; Javle et al. 2016]. Also, a patient with MET amplification confirmed by NGS experienced a metabolic response with a MET inhibitor [Churi et al. 2014]. In a xenograft mouse model study, LY2801653, a small-molecule inhibitor with potent activity against MET kinase, led to suppression of the proliferation of cholangiocarcinoma cell line [Barat et al. 2016]. -
(7) RAS/RAF/MEK/ERK pathway
Aberrant signaling of RAS/RAF/MEK/ERK pathway is frequent in BTC [O’Neill and Kolch, 2004], and alteration of this pathway including KRAS mutation may confer a poor prognosis [Javle et al. 2016; Chong and Zhu, 2016]. A phase II study of selumetinib, an MEK inhibitor, in metastatic BTC showed that 3 (12%) of 28 patients demonstrated objective response and 17 (68%) had stable disease [Bekaii-Saab et al. 2011]. A recent phase Ib study of selumetinib in combination with gemcitabine and cisplatin in advanced or metastatic BTC showed manageable toxicities [Bridgewater et al. 2016]. Also, patients with BRAF V600E-mutated BTC showed partial response to treatment with BRAF-inhibitor [Churi et al. 2014]. Moreover, a patient with BRAF-mutated ICC showed a dramatic response to dual inhibition of BRAF (dabrafenib) and MEK (trametinib) [Loaiza-Bonilla et al. 2014]. Although widely anticipated, the development of an effective direct KRAS inhibitor for diseases such as BTC is yet to be achieved. -
(8) PI3K/AKT/mTOR pathway
A study with 39 patients with advanced, metastatic or recurrent BTC that progressed despite chemotherapy (mostly combination of gemcitabine and oxaliplatin) were enrolled in a phase II trial of mTOR inhibitor everolimus. One patient showed partial response and one showed complete response that sustained up to 8 months, with a favorable toxicity profile [Buzzoni et al. 2014].A recent experiment using human cholangiocarcinoma cell line demonstrated that targeting AKT by MK2206, an AKT inhibitor, resulted in suppression of cellular growth [Wilson et al. 2015]. In another cell-line study, combined targeting of AKT and mTOR by MK2206 and RAD001 enhanced the efficacy of BTC treatment [Ewald et al. 2013].The oral PI3K inhibitor BKM120 in combination with mFOLFOX6 (5-fluorouracil/leucovorin + oxaliplatin) was administered to 17 patients with advanced solid tumors, including four patients with BTC. Although one patient with ICC showed stable disease and remained on treatment for 26 weeks, further study was not recommended due to intolerable toxicity [McRee et al. 2015].
Figure 5.
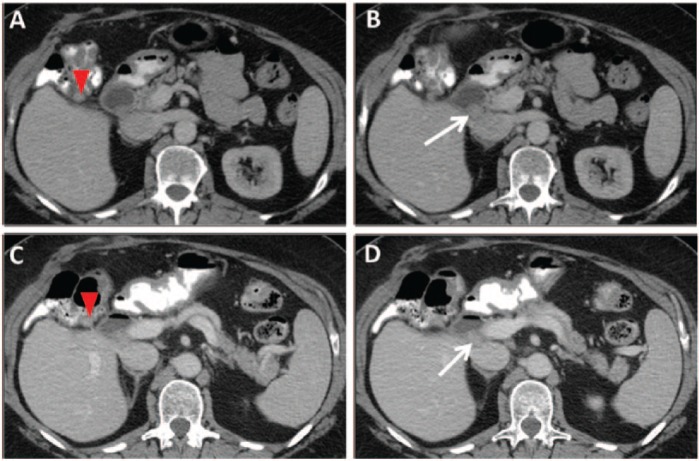
ERBB2 amplified gallbladder carcinoma responds to combination therapy of trastuzumab with chemotherapy. A 64-year-old female with recurrent gallbladder carcinoma. Axial contrast-enhanced computed tomography images demonstrate (A) a 1.2 cm nodule in the gallbladder fossa adjacent to the hepatic flexure, and (B) a 1.6 cm nodule in the portocaval region. Both nodules were new from the postoperative scan (following resection of recurrent tumor in the gallbladder fossa), in keeping with recurrence. In (C) and (D), 8 months later, both nodules are stable. (Case provided by Dr Milind Javle, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.)
Figure 6.
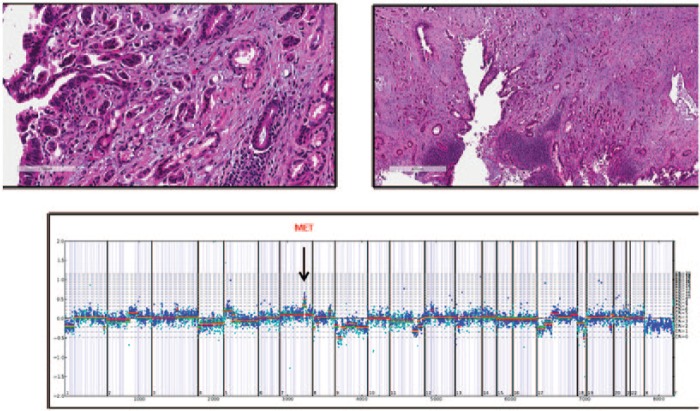
MET amplification of grade 3 Stage III gallbladder carcinoma in a female patient. MET amplification was detected using the comparative genomic hybridization plot (shown) generated by the Illumina Hi-Seq system and the FMI copy number algorithm (arrow: MET amplification spike at chromosome 7) (Case provided by Foundation Medicine, Inc. Cambridge, MA, USA).
Mutational burden and immunotherapy
Some BTCs are enriched with significantly high mutational load. Transcriptome sequencing and hierarchical clustering of gene expression levels classified BTC into four subgroups with prognostic implication. Notably, the tumors in the worst prognosis group (39%; 74 of 188 tumors) showed high mutational burden, with increased expression of immune-checkpoint molecules and enrichment for the genes involved in cytokine activity and antiapoptosis [Nakamura et al. 2015]. Recent studies have confirmed that patients with hypermutated tumors have greater chance of benefitting from immune-checkpoint inhibitors (ICPI) in non-small cell lung cancer, melanoma, and bladder cancer [Reck et al. 2016]. Therefore, the BTC patients whose tumors demonstrate a high mutational burden may be candidates for ICPI immunotherapy.
While mismatch repair protein (MMR)-deficient (microsatellite instability [MSI] high) tumors with their uniformly high mutational burden are also considered to be good targets for ICPI treatment, <10% of BTC was reported to be MMR deficient by immunohistochemistry [Goyal et al. 2014]. A study of PD-1 blockade in tumors with MMR deficiency enrolled one patient with MMR-deficient BTC. This patient showed partial response with 93% of biochemical response [Le et al. 2015]. Currently ongoing phase II study of pembrolizumab (ML-3495; anti PD-1 antibody) in noncolorectal MMR-deficient tumors includes three patients with BTC. Pembrolizumab appears to be well tolerated in patients with advanced BTC [Chong and Zhu, 2016].
In addition, recent studies in colorectal cancer suggest that tumor mutation burden (TMB), the algorithm-based calculation of nongermline mutations per megabase of sequenced DNA, will outperform MMR status as a biomarker for predicting response to checkpoint-inhibitor-based therapies, and tumors with high TMB may be a potential cohort for immunotherapy [George et al. 2016]. When subjected to a hybrid capture-based CGP assay, the prevalence of high TMB, defined by a >20 mutations per megabase TMB score in BTC, was quite low at 2% (unpublished data, Table 2).
Table 2.
Tumor mutation burden of biliary tract cancer. (Data provided by Foundation Medicine, Inc., Cambridge, MA).
| Tumor type | Case number | % > 10 mut/Mb | % > 20 mut/Mb |
|---|---|---|---|
| ICC | 1018 | 3.4% | 1.7% |
| ECC | 176 | 5.7% | 1.7% |
| Gallbladder carcinoma | 344 | 5.5% | 1.2% |
mut, mutations; Mb, megabase; ICC, intrahepatic cholangiocarcinoma; ECC, extrahepatic cholangiocarcinoma.
Genomic alteration and resistance to chemotherapy
Genomic alterations in BTC may serve as biomarkers in predicting response to chemotherapy. Current standard regimen for advanced or recurrent BTC constitutes a combination of gemcitabine and platinum, with a variable and generally overall poor response. The mechanism of chemoresistance in BTC and relevant genes can be divided into five categories; (1) by reduction of the amount of intracellular drug: SLCO2A1, SLC22A3, SLC29A1, SLC28 A1, SLC31 A1, ABCB1, ABCC1, ABCC3, ABCC4, ABCC5, (2) by decreased activation of prodrug or inactivation of active agents: TYMP, UPP1, UMPS, GSTP1, (3) by changing molecular targets: TYMS, ESR1, ESR2, EGFR, IGF1R, (4) by interfering drug-induced DNA lesions: ERCC1, RAD51, MSH2, MSH3, MSH6, MLH1, PMS2, RRM2B, TLK1, and (5) by downregulation of apoptosis: NK4, MET, TNFSF10, FAS, TP53, BCL2, XIAP, BIRC5, AKT1 [Banales et al. 2016].
Ahn and colleagues profiled 183 BTC using NGS. Nine common somatic mutations were selected, and their association with overall survival was studied. While mutations in CDKN2A, TP53 and ARID1A were mostly mutually exclusive, dual loss of function mutations of these were associated with PFS and overall survival in patients treated with gemcitabine and platinum-based therapy. For example, patients with dual mutations in CDKN2A and TP53 with wild-type ARID1A showed shorter PFS compared with those who were wild type for all three. On the other hand, ARID1A mutation slightly improved PFS in patients with TP53 and/or CDKN2A mutation. A single patient with all three mutations demonstrated greatly improved PFS. KRAS mutation did not show prognostic relevance [Ahn et al. 2016].
Summary
BTC is a relatively rare but aggressive form of cancer which typically presents at an advanced clinical stage and is refractory to standard chemotherapy regimens. However, recent advancement of CGP revealed that BTC is enriched with multiple targetable genomic alterations and that the three types of BTC, ICC, ECC and GBCA, differ greatly in their molecular signatures. While its rarity makes it challenging to design robust clinical trials, BTC may be an ideal type of tumor to apply and test targeted therapy and precision medicine given its diverse genomic landscape.
Limitations of CGP and its application are firstly, many genomic alterations are not targetable; secondly, identified clinical trials may not be locally available and thirdly, CGP has not, to date, been proven to predict responses to chemo- or radiation therapy and has not been used to predict combination therapy benefits. Lastly, long-term clinical-outcome data are required to show true impact of targeted therapy.
Incorporation of genomic profiling in clinical practice and multidisciplinary approach to this intriguing tumor will enhance our knowledge about it and lead to the accumulation of long-term outcome data.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: HL declares that there is no conflict of interest. JSR has employment, stock ownership, a leadership position and research support from Foundation Medicine.
Contributor Information
Hwajeong Lee, Department of Pathology and Laboratory Medicine, Albany Medical College, 47 New Scotland Ave, MC81, Albany, NY 12208, USA.
Jeffrey S. Ross, Department of Pathology and Laboratory Medicine, Albany Medical College, Albany, NY, USA Foundation Medicine, Cambridge, MA, USA
References
- Abou-Alfa G., Anderson J., Chapman W., Choti M., Forbes S., Gores G., et al. (2016) Advances in cholangiocarcinoma research: report from the third Cholangiocarcinoma Foundation Annual Conference. J Gastrointest Oncol 7: 819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn D., Javle M., Ahn C., Jain A., Mikhai S., Noonan A., et al. (2016) Next-generation sequencing survey of biliary tract cancer reveals the association between tumor somatic variants and chemotherapy resistance. Cancer 122: 3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shamsi H., Anand D., Shroff R., Jain A., Zuo M., Conrad C., et al. (2016) BRCA-associated protein 1 mutant cholangiocarcinoma: an aggressive disease subtype. J Gastrointest Oncol 7: 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andia M., Hsing A., Andreotti G., Ferreccio C. (2008) Geographic variation of gallbladder cancer mortality and risk factors in Chile: a population-based ecologic study. Int J Cancer 123: 1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang C. (2015) Role of the fibroblast growth factor receptor axis in cholangiocarcinoma. J Gastroenterol Hepatol 30: 1116–1122. [DOI] [PubMed] [Google Scholar]
- Arai Y., Totoki Y., Hosoda F., Shirota T., Hama N., Nakamura H., et al. (2014) Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 59: 1427–1434. [DOI] [PubMed] [Google Scholar]
- Augustine M., Fong Y. (2014) Epidemiology and risk factors of biliary tract and primary liver tumors. Sur Oncol Clin N Am 23: 171–188. [DOI] [PubMed] [Google Scholar]
- Banales J., Cardinale V., Carpino G., Marzioni M., Andersen J., Invernizzi P., et al. (2016) Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 13: 261–280. [DOI] [PubMed] [Google Scholar]
- Bang Y., Van Cutsem E., Feyereislova A., Chung H., Shen L., Sawaki A., et al. (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687–697. [DOI] [PubMed] [Google Scholar]
- Barat S., Bozko P., Chen X., Scholta T., Hanert F., Götze J., et al. (2016) Targeting c-MET by LY2801653 for treatment of cholangiocarcinoma. Mol Carcinog 55: 2037–2050. [DOI] [PubMed] [Google Scholar]
- Bekaii-Saab T., Phelps M., Li X., Saji M., Goff L., Kauh J., et al. (2011) Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 29: 2357–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borger D., Tanabe K., Fan K., Lopez H., Fantin V., Straley K., et al. (2012) Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 17: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodovsky A., Salmasi V., Turcan S., Fabius A., Baia G., Eberhart C., et al. (2013) 5-azacytidine reduces methylation, promotes differentiation and induces tumor regression in a patient-derived IDH1 mutant glioma xenograft. Oncotarget 4: 1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose R., Kavuri S., Searleman A., Shen W., Shen D., Koboldt D., et al. (2013) Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 3: 224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater J., Lopes A., Beare S., Duggan M., Lee D., Ricamara M., et al. (2016) A phase 1b study of selumetinib in combination with cisplatin and gemcitabine in advanced or metastatic biliary tract cancer: the ABC-04 study. BMC Cancer 16: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzoni R., Pusceddu S., Bajetta E., De Braud F., Platania M., Iannacone C., et al. (2014) Activity and safety of RAD001 (everolimus) in patients affected by biliary tract cancer progressing after prior chemotherapy: a phase II ITMO study. Ann Oncol 25: 1597–1603. [DOI] [PubMed] [Google Scholar]
- Chan-On W., Nairismägi M., Ong C., Lim W., Dima S., Pairojkul C., et al. (2013) Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet 45: 1474–1478. [DOI] [PubMed] [Google Scholar]
- Chong D., Zhu A. (2016) The landscape of targeted therapies for cholangiocarcinoma: current status and emerging targets. Oncotarget 7: 46750–46767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churi C., Shroff R., Wang Y., Rashid A., Kang H., Weatherly J., et al. (2014) Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 9: e115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald F., Grabinski N., Grottke A., Windhorst S., Nörz D., Carstensen L., et al. (2013) Combined targeting of AKT and mTOR using MK-2206 and RAD001 is synergistic in the treatment of cholangiocarcinoma. Int J Cancer 133: 2065–2076. [DOI] [PubMed] [Google Scholar]
- George T., Frampton G., Sun J., Gowen K., Kennedy M., Greenbowe J., et al. (2016) Tumor mutational burden as a potential biomarker for PD-1/PD-L1 therapy in colorectal cancer. J Clin Oncol 34: abstract; 3587. [Google Scholar]
- Global Burden of Disease Cancer Collaboration (2015) The Global Burden of Cancer 2013. JAMA Oncol 1: 505–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal L., Deshpande V., Chung D., Groeschl R., Gamblin T., Zhu A. (2014) Mismatch repair protein loss and microsatellite instability in cholangiocarcinoma. J Clin Oncol 32: abstract 237. [Google Scholar]
- Graham R., Barr Fritcher E., Pestova E., Schulz J., Sitailo L., Vasmatzis G., et al. (2014) Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol 45: 1630–1638. [DOI] [PubMed] [Google Scholar]
- Jain A., Javle M. (2016) Molecular profiling of biliary tract cancer: a target rich disease. J Gastrointest Oncol 7: 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Kwong L., Javle M. (2016) Genomic profiling of biliary tract cancers and implications for clinical practice. Curr Treat Options in Oncol 17: 58. [DOI] [PubMed] [Google Scholar]
- Jang S., Chun S., Hong S., Sung C., Park H., Kang H., et al. (2014) High throughput molecular profiling reveals differential mutation patterns in intrahepatic cholangiocarcinomas arising in chronic advanced liver diseases. Mod Pathol 27: 731–739. [DOI] [PubMed] [Google Scholar]
- Javle M., Bekaii-Saab T., Jain A., Wang Y., Kelley R., Wang K., et al. (2016) Biliary cancer: utility of next-generation sequencing for clinical management. Cancer 122: 3838–3847. [DOI] [PubMed] [Google Scholar]
- Javle M., Churi C., Kang H., Shroff R., Janku F., Surapaneni R., et al. (2015) HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol 8: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Pawlik T., Anders R., Selaru F., Streppel M., Lucas D., et al. (2013) Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 45: 1470–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Toledano M., Taylor-Robinson S. (2008) Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 10: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Jang K., Lee J., Jang H., Choi H., Jang H., et al. (2015) Molecular subgroup analysis of clinical outcomes in a phase 3 study of gemcitabine and oxaliplatin with or without erlotinib in advanced biliary tract cancer. Transl Oncol 8: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongpetch S., Jusakul A., Ong C., Lim W., Rozen S., Tan P., et al. (2015) Pathogenesis of cholangiocarcinoma: from genetics to signalling pathways. Best Pract Res Clin Gastroenterol 29: 233–244. [DOI] [PubMed] [Google Scholar]
- Le D., Uram J., Wang H., Bartlett B., Kemberling H., Eyring A., et al. (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Wang K., Johnson A., Jones D., Ali S., Elvin J., et al. (2016) Comprehensive genomic profiling of extrahepatic cholangiocarcinoma reveals a long tail of therapeutic targets. J Clin Pathol 69: 403–408. [DOI] [PubMed] [Google Scholar]
- Lee J., Capanu M., O’Reilly E., Ma J., Chou J., Shia J., et al. (2013) A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br J Cancer 109: 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Park S., Chang H., Kim J., Choi H., Lee M., et al. (2012) Gemcitabine and oxaliplatin with or within erlotinib in advanced biliary-tract cancer: a multicenter, open-label, randomised, phase 3 study. Lancet Oncol 13: 181–188. [DOI] [PubMed] [Google Scholar]
- Li M., Zhang Z., Li X., Ye J., Wu X., Tan Z., et al. (2014) Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet 46: 872–876. [DOI] [PubMed] [Google Scholar]
- Loaiza-Bonilla A., Clayton E., Furth E., O’Hara M., Morrissette J. (2014) Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience 8: 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H., Gores G. (2006) Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol 45: 856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei D., Kelich S., Cao L., Menning N., Emerson R., Rao J., et al. (2007) PDGF BB induces VEGF secretion in ovarian cancer. Cancer Biol Ther 6: 1951–1959. [DOI] [PubMed] [Google Scholar]
- McRee A., Sanoff H., Carlson C., Ivanova A., O’Neil B. (2015) A phase I trial of mFOLFOX6 combined with the oral PI3K inhibitor BKM120 in patients with advanced refractory solid tumors. Invest New Drugs 33: 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesir J., Willekens C., Touat M., de Botton S. (2016) IDH1 and IDH2 mutations as novel therapeutic targets: current perspectives. J Blood Med 7: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Arai Y., Totoki Y., Shirota T., Elzawahry A., Kato M., et al. (2015) Genomic spectra of biliary tract cancer. Nat Genet 47: 1003–1010. [DOI] [PubMed] [Google Scholar]
- Nakanuma Y., Curado M., Franceschi S., et al. (2010) Intrahepatic cholangiocarcinoma. In: Bosman F., Carneiro F., Hruban R., Theise N.(eds) WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press, pp.217–224. [Google Scholar]
- Nathan H., Pawlik T., Wolfgang C., Choti M., Cameron J., Schulick R. (2007) Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg 11: 1488–1496. [DOI] [PubMed] [Google Scholar]
- O’Neill E., Kolch W. (2004) Conferring specificity on the ubiquitous Raf/MEK signaling pathway. Br J Cancer 90: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C., Subimerb C., Pairojkul C., Wongkham S., Cutcutache I., Yu W., et al. (2012) Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet 44: 690–693. [DOI] [PubMed] [Google Scholar]
- Poomphakwaen K., Promthet S., Kamsa-Ard S., Vatanasapt P., Chaveepojnkamjorn W., Klaewkla J., et al. (2009) Risk factors for cholangiocarcinoma in Khon Kaen, Thailand: a nested case-control study. Asian Pac J Cancer Prev 10: 251–258. [PubMed] [Google Scholar]
- Reck M., Rodríguez-Abreu D., Robinson A., Hui R., Csőszi T., Fülöp A., et al. (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- Roa I., de Toro G., Schalper K., de Aretxabala X., Churi C., Javle M., et al. (2014) Overexpression of the HER2/neu gene: a new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointest Cancer Res 7: 42–48. [PMC free article] [PubMed] [Google Scholar]
- Rosen C., Heimbach J., Gores G. (2010) Liver transplantation for cholangiocarcinoma. Transpl Int 23: 692–697. [DOI] [PubMed] [Google Scholar]
- Ross J., Wang K., Gay L., Al-Rohil R., Rand J., Jones D., et al. (2014) New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 19: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scagliotti G., von Pawel J., Novello S., Ramlau R., Favaretto A., Barlesi F., et al. (2015) Phase III multinational, randomized, double-blind, placebo-controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 33: 2667–2674. [DOI] [PubMed] [Google Scholar]
- Schumacher T., Bunse L., Pusch S., Sahm F., Wiestler B., Quandt J., et al. (2014) A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 512: 324–327. [DOI] [PubMed] [Google Scholar]
- Shin H., Oh J., Masuyer E., Curado M., Bouvard V., Fang Y., et al. (2010) Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci 101: 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbolo M., Fassan M., Ruzzenente A., Mafficini A., Wood L., Corbo V., et al. (2014) Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget 5: 2839–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipworth J., Olde Damink S., Imber C., Bridgewater J., Pereira S., Malagó M., et al. (2011) Review article: surgical, neo-adjuvant and adjuvant management strategies in biliary tract cancer. Aliment Pharmacol Ther 34: 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigel D., Ervin T., Ramlau R., Daniel D., Goldschmidt J., Jr., Blumenschein G., Jr. (2013) Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 31: 4105–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theelen W., Mittempergher L., Willems S., Bosma A., Peters D., van der Noort V., et al. (2016) FGFR1, 2 and 3 protein overexpression and molecular aberrations of FGFR3 in early stage non-small cell lung cancer. J Pathol Clin Res 2: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle J., Wasan H., Lopes A., Backen A., Palmer D., Morris K., et al. (2015) Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary cancer (ABC-03): a randomized phase 2 trial. Lancet Oncol 16: 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle J., Wasan H., Palmer D., Cunningham D., Anthoney A., Maraveyas A., et al. (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362: 1273–1281. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ding X., Wang S., Moser C., Shaleh H., Mohamed E., et al. (2016) Antitumor effect of FGFR inhibitors on a novel cholangiocarcinoma patient derived xenograft mouse model endogenously expressing an FGFR2-CCDC6 fusion protein. Cancer Lett 380: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Dong Q., Zhang C., Kuan P., Liu Y., Jeck W., et al. (2013) Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene 32: 3091–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzel T., McGlynn K., Hsing A., O’Brien T., Pfeiffer R. (2006) Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 98: 873–875. [DOI] [PubMed] [Google Scholar]
- Wilson J., Kunnimalaiyaan S., Kunnimalaiyaan M., Gamblin T. (2015) Inhibition of the AKT pathway in cholangiocarcinoma by MK2206 reduces cellular viability via induction of apoptosis. Cancer Cell Int 15: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Wang B., Chen Y., Li H., Hu J., Zou S. (2013) Efficacy of gemcitabine plus platinum agents for biliary tract cancers: a meta-analysis. Anticancer Drugs 24: 871–877. [DOI] [PubMed] [Google Scholar]
- Yang X., Wang W., Wang C., Wang L., Yang M., Qi M., et al. (2014) Characterization of EGFR family gene aberrations in cholangiocarcinoma. Oncol Rep 32: 700–708. [DOI] [PubMed] [Google Scholar]
- Yoshikawa D., Ojima H., Iwasaki M., Hiraoka N., Kosuge T., Kasai S., et al. (2008) Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 98: 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhao Y., Li B., Huang J., Wu L., Xu D., et al. (2012) Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer 12: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S., Li J., Zhou H., Frech C., Jiang X., Chu J., et al. (2014) Mutational landscape of intrahepatic cholangiocarcinoma. Nature Commun 5: 5696. [DOI] [PubMed] [Google Scholar]