Abstract
Background:
Percutaneous transhepatic biliary drainage (PTBD) is widely performed as a salvage procedure in patients with unresectable malignant obstruction of the common bile duct (CBD) after failed endoscopic retrograde cholangiopancreatography (ERCP) or in case of surgically altered anatomy. Endoscopic ultrasound-guided hepaticogastrostomy (EU-HGS) is a more recently introduced alternative to relieve malignant obstructive jaundice. The aim of this prospective observational study was to compare the outcome, efficacy and adverse events of EU-HGS and PTBD.
Methods:
From April 2012 to August 2015, consecutive patients with malignant CBD obstruction who underwent EU-HGS or PTBD in two tertiary-care referral centers were included. The primary endpoint was the clinical success rate. Secondary endpoints were technical success, overall survival, procedure-related adverse events, incidence of adverse events, and reintervention rate.
Results:
A total of 51 patients (EU-HGS, n = 31; PTBD, n = 20) were included. Median survival was 71 days (range 25–75th percentile; 30–95) for the EU-HGS group and 78 days (range 25–75th percentile; 42–108) for the PTBD group (p = 0.99). Technical success was achieved in all patients in both groups. Clinical success was achieved in 25 (86%) of 31 patients in the EU-HGS group and in 15 (83%) of 20 patients in the PTBD group (p = 0.88). There was no difference in adverse events rates between the two groups (EU-HGS: 16%; PTBD: 10%) (p = 0.69). Four deaths within 1 month (two hemorrhagic and two septic) were considered procedure related (two in the EU-HGS group and two in the PTBD group). Overall reintervention rate was significantly lower after EU-HGS (n = 2) than after PTBD (n = 21) (p = 0.0001). Length of hospital stay was shorter after EU-HGS (8 days versus 15 days; p = 0.002).
Conclusions:
EU-HGS can be an effective and safe mini invasive-procedure alternative to PTBD, with similar success and adverse-event rates, but with lower rates of reintervention and length of hospitalization.
Keywords: EUS, PTBD, ERCP, pancreatic cancer, jaundice
Introduction
The instrumental treatment of jaundice secondary to a malignancy obstructing the common bile duct (CBD) generally involves stenting during endoscopic retrograde cholangiopancreatography (ERCP).1–3 However, 3–5% of cases cannot be managed by ERCP due to various impediments, such as ampullary infiltration, modified anatomy (gastrectomy with Roux-en-Y anastomosis, Whipple’s resection, etc.), duodenal obstruction or to the tightness of luminal occlusion.4,5 In such cases, percutaneous transhepatic biliary drainage (PTBD) is most commonly performed. However, PTBD is associated with complications such as bleeding, cholangitis, and pneumothorax.6,7 The common practice of temporary external drainage also has an adverse impact on the quality of life (QOL) of patients who are already treated for an incurable disease. Recently, interventions using endoscopic ultrasound (EUS) have been developed, not only to obtain histopathologic proof of malignancy, but also to achieve biliary drainage. EUS-guided biliary drainage (EU-BD), first reported by Giovannini et al.,8 broadly includes three different access routes, namely EUS-guided transpapillary rendez-vous,9,10 EUS-guided choledochoduodenostomy11 and EUS-guided hepaticogastrostomy [from hereinafter abbreviated as endoscopic ultrasound-guided hepaticogastrostomy (EU-HGS) in this paper].12 Because of guidewire manipulation difficulties, the rendez-vous manoeuver was reported to have a low success rate ranging from 40% to 80%,13–15 with prolonged procedure time and fluoroscopic exposure.13,16–18 EUS-guided choledochoduodenostomy is also indicated in case of failed ERCP, however, this technique cannot always be performed in case of surgically altered anatomy or duodenal bulb obstruction. Transduodenal and transhepatogastric access routes are reported to have similar feasibility, but EU-HGS has currently the broadest range of indications among EU-BD procedures, with a good feasibility in a recent prospective study.19 To date, many case reports, small series, retrospective and prospective comparisons between the different EU-BD methods have been reported.20 Despite this literature, no study has specifically compared EU-HGS and PTBD in case of failed ERCP for malignant obstructive jaundice.
Since both EU-BD and PTDB are technically available alternatives in case of failed ERCP, it is important to compare their outcomes in order to select the best option for those patients. Until now, only four small studies21–24 have compared EU-BD and PTBD but studied mostly the transduodenal route.
We thus conducted a retrospective analysis from two prospectively maintained databases to compare EU-HGS and PTDB after failed ERCP for malignant obstructive jaundice.
Patients and methods
Patients
All therapeutic EU-BD procedures were performed in Cochin Hospital (Paris, France), whereas all PTBD procedures were performed in the University hospital of Strasbourg. All procedures and data collection were done after obtaining the patients’ informed consent. Information on patients were retrieved from two similarly constructed databases prospectively implemented in each center.
We included all consecutive patients presenting with malignant obstructive jaundice not exceeding Bismuth stage I (i.e. involving only the distal bile duct or the upper part of the common hepatic duct with no involvement of the main confluence), who underwent EU-HGS or PTBD with stent placement after one (or several) failed ERCP, as well as patients with previous surgery precluding ERCP. All patients had histologically proven malignancy deemed unresectable after radiologic assessment (computerized tomography, EUS, or magnetic resonance imaging) and multidisciplinary staff agreement. Exclusion criteria were resectable, borderline or potentially resectable tumors, hilar obstruction involving the main confluence, large-volume ascites, and blood coagulation disorders. We obtained specific informed consent from each patient in both groups. The study received IRB approval in both centers.
Study endpoints
Data: Patient history, biochemical data at the time of intervention and during follow up, the radiological database, procedural records, and pathological and clinical follow up were reviewed. A patient’s demographics, histological diagnosis, WHO status, procedure details, characteristics and reasons for failed ERCP, clinical success, technical success, adverse events, inpatient length of stay, number of reinterventions during follow up and survival were recorded. Follow up was continued until death or date of transfer to a palliative care center. QOL was not analyzed due to insufficient data.
Outcomes were defined as follows: Clinical success was defined as a drop in the plasma bilirubin level of >50% at 1 week. Technical success was defined as a successful hepatic bile duct puncture with guidewire insertion and stent or drain placement in the desired location as determined endoscopically and/or radiographically. Procedural mortality was defined as death occurring within 7 days of the procedure. Reinterventions were split between scheduled reinterventions (for tube upsizing/exchange) and unscheduled reinterventions (for adverse-event management).
Endpoints: The primary endpoint of the study was the clinical success rate. Secondary endpoints were technical success of the intervention, overall survival, procedure-related adverse events within 30 days, number of reinterventions until death and length of hospital stay (from the date of procedure to discharge).
Procedures
All procedures were performed with monitored anesthesia and general anesthesia. Prophylactic antibiotics (generally second- or third-generation cephalosporin) were administered perioperatively in all patients.
Endoscopic ultrasound-guided hepaticogastrostomy
By using a curved linear array echoendoscope (Olympus GF-UCT 140–180, Tokyo, Japan), access to the left hepatic ducts was identified from the proximal gastric body/cardia and punctured with a 19-gauge needle (EUSN-19A, Cook Endoscopy, Winston-Salem, North Carolina, USA). Color Doppler was used to detect any intervening vessel. After suction sampling for bile bacteriology analysis, contrast was injected to obtain a cholangiogram and a 0.035-inch guidewire (Jagwire, Boston Scientific, Natick, Ma, USA) was advanced into the intrahepatic biliary ducts. The needle shaft was exchanged over the wire for a 6-French (6 Fr) cystostome (Endo-Flex®, GmbH, Voerde Düsseldorf, Germany) and the needle tract was coagulated using Endocut I–Effet1/2-2 W (Erbe VIO 200 D, GmbH, Tübingen, Germany) before inserting a fully covered self-expandable metal stent in the left hepatic duct (Wallflex RX Permalume®, Boston Scientific, Natick, Ma, USA). Usually, at least 2 cm from the proximal stent end were left in the stomach (Figure 1). When the stent was released in the distal esophagus, it was gently pushed down in the gastric cavity. No additional antegrade transpapillary stent was placed. A Hemostatic clip (Instinct, Cook Medical or Resolution, Boston Scientific, Natick, Ma, USA) was most often used in order to secure the stent position and reduce the risk of stent migration. All procedures were performed by one interventional endoscopist (FP) with expertise in ERCP and interventional EUS (FP: 5-years’ experience with EU-BD before the first inclusion in the study, >300 therapeutic EUS procedure/years and >1000 ERCPs/year).
Figure 1a.
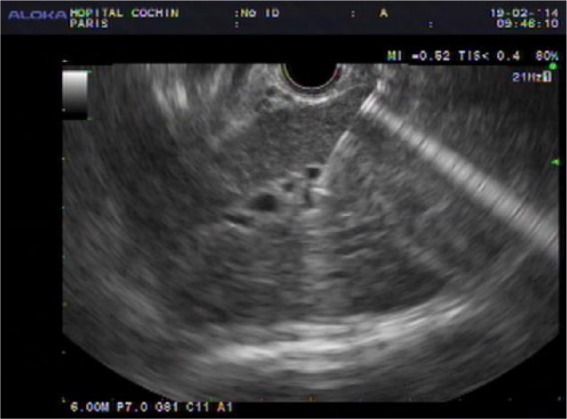
Biliary puncture under endoscopic ultrasound control.
Figure 1b.
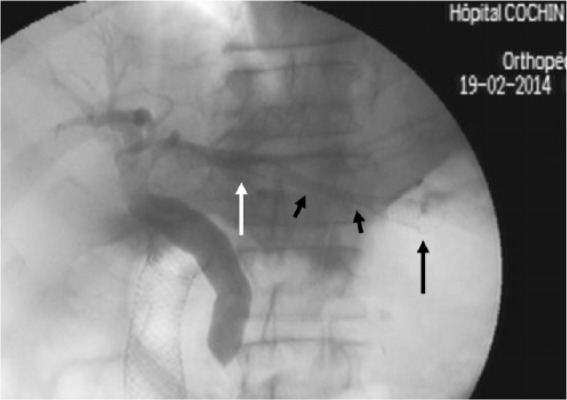
Cholangiographic view after transhepatogastric stent release in a patient with previously stented duodenal obstruction and distal biliary stricture. With arrow: intraductal stent end; long black arrow: intragastric stent end; short black arrows: stent portion traversing the liver parenchyma.
Figure 1c.
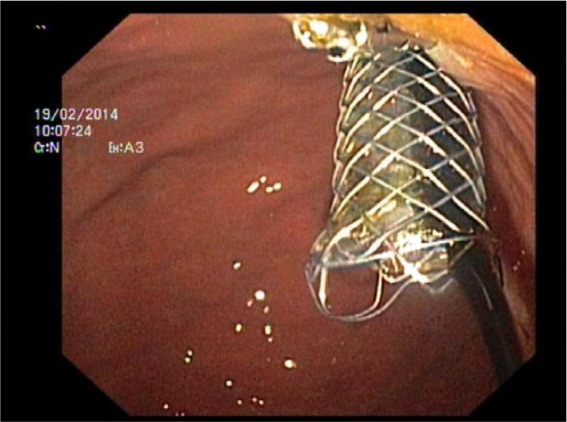
Endoscopic view of a transhepatogastric fully covered self-expanding metal stents (FCSEMS) with bile outflow inserted through the posterior and upper gastric wall and stitched with a clip to the mucosa.
Percutaneous transhepatic biliary drainage
The biliary tree was punctured under ultrasound guidance. Contrast injection was performed to obtain a cholangiogram. The biliary tree was then catheterized with a guide wire under fluoroscopic guidance and a 7 Fr introducer placed at entry of the intrahepatic bile duct. After crossing the tumor obstruction, a self-expandable metallic stent (Bard E. Luminex Stent 10 mm diameter, Angiomed GmbH & Co., Karlsruhe, Germany) was inserted in a one-step procedure. In case of impassable obstruction or poor condition of the patient due to infection, an 8 Fr externally locked drain was placed. After 1 week, or if the patient improved, a second attempt of metallic stent placement was performed. In case of lower bile duct obstruction, the distal end of the stent was placed across the ampulla in order to ensure maximal biliary drainage and reduce the risk of post-procedure cholangitis. An 8 Fr external drain was left along the PTB track following stent placement and removed 2–7 days later if the drainage from the metallic stent was effective. All cases were performed by one interventional radiologist with expertise in interventional radiology (MG: 15-years’ experience with PTBD, with 200 procedures/year).
Follow up
The patients were followed up after the procedure, with scheduled visits for clinical assessment and laboratory tests. The date of death or date of transfer to the palliative care unit was prospectively recorded. In case of missing data, medical records were reviewed and eventually, referring physicians were called on.
Statistical analysis
Patient characteristics are expressed as proportions and median ± interquartile range. Association between outcomes (clinical success, technical success, adverse event) and qualitative variables was determined using Fisher’s exact test. Comparison between continuous variables was performed using the Kruskal–Wallis test. Median survival times were estimated with their 95% confidence interval using the Kaplan–Meier’s method, and compared between procedure groups using the log-rank test. A p value < 0.05 (two sided) was considered significant.
Results
Characteristics
Out of 3159 ERCP procedures for obstructive jaundice performed at Cochin Hospital during the study period, 51 (1.6%) had EU-BD performed for malignant obstructive jaundice after a failed ERCP. Of those 51 patients, 31 (61%) underwent EU-HGS, 15 (30%) underwent EUS rendez-vous and 5 (9%) patients underwent EUS-guided choledocoduodenostomy. Only patients with EU-HGS were included.
A total of 214 PTB procedures were performed at University Hospital, Strasbourg, for malignant biliary obstruction during the same period. Out of those, 20 patients underwent PTBD after a failed ERCP. Three patients only had an external drain for poor tumor prognosis (Figure 2).
Figure 2.
Flowchart of patient selection.
EUS, endoscopic ultrasound; ERCP, endoscopic retrograde cholangiopancreatography.
A total of 51 patients (mean age 71 year ± 13.6, female 24) with malignant CBD biliary obstruction were thus included after either EU-HGS (n = 31) or PTBD (n = 20). Patient demographics, clinical information and reason for ERCP failure were similar in both groups. Clinical indications for biliary drainage and stenting according to technique are depicted in Table 1. There was no statistical difference between groups.
Table 1.
Patients characteristics at inclusion.
| Characteristic | EU-HGS (n = 31) | PTDB (n = 20) | p |
|---|---|---|---|
| Age, mean (SD), years | 69.2 | 67.7 | 0.55 |
| Sex | 0.40 | ||
| Female | 14 | 11 | |
| Male | 17 | 9 | |
| WHO status (median) | 2 (1–3) | 2 (1–3) | 0.10 |
| Indication | 0.90 | ||
| Pancreatic adenocarcinoma n (%) | 22 (71) | 15 (75) | |
| Metastatic lymphadenopathy n (%)* | 5 (16) | 2 (10) | |
| Cholangiocarcinoma n (%) | 3 (10) | 2 (10) | |
| Periampullary cancer n (%) | 1 (3) | 1 (5) | |
| Reason for unsuccessful ERCP | 0.89 | ||
| Roux-en-Y n (%) | 13 (42) | 10 (50) | |
| Duodenal stenosis n (%) | 9 (30) | 6 (30) | |
| Periampullary tumor infiltration n (%) | 5 (16) | 2 (10) | |
| Impassable stricture n (%) | 4 (13) | 2 (10) |
Colorectal cancer (n = 4), renal cancer (n = 1), lung cancer (n = 1), and ovarian cancer (n = 1).
EU-HGS, endoscopic ultrasound-guided hepaticogastrostomy; PTBD, percutaneous transhepatic biliary drainage; SD, standard deviation; WHO, World Health Organization; ERCP, endoscopic retrograde cholangiopancreatography.
Causes of endoscopic retrograde cholangiopancreatography failure
A previous Whipple’s resection for pancreatic cancer induced ERCP failure in 19 patients (11 in the EU-HGS group and 8 in the PTBD group). Other surgical bypass interventions were Roux-en-Y reconstruction following gastrectomy in two patients and Roux-en-Y hepaticojejunostomy for cholangiocarcinoma in two patients. Tight duodenal stenosis prevented access to the papilla due to pancreatic carcinoma in 13 patients, and retroperitoneal adenopathy in 3 patients (ovarian cancer n = 1, renal cancer n = 1, colorectal cancer n = 1). Biliary cannulation failed as a consequence of periampullary tumor infiltration associated with pancreatic head carcinoma in five patients and ampullary cancer in two patients; in six patients, complex and tight biliary stricture was the reason for failed ERCP.
Outcomes
Technical success of biliary stent placement was achieved in 100% of cases in both groups after successful cannulation (Table 2).
Table 2.
Clinical, technical success and adverse events according to the procedure.
| Variable | EU-HGS (n = 31) | PTBD (n = 20) | p |
|---|---|---|---|
| Technical success n (%) | 100 (31) | 100 (20) | 1.00 |
| Clinical success n (%) | 25 (86) | 15 (83) | 0.88 |
| Adverse events | 0.69 | ||
| Immediate adverse events, no. (%) | 5 (16) | 2 (10) | |
| Severe sepsis | 2 | 2 | |
| Bleeding | 1 | – | |
| Bile leak | 2 | – | |
| Perforation | 0 | – | |
| Procedure-related death | 2 | 2 | 0.87 |
| Reintervention | |||
| Unscheduled reintervention | 2 | 4 | 0.19 |
| Scheduled reintervention | 0 | 17 | 0.0001 |
| Overall reintervention | 2 | 21 | 0.0001 |
| Length of hospital stay (days) | 8 | 15 | 0.0002 |
EU-HGS, endoscopic ultrasound-guided hepaticogastrostomy; PTBD, percutaneous transhepatic biliary drainage.
Clinical success was achieved in 25 (86%) of 31 patients in the EU-HGS group and in 15 (83%) of 20 patients in the PTBD group, with no statistical difference between groups as shown in Table 2.
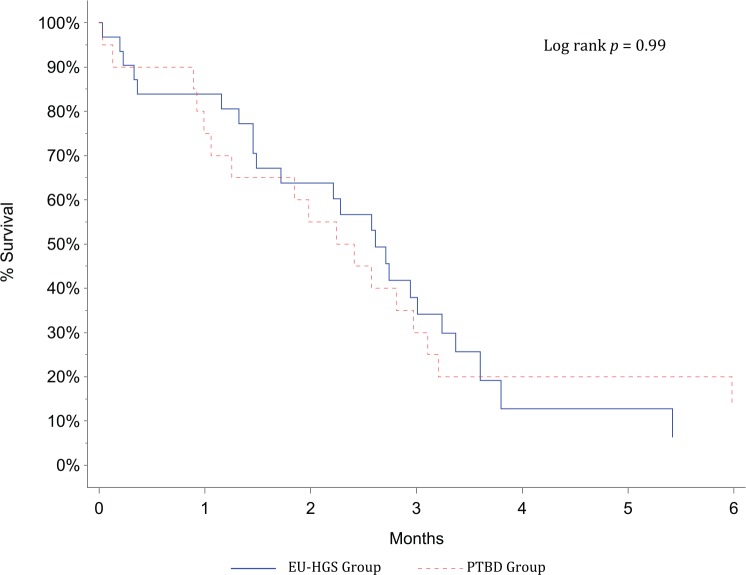
Median survival was 71 days (range 25–75th percentile; 30–95) for the EU-HGS group and 78 days (range 25–75th percentile; 42–108) for the PTBD group (p = 0.99), without significant difference between both groups (p = 0.99) (Figure 3). All patients died as a consequence of the underlying disease progression. The exact date of death was known in 43 (84%) patients. The date of transfer to a palliative care unit was known for five (10%) patients, and only the date of the last visit to the clinic for three patients (6%), in whom it was approximated as the presumed date of death (two in the EU-HGS group, one in the PTBD group).
Figure 3.
Survival after biliary drainage according to procedure (Kaplan–Meier’s survival calculation).
The overall rate of adverse events was 16% (five adverse events) in the EUS-HGS group and 10% (two adverse events) in the PTBD group, with no statistical difference between groups as shown in Table 2.
Mortality at day 7, deemed potentially procedure related, was 6% (two patients) in the EU-HGS group (including one severe sepsis at day 1 and a major bleeding at day 5 from the puncture site) and 10% (two patients) in the PTBD group (two with severe sepsis at day 1 and day 3) (Table 2). Patients with bile leak required percutaneous biloma drainage. All other patients were managed conservatively.
Reinterventions
During long-term follow up, a total of two and four unscheduled reinterventions were required in the EU-HGS and PTBD groups (p = 0.19), respectively (Table 2). No patient presented with stent migration in the EU-HGS group, but two patients who developed stent occlusion were successfully treated by stent-in-stent insertion using uncovered metal stents. Four patients presented with stent occlusion in the PTBD group: two patients initially had a 10 Fr plastic stent for a poor carcinologic prognosis. Treatment consisted of changing the 10 Fr plastic stent. In two other patients, stent occlusion was successfully treated by stent-in-stent with uncovered metal stents.
In the EU-HGS group, no scheduled reintervention was required, whereas 17 patients needed drain exchange or removal in the PTBD group (p = 0.0001) (Table 2).
The overall reintervention rate (scheduled and unscheduled) was significantly higher in the PTBD group (2 versus 21; p = 0.0001).
Length of hospital stay
The length of stay was longer in the PTDB group (15 days versus 8 days; p = 0.002) due to the number of scheduled reinterventions and the need to keep the patient for 2–7 days before removing the 8 Fr external drain following metallic stent placement.
Discussion
Percutaneous transhepatic cholangiographic access is often preferred for the palliation of malignant obstructive jaundice only after a first unsuccessful attempt at ERCP, because PTBD remains associated with relatively more adverse events than endoscopic access to the biliary tract: a recent meta-analysis found a perioperative morbidity rate of up to 60%,25 suggesting the need of a less iatrogenic procedure.
EU-BD was first described in 2001.8 Following this paper, several groups reported on the efficacy of EU-BD as an alternative biliary drainage method after unsuccessful ERCP. Interventional EUS techniques have only very recently gained some momentum, because devices designed to create a transparietal tract, manipulate guidewires and secure stent positioning have been made available during the last 5–8 years.
Both EU-BD and PTBD are associated with a risk of injury to intervening vessels and additional hazards in case of ascites.
Beside EUS-based techniques, endoscopic approaches for pancreatobiliary diseases in patients with surgically altered gastrointestinal anatomy also include the use of enteroscopy-assisted ERCP. Recently an international multicenter trial compared EUS-guided biliary drainage versus enteroscopy-assisted ERCP in patients with biliary obstruction and previous surgery precluding standard ERCP.26 The authors reported higher technical (98% versus 65.3%) and clinical (88% versus 60.4%) success rates in the EUS-BD group versus the enteroscopy-assisted group, respectively. The study concluded that EU-BD offers multiple advantages over enteroscopy-assisted ERCP in patients with surgically altered upper gastrointestinal anatomy, including not only success rates, but also reduced procedural times.
Four previous studies have compared EU-BD and PTBD. Their main results are summarized in Table 3 . Artifon et al. randomized 25 patients to receive either EUS-guided choledocoduodenostomy or PTBD21 with clinical and technical success in all patients of both groups. There were no differences in terms of adverse events between groups.
Table 3.
Studies comparing endoscopic ultrasound-guided biliary drainage and percutaneous drainage for malignant distal biliary obstruction.
| Study | Type | Patients EUBD/PTBD (n) | EUS site puncture EUHGS/EUCD | Technical success (%) | Clinical success (%) | Adverse events EUBD/PTBD (%) | Reintervention EUBD/PTBD (%) | LOS EUBD/PTBD (days) |
|---|---|---|---|---|---|---|---|---|
| Artifon et al.21 | Prospective | 13/12 | 0/12 | 100/100 | 100/100 | 15/25 | N/A | N/A |
| Bapaye et al.22 | Retrospective | 25/26 | 13/10 | 92/46 | N/A | 20/46 | N/A | N/A |
| Khashab et al.23 | Retrospective | 22/51 | 3/19 | 86/100 | 86/92 | 18/39 | 15/80 | N/A |
| Lee et al.24 | Prospective | 34/32 | 25/9 | 94/96 | 87/87 | 8/31 | 25/54 | 6/12 |
| Current study | Retrospective | 31/20 | 31/0 | 100/100 | 86/83 | 16/10 | 6/100 | 8/15 |
EUBD, endosonography-guided biliary drainage; PTBD, percutaneous transhepatic biliary drainage; EUCD, endosonography-guided choledochoduodenostomy; EUHGS, endosonography-guided hepaticogastrostomy; LOS, length of hospital stay; N/A, data not available.
Two retrospective studies by Bapaye22 and Khashab23 compared PTBD and EU-BD and found significantly more adverse events after PTBD, although clinical success rates were similar. Finally, a recently published study by Lee et al24 compared in a randomized fashion a mixture of EU-CD and EU-HGS procedures versus PTBD and found similar clinical results but more adverse events after PTBD.
We decided to compare only EU-HGS and PTBD cases for two reasons: first, EU-HGS is the only technique that allows biliary drainage after surgical bile duct resection or disconnection, as long as left hepatic ducts are dilated; second, and above all, because EU-HGS shares with PTBD the use of an antegrade transhepatic route, allowing a rather peripheral puncture of the bile ducts.
It is generally considered that a randomized controlled trial with inclusions for all arms in the same center is the best methodology when interventions can be achieved with the same level of quality (i.e. when comparing two drugs or two endoscopic procedures for which endoscopists have the same level of expertise). However, when comparing EUS-guided and percutaneous techniques, it is uncommon to find the same level of expertise in one center for both techniques, since most difficult cases are referred to the specialist with the best available expertise (i.e. the radiologist or the endoscopist), which can be seen as discouraging the development of the alternative technique.27 A valid method to avoid skewing a study in favor of one technique (arguably in favor of EUS in previously published studies) may be the retrospective expertise-based study, which may minimize bias resulting from differences in technical competency and endoscopist’s preference, and decrease crossover from one intervention to the other.28 The present study is a retrospective expertise-based study from two centers, in which data were collected on a prospectively maintained database, inclusion criteria were strictly verified and experts of each technique were available. This study is therefore the first comparing EUS using exclusively the transgastric approach to the standard technique of PTBD, both performed by experts, when ERCP has failed to gain access to the obstructed bile duct. No differences were seen in preprocedure patient characteristics between the two study groups and all the patients had unresectable malignant biliary obstruction. Technical success with stent placement in the desired location was successful in all the patients in both groups. A similarly high clinical success rate was achieved in both PTBD and EU-HGS groups, and survival was similar. Furthermore, no significant difference was noted with regard to procedure-related adverse events. The overall PTBD rate of adverse events, lower than in some other reports,21,23 can be biased by the performance of all procedures by the same operator with expertise in interventional radiology, but the same is true of the EU-HGS group.
The only significant difference between EU-HGS and PTBD was found to be the overall number of reinterventions, which was 10 times lower in the EU-HGS group. Our data suggest that EU-HGS may be as effective and safe as the regular PTBD approach, with the key advantage of EU-HGS that the procedure can be done during the same session as the failed ERCP without the pain and inconvenience of an external catheter. EU-HGS also maintains bile within the gastrointestinal tract to ensure proper digestion and absorption of nutrients and avoid compensation for the loss of alkali during external bile drainage. A major disadvantage of PTBD compared with EU-HGS is the need for reinterventions, especially when an external drain is inserted in the first place. It was a standard operating procedure for radiologists in our study to place an external or internal–external plastic drain for at least 5 days before, and 3 days after metal stenting. Single-step PTBD, with immediate self-expanding metal stents (SEMS) stenting and no external drainage has been reported7 but has also been associated with significantly more iatrogenic events in septic cases, such as biloma, hemorrhage and cholangitis than the three-step procedure (prestenting drainage, stent insertion, poststenting drain removal). QOL has not been thoroughly assessed in this study, but World Health Organization status was at least equal or better shortly after the procedure in all patients with successful EUS-HGS. However, due to the advanced course of the disease in most patients and their poor prognosis, QOL tended to decline in both groups after a few weeks. It seems reasonable to estimate that the external biliary drainage, even for a limited period of time, is a significant hindrance to the rapid QOL improvement that is expected after those palliative procedures. A limitation of EU-HGS procedure is the left lobar access route, which can be inadequate or insufficient in patients with advanced hilar obstruction.29 Complex hilar obstruction was excluded from the present study, and we are now investigating the value of EU-HGS in such patients.
Another impediment to the development of EU-HGS procedure is that expertise in interventional EUS is not widespread, whereas PTBD can be performed by most interventional radiologists and is routinely available in more centers than interventional EUS. The overall rate of adverse events in the EUS-HGS group is consistent with recent studies and illustrates the relatively good safety profile of EU-BD compared with ERCP, as shown by one recent study comparing the clinical efficacy and safety of EU-BD and ERCP for patients with distal malignant biliary obstruction.30
Finally, despite these limitations and a relatively small sample size, the strengths of this study, similar patients in both groups, homogeneity of techniques with only one expert involved in each group, full survival follow up, suggest that EU-HGS is an effective and safe technique and may provide endoscopists with an additional alternative biliary drainage method when ERCP is precluded or unsuccessful. Larger multicenter, randomized trials would be welcome to establish the therapeutic and safety profiles of the EU-HGS procedure before this technique is accepted as a standard option.
Conclusion
EU-HGS is an effective and safe minimally invasive procedure alternative to PTBD with similar success and adverse event rates. However, EU-HGS is associated with a lower number of reinterventions and a shorter hospital stay. Our results suggest that EU-HGS can be a technique of choice after unsuccessful ERCP at institutions with experienced interventional endosonographers.
Footnotes
Author’s contributions: A. Sportes: inclusion of patients, collection of data, preparation of the manuscript.
M. Camus: inclusion of patients, preparation of the manuscript.
M. Greget: performance of percutaneous transhepatic drainage.
S. Leblanc: inclusion of patients, preparation of the manuscript.
R. Coriat: revision of the manuscript.
J. Hochberger: inclusion of patients.
S. Chaussade: revision of the manuscript.
S. Grabar: statistical analysis.
F. Prat: design of the protocol, revision of the manuscript, performance of EUS-guided hepaticogastrostomy.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Adrien Sportes, Gastroenterology Unit, Hôpital Cochin (AP-HP), University Paris Descartes, Paris, France.
Marine Camus, Gastroenterology Unit, Hôpital Cochin (AP-HP), University Paris Descartes, Paris, France.
Michel Greget, Interventional Radiology Unit CHRU Strasbourg, University of Strasbourg, France.
Sarah Leblanc, Gastroenterology Unit, Hôpital Cochin (AP-HP), University Paris Descartes, Paris, France.
Romain Coriat, Gastroenterology Unit, Hôpital Cochin (AP-HP), University Paris Descartes, Paris, France.
Jürgen Hochberger, Gastroenterology Unit, Nouvel Hôpital Civil CHRU Strasbourg, University of Strasbourg, France.
Stanislas Chaussade, Gastroenterology Unit, Hôpital Cochin (AP-HP), University Paris Descartes, Paris, France.
Sophie Grabar, Biostatistics and Epidemiology, Hôtel Dieu (AP-HP), University Paris Descartes, Paris, France.
Frédéric Prat, Gastroenterology unit, Hôpital Cochin (AP-HP), 27 Rue du Faubourg Saint-Jacques, 75014 Paris, France.
References
- 1. Cotton PB. Cannulation of the papilla of Vater by endoscopy and retrograde cholangiopancreatography (ERCP). Gut 1972; 13: 1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ASGE guidelines for clinical application. The role of ERCP in diseases of the biliary tract and pancreas. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc 1999; 50: 915–920. [DOI] [PubMed] [Google Scholar]
- 3. Fogel EL, Sherman S, Devereaux BM, et al. Therapeutic biliary endoscopy. Endoscopy 2001; 33: 31–38. [DOI] [PubMed] [Google Scholar]
- 4. Ekkelenkamp VE, de Man RA, Ter Borg F, et al. Prospective evaluation of ERCP performance: results of a nationwide quality registry. Endoscopy 2015; 47: 503–507. [DOI] [PubMed] [Google Scholar]
- 5. Baron TH, Petersen BT, Mergener K, et al. Quality indicators for endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 2006; 63 (Suppl. 4): S29–S34. [DOI] [PubMed] [Google Scholar]
- 6. Li M, Bai M, Qi X, et al. Percutaneous transhepatic biliary metal stent for malignant hilar obstruction: results and predictive factors for efficacy in 159 patients from a single center. Cardiovasc Intervent Radiol 2015; 38: 709–721. [DOI] [PubMed] [Google Scholar]
- 7. Winick AB, Waybill PN, Venbrux AC. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol 2001; 4: 200–206. [DOI] [PubMed] [Google Scholar]
- 8. Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy 2001; 33: 898–900. [DOI] [PubMed] [Google Scholar]
- 9. Isayama H, Nakai Y, Kawakubo K, et al. The endoscopic ultrasonography-guided rendezvous technique for biliary cannulation: a technical review. J Hepato-Biliary-Pancreat Sci 2013; 20: 413–420. [DOI] [PubMed] [Google Scholar]
- 10. Dhir V, Bhandari S, Bapat M, et al. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos). Gastrointest Endosc 2012; 75: 354–359. [DOI] [PubMed] [Google Scholar]
- 11. Song TJ, Hyun YS, Lee SS, et al. Endoscopic ultrasound-guided choledochoduodenostomies with fully covered self-expandable metallic stents. World J Gastroenterol 2012; 18: 4435–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park DH. Endoscopic ultrasonography-guided hepaticogastrostomy. Gastrointest Endosc Clin N Am 2012; 22: 271–280. [DOI] [PubMed] [Google Scholar]
- 13. Shah JN, Marson F, Weilert F, et al. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc 2012; 75: 56–64. [DOI] [PubMed] [Google Scholar]
- 14. Khashab MA, Valeshabad AK, Modayil R, et al. EUS-guided biliary drainage by using a standardized approach for malignant biliary obstruction: rendezvous versus direct transluminal techniques (with videos). Gastrointest Endosc 2013; 78: 734–741. [DOI] [PubMed] [Google Scholar]
- 15. Kim YS, Gupta K, Mallery S, et al. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: cumulative experience at a single center. A case series. Endoscopy 2010; 42: 496–502. [DOI] [PubMed] [Google Scholar]
- 16. Khashab MA, Dewitt J. EUS-guided biliary drainage: is it ready for prime time? Yes! Gastrointest Endosc 2013; 78: 102–105. [DOI] [PubMed] [Google Scholar]
- 17. Henry WA, Singh VK, Kalloo AN, et al. Simultaneous EUS-guided transbulbar pancreaticobiliary drainage (with video). Gastrointest Endosc 2012; 76: 1065–1067, e1–e2. [DOI] [PubMed] [Google Scholar]
- 18. Itoi T, Yamao K. EUS 2008 Working Group. EUS 2008 Working Group document: evaluation of EUS-guided choledochoduodenostomy (with video). Gastrointest Endosc 2009; 69 (Suppl. 2): S8–S12. [DOI] [PubMed] [Google Scholar]
- 19. Artifon ELA, Marson FP, Gaidhane M, et al. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: is there any difference? Gastrointest Endosc 2015; 81: 950–959. [DOI] [PubMed] [Google Scholar]
- 20. Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: a systematic review. Gastrointest Endosc 2015; 83: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 21. Artifon ELA, Aparicio D, Paione JB, et al. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol 2012; 46: 768–774. [DOI] [PubMed] [Google Scholar]
- 22. Bapaye A, Dubale N, Aher A. Comparison of endosonography-guided vs. percutaneous biliary stenting when papilla is inaccessible for ERCP. United Eur Gastroenterol J 2013; 1: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khashab MA, Valeshabad AK, Afghani E, et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci 2015; 60: 557–565. [DOI] [PubMed] [Google Scholar]
- 24. Lee TH, Choi J-H, Park DH, et al. Similar efficacies of endoscopic ultrasound-guided transmural and percutaneous drainage for malignant distal biliary obstruction. Clin Gastroenterol Hepatol 2016; 14: 1011–1019.e3. [DOI] [PubMed] [Google Scholar]
- 25. Fang Y, Gurusamy KS, Wang Q, et al. Meta-analysis of randomized clinical trials on safety and efficacy of biliary drainage before surgery for obstructive jaundice. Br J Surg 2013; 100: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 26. Khashab MA, El Zein M, Sharzehi K, et al. International multicenter comparative trial of EUS-guided biliary drainage vs. enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction. United Eur Gastroenterol J 2015; 3 (Suppl. 5): 1–145. [Google Scholar]
- 27. Kao LS, Tyson JE, Blakely ML, et al. Clinical research methodology I: introduction to randomized trials. J Am Coll Surg 2008; 206: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Devereaux PJ, Bhandari M, Clarke M, et al. Need for expertise based randomised controlled trials. Br Med J 2005; 330: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giovannini M, Bories E. EUS-guided biliary drainage. Gastroenterol Res Pract 2012; 2012: 348719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawakubo K, Kawakami H, Kuwatani M, et al. Endoscopic ultrasound-guided choledochoduodenostomy vs. transpapillary stenting for distal biliary obstruction. Endoscopy 2016; 48: 164–169. [DOI] [PubMed] [Google Scholar]