Abstract
Placenta accreta is now the chief cause of postpartum hemorrhage resulting in maternal and neonatal morbidity. Prenatal diagnosis decreases blood loss at delivery and intra and post-partum complications. Ultrasound is critical for diagnosis and MRI is a complementary tool when the diagnosis is uncertain. Peripartum hysterectomy has been the standard of therapy but conservative management is increasingly being used. The etiology of accreta is due to a deficiency of maternal decidua resulting in placental invasion into the uterine myometrium. The molecular basis for the development of invasive placentation is yet to be elucidated but may involve abnormal paracrine/autocrine signaling between the deficient maternal decidua and the trophoblastic tissue. The interaction of hormones such as Relaxin which is abundant in maternal decidua and INSL4, an insulin like peptide found in placental trophoblastic tissue may play role in the formation of placenta accreta.
INTRODUCTION
The morbidly adherent placenta is now a significant obstetric challenge. Placenta accreta is often used as a general term but is defined by the levels of invasion of chorionic villi into maternal myometrium. Once a rare diagnosis, it is now the leading cause of postpartum hemorrhage and indication for a gravid hysterectomy (1). Traditionally, abnormal placentation has been classified into accreta, increta and percreta based on the depth of myometrial invasion: superficial, deep, and through the uterine serosa respectively and the greater the invasion, the greater the risks for hemorrhage and maternal morbidity (2). Analyzing a single institution’s Cesarean hysterectomy specimens over a ten year period, Miller et al showed that the majority of cases (75%) will be accretas, 18% incretas and 7% percretas. Initially thought to be a rare complication of pregnancy, there has been a substantial increase in the occurrence of placenta accreta in the last 20 years with an incidence as high 1/533 pregnancies (3).
PROCEDURE
A Pub Med search was initially performed using the search terms Placenta, growth factors. An additional search was also performed using the terms: placenta accreta, diagnosis, management. Both searches were limited to English publications in the attempt to produce both a clinical state of the science review and a review of the molecular biology of placenta accreta.
RISK FACTORS FOR ACCRETA
All invasive procedures on the uterus or the uterine cavity have been associated with the subsequent development of placenta accreta including uterine curettage, hysteroscopic surgery, endometrial ablation, uterine artery embolization and myomectomy (2). However, the most important risk factor for the development of placenta accreta is a prior Cesarean delivery (4) and the continued rise in the Cesarean section rates worldwide ensures that accretas will remain a troublesome clinical issue. Accretas are present in 0.24%, 0.31%, 0.57%, 2.13%, 2.33%, and 6.74% of women undergoing their first, second, third, fourth, fifth, and sixth or more cesarean deliveries, respectively (4). The risk for accreta formation are markedly increased with a history of a prior Cesarean delivery and the presence of a placenta previa (4). The MFMU network showed that with a history of prior Cesarean sections and a concurrent previa, the risk for placenta accreta was 3%, 11%, 40%, 61%, and 67% for first, second, third, fourth, and fifth or more repeat cesarean deliveries, respectively.
SCREENING FOR PLACENTA ACCRETA
Ultrasound
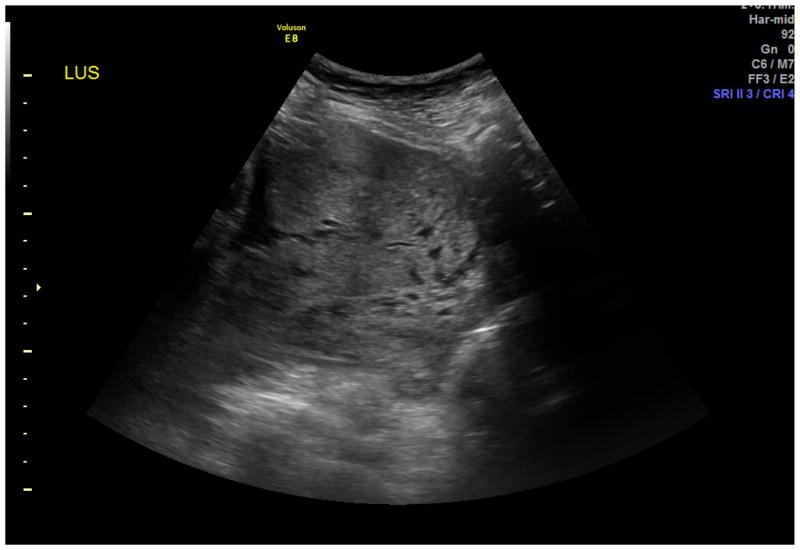
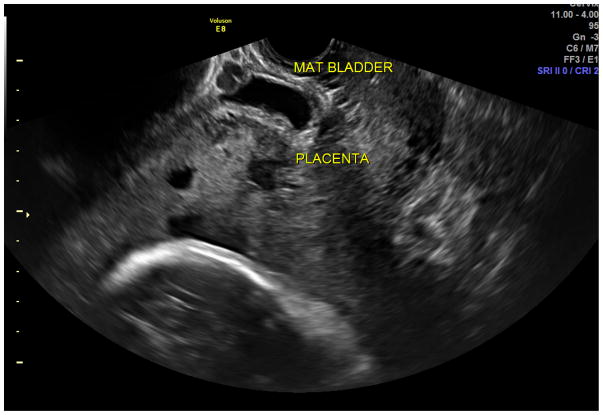
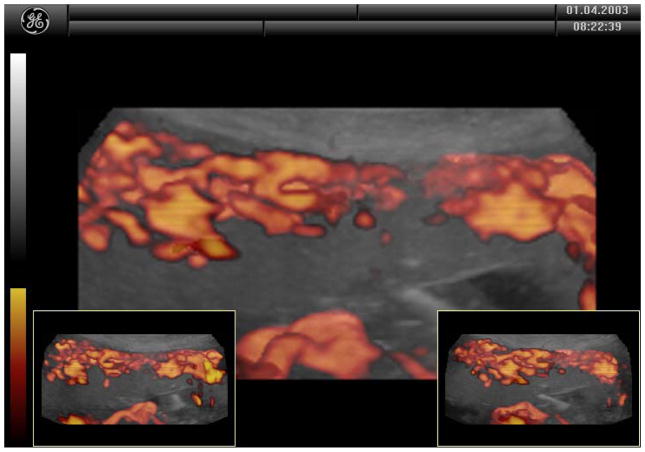
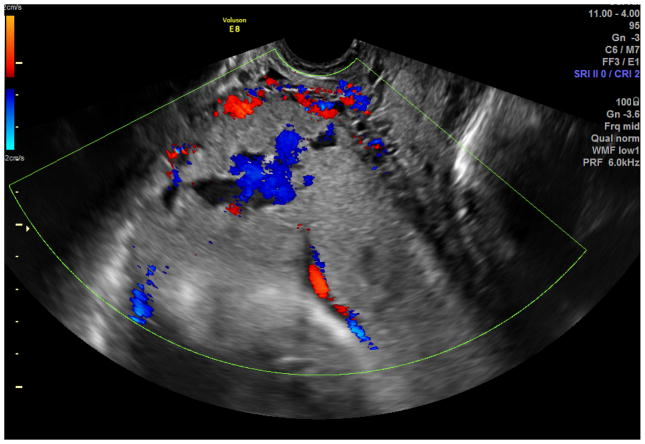
Standard transabdominal ultrasonography is a reliable tool for diagnosing invasive placentation and is the primary tool for the antenatal diagnosis of the morbidly adherent placenta (Figure 1). Several US features have been documented to be associated with a higher risk of placenta accreta, including the presence of placental lacunae (irregular vascular spaces resulting in a “Swiss cheese” appearance), retroplacental myometrial thickness (less than 1 mm), loss of the normal hypoechoic retroplacental zone, and anomalies of the bladder-myometrium interface (5). Comstock et al performed the largest prospective study of gray scale ultrasound for the diagnosis of abnormal placentation (6). Over 163,000 patients were scanned over a 12 year period and 2002 had dual risk factors of placenta previa and a prior cesarean delivery. 33 of these patients had ultrasound findings suspicious for a placenta accreta. The sensitivity of ultrasound was 100%, with the presence of placental lacunae having the highest sensitivity (93%). Eight six percent of patients had abnormal findings between 15 and 20 weeks, which suggests that the diagnosis can be made at the routine anatomic scan. Yang et al used transvaginal sonography in patients at risk for morbidly adherent placentation to grade intraplacental lacunae (Grade 0 for no lacunae, Grade 1 for one to three, Grade 2 for four to six and Grade 3 for large and irregular lacunae) (7). They noted a sensitivity of 87% with Grade 1 lacunae and 100% with Grade 3. Twickler et al used ultrasound color flow mapping on placental implantations in proximity to the prior hysterotomy scar and showed that the measurement of <1 mm for the smallest myometrial thickness or the presence of large intraplacental lakes was 100% sensitive in predicting myometrial invasion (specificity 72%, PPV 72%, NPV 100%) (8). Transvaginal ultrasound allows for a more detailed assessment of a placenta’s invasiveness, improving diagnostic accuracy in invasive placentation (9) (Figure 2). Using linear logistic regression and multiparametric analyses, Rac et al were able to exclude the loss of retroplacental clear zone, irregularity and width of the bladder-uterine interface as significant variables in accreta prediction (10). Applying a value to the parameters of smallest sagittal myometrial thickness, lacunae, bridging vessels, number of Cesarean deliveries and placental location, this group were able to establish a graded probability of invasion unique to each individual patient via a Placenta Accreta Index (10). 3D power Doppler ultrasound has the ability to differentiate between the degrees of placental invasion (11)(Figure 3). Irregular intraplacental vascularization with tortuous confluent vessels affecting the entire placental width and hypervascularity of the entire serosa-bladder wall interface were important markers in differentiating accreta from percreta in a prospective evaluation of at-risk patients (FIgure 4). In a retrospective study of 40 histopathologically confirmed accretas, Chalubinski et al used transvaginal ultrasound, 2D grey scale, color and power Doppler and were able to differentiate between normal placentation/accreta and increta/percreta with a 100% accuracy (12). A recent meta analysis by D’Antonio et al compared twenty three studies involving over 3700 patients found that that the sensitivity of individual studies for diagnosing morbidly adherent placentation ranged from 61% to 100% with a pooled sensitivity of 91% (13). They found that abnormal vasculature on color Doppler ultrasound had the best combination of sensitivity and specificity and that abnormalities on the uterine-bladder interface had the best specificity for predicting accreta. This study showed that the presence of lacunae and the lack of a clear space did not perform as well.
Figure 1.
Transabdominal ultrasound of placenta accreta. The diagnosis was based on the presence of placental lacunae - irregular vascular spaces resulting in a “swiss cheese” appearance and loss of the normal hypoechoic retroplacental zone.
Figure 2.
Transvaginal ultrasound allows for a more detailed assessment of a placenta’s invasiveness. MAT BLADDER on the picture indicated placenta percreta at the level of the bladder serosa. The retroplacental myometrial thickness was less than 1 mm and normal bladder-myometrium interface was not visualized.
Figure 3.
3D power Doppler ultrasound of placenta accreta. Disorganized and high velocity myometrium blood flow was visualized.
Figure 4.
Color Doppler ultrasound in the patient with placenta percreta. Irregular intraplacental vascularization with tortuous confluent vessels affecting the entire placental width and hypervascularity of the entire serosa- bladder wall interface.
Magnetic Resonance Imaging
Three MRI findings have been found abnormal placentation: 1. Abnormal Uterine bulging, 2. Heterogeneity of signal intensity within the body of the placenta, 3. Presence of dark intraplacental bands on T2 weighted images (14). Warshak et al suggest a two stage protocol in evaluating a patient at risk for abnormal placentation using ultrasonography first, then MRI for cases that are inconclusive (15). Riteau et al showed that ultrasound remains the more sensitive imaging modality for diagnosing accreta and suggest that MRI be complementary to ultrasound, especially in cases where there are few ultrasound signs (16). It has been suggested that MRI be performed if there is a suspicion for invasion into the parametrium or surrounding organs or in suspected posterior placenta accreta (1).
CLINICAL OUTCOMES
Hemorrhage is the most common complication at the time of delivery in a patient with placenta accreta. There is a potential for massive blood loss potentially resulting in consumptive coagulopathy, renal failure, acute respiratory distress syndrome, the need for re-operation and death (1). Miller et al showed that the estimated blood loss exceeded 2000cc in 66% of their cohort, 5000cc in 15% of cases and 10,000cc in 6.5% (2). 55% of women required transfusion and 21% of the patients required more than 5U units of blood.
Other well described complications of peripartum hysterectomies for placenta accretas include infection, cystotomy, ureteral injury and the need for re-operation for hemoperitoneum (2).
MANAGEMENT
Prenatal Diagnosis and Timing of Delivery
Antenatal diagnosis of invasive placentation is critical (17). Pre-delivery diagnosis is associated with decreased maternal hemorrhagic morbidity. Warshak et al identified 99 women with placenta accreta in their cohort, 62 of whom were diagnosed pre-delivery and 37 of whom were diagnosed intrapartum. The prenatally diagnosed group received fewer units of PRBCs (4.7 units compared to 6.9) and had a lower estimated blood loss (2,344 cc vs 2,951cc). The prenatally diagnosed group was delivered electively at 34 to 35 weeks to reduce the morbidity associated with emergent hysterectomy. This practice was not associated with increased neonatal morbidity (NICU length of stay, RDS, need for surfactant administration or intubation) which may have been related to the increased use of antenatal steroid administration given to the prenatally diagnosed group. Eller et al also advocate an electively scheduled Cesarean delivery and hysterectomy at 34 weeks after antenatal steroids to decrease morbidity associated with vaginal bleeding and emergent delivery (18). In their cohort of 69 patients (57 prenatally diagnosed versus 17 unsuspected) they showed that prenatal diagnosis resulted in lower ICU admission rates (23 versus 43%), lower large volume of blood transfusions (5 versus 9%), less ureteric injury (5 versus 9%), less intra-abdominal infection (6 versus 9%), decreased hospital readmission (5 versus 18%) and less vesicovaginal fistula formation (0 versus 6%).
Placental Removal
Eller et al also showed that attempts at placental removal prior to hysterectomy had significantly increased early morbidity compared to those who underwent Cesarean hysterectomy with the placenta left undisturbed in situ (18). Belfort et al suggest delivery of the fetus via a fundal or high classical incision and leaving the placenta in-situ before proceeding onto hysterectomy (1). Warshak et al compared ninety patients, sixty two of whom had prenatal diagnosis and thirty seven of whom were diagnosed intrapartum (19). The antenatally diagnosed group had planned cesarean hysterectomies and this group had lower transfusion rates and blood loss. A recent review by Bowman et al does underscore the fact that diagnosis of accreta with ultrasound may not be straightforward (20). This study noted an up to 5.9% false positive rate, 16% false negative rate and a 12.3% uncertain diagnosis rate for patients at risk for accreta. Given the morbidity of Cesarean hysterectomy, placentas that detach easily in patients suspected to be at risk for morbidly adherent placentation may be candidates for placental removal or conservative management.
Conservative Management
Cesarean hysterectomy has been the management of choice for placenta accreta. It is associated with significant morbidity as documented above as well as the psychological consequences of the loss of fertility. There are now multiple studies and case reports for the conservative management of abnormal placentation. These include expectant management by leaving the placenta in situ, uterine artery embolization followed by expectant management and adjuvant medical therapy including Methotrexate, Misoprostol, Mifepristone and GnRH analogues. Sentilhes et al recently published a retrospective multi-center cohort study in France assessing maternal outcome after conservative treatment for placenta accreta (21). Of the 311 women initially studied in this trial, 144(46.3%) were excluded because they underwent partial removal of the placenta or cesarean hysterectomy. Conservative treatment was successful in 131 out of 167 women (78.4%). 36 patients needed a hysterectomy either primarily or delayed. Severe maternal morbidity was noted in 10 patients (6%). Complications for the conservatively managed cohort included pulmonary edema, septic shock, acute renal failure, infection, DVT or pulmonary embolism and secondary postpartum hemorrhage. There was one maternal death related to myelosuppression and nephrotoxicity related to Methotrexate administration.
Sentilhes et al studied fertility and obstetric outcome after conservative management of placenta accreta (22). 46 patients out of a cohort of 73 patients with placenta accreta were treated conservatively without extirpative measures, all of whom had residual placenta in situ. Additional treatments to conserve fertility included bilateral hypogastric artery ligation, uterine artery ligation, uterine sutures, embolization, methotrexate, oxytocin and/or prostaglandins. The median time of follow-up for the cohort was 65 months. The median time for the resumption of menses was 130 days and none had amenorrhea. 12/14 patients desiring another pregnancy got pregnant for a total of 15 pregnancies and 2 had recurrent placenta accreta. Five spontaneous abortions were noted and the median term of delivery was 37 weeks.
Surgical Management
Eller at al studies 76 cases of placenta accretas (18). When accreta was diagnosed prenatally, scheduled cesarean hysterectomies were performed without attempted placental removal. A significant decrease in morbidity was noted in comparison to patients were placental removal was attempted. The pre or peri-operative use of ureteral stents was noted to decrease the risk of ureteral injury and to significantly reduce the rate of post operative morbidity (p=0.02). Hypogastric artery ligation was not noted to decrease the mean blood loss or the need for large volume of blood transfusions. They concluded that scheduled cesarean hysterectomy with preoperative ureteral stent placement and avoiding peacemeal placental removal was the optimal management strategy for prenatally diagnosed placenta accretas.
Angstmann et al retrospectively analyzed a cohort of 26 cases of placenta accreta. Eight cases were managed using a staged embolization procedure with hysterectomy and outcomes were compared to routine cesarean hysterectomies (23). Compared to planned cesarean hysterectomies, a staged procedure involving preoperative use of Interventional radiological embolization followed by a hysterectomy resulted in significant blood loss reductions (553 vs 4517 ml; p=0.0001), need for transfusion (2 vs 16; p=0.001) and units of blood transfused (0.5 vs 7.9; p=0.0013).
The role of cell saver technology in obstetric practice is controversial. Elagamy et al conducted a prospective observational study on 41 patients with placenta accreta and the role of cell saver technology in reducing allogenic transfusion (24). They found that their cell saver cohort that the mean volume of re-infused salvaged blood was 1476 cc and that 87% of their cohort did not require allogenic red blood cell transfusion. There was no incidence of amniotic fluid embolism, hypotension, sepsis or coagulopathy in the cell saver cohort.
The urinary tract is the most common affected pelvic structure with placenta accreta (25). Tam Tam et al reviewed 285 pregnancies complicated by morbidly adherent placentas that were managed with hysterectomy and found 29% chance of urinary tract injury. Findings from this cohort were that prenatal diagnosis significantly lowered the incidence of urinary tract injury (p<0.04). Suggested approaches after analysis from their cohort include: 1) Using pre-op cystoscopy to check for obvious bladder wall involvement to assure urologic backup for bladder preservation. 2) Placement of large bore ureteral stents to make for easier palpation. 3) Placing the patients in Allen stirrups to allow three surgeons to be at the operative field. 4) Filling the bladder with sterile milk prior to bladder mobilization.
ETIOLOGY
There is good evidence that placenta accreta forms due to the failure of a normal decidua to form, either because the endometrium is deficient or because it cannot transform (26). Placenta accreta is a regular finding in abdominal and ectopic pregnant where there is also no normal endometrium to transform into decidua. The placenta forms differently from other organs. It is created by cellular interactions of maternal cells and fetal trophoblastic cells, each directed by different genomes. The placental interaction with endometrium begins at implantation. Early on, trophoblasts invade the veins and stroma of maternal tissues, allowing the placenta to grow into the uterine cavity. Nitabuch’s fibrinoid lies between the placenta and the uterine tissues. It is an amorphous eosinophilic matrix containing trophoblastic cell proteins and maternal fibrin. Without a normal decidual plate and Nitabuch’s layer, the villous trophoblast has direct access to the maternal myometrium. Tseng et al theorize that trophoblastic migration and invasion during normal placental development must be interdependently influenced by different kinds of molecules such as growth factors and their receptors, cytokines, hormones, adhesion molecules and enzymes in an autocrine or paracrine manner and that normal placentas do not proceed beyond the inner third of the myometrium through tight spatial and temporal regulation (27). The role of decidua in preventing abnormal placentation is probably by a autocrine/paracrine feedback. Decidual Natural Killer cells play an important role in the immune regulation of trophoblastic invasion; Laban et al showed via immunohistochemistry that decidual natural killer cells were significantly decreased in placenta accreta (28).
Insulin and the insulin-like growth factors belong to a family of peptides essential for fetal growth and development. Relaxin (RLN) belongs to the insulin-like growth factor family and may also play a role as a regulator of cell growth in fetal membranes and placenta via autocrine/paracrine interactions and its site of action in the uterus has been shown to be primarily in the decidua (29). Another insulin like growth factor INSL4 (Insulin-like 4) has been shown to play a role in the concerted regulation of placental development and fetal growth via apoptosis. (30) Goh et al showed by gene expression and immunohistochemistry that INSL4 expression was significantly decreased in trophoblastic tissue in both invasive and non invasive areas in placenta accreta when compared to gestational aged matched controls. They suggest that accreta formation is not a local trophoblastic problem but a more generalized phenomenon and that the pathophysiology of trophoblastic invasiveness is related to reduced trophoblast apoptosis (31). This group also showed that increased Relaxin gene and protein expression was associated with significant antepartum bleeding in accreta patients.
CONCLUSION
The incidence of placenta accreta is rising with the rates as high as 1/533 pregnancies. Accretas are now the chief cause of postpartum hemorrhage and are a significant cause of both maternal and neonatal morbidity and occasionally mortality. The main risk factor for the development of placenta accreta is a history of a prior cesarean delivery. The prenatal diagnosis of accreta is crucial and is associated with a significant reduction in maternal blood loss and of post partum complications. Ultrasound findings suggestive of accreta include placental lacunae, myometrial thinning to less than 1 mm, the loss of a placental-uterine interface and an abnormal uterine bladder interface. The use of color and 3D Doppler ultrasound is also helpful in diagnosing placenta accreta. MRI may be helpful when the diagnosis is uncertain, if posterior invasion is concerned, or if percreta/invasion of other surrounding organs is suspected. The trend towards conservative and management of placenta accreta using embolization techniques, methotrexate and observation must be balanced with a significant rate of complications such as infection and DIC. Histologically, the absence of a maternal decidual layer allows placental trophoblastic tissue to invade into the maternal myometrium. Many peptides and growth factors have been examined in placenta accreta and there is likely a defect in the paracrine/autocrine feedback between the placental trophoblast and maternal decidua. The interaction of hormones such as Relaxin which is abundant in maternal decidua and INSL4, an insulin like peptide found in placental trophoblastic tissue may play role in the formation of placenta accreta.
Acknowledgments
Special thanks to NIH/NCRR (grant 5P20RR0242206), Gillian Bryant Greenwood PhD and the Institute of Biogenesis Research (University of Hawaii-Manoa) for their mentorship and grant support.
Footnotes
DECLARATION OF INTEREST
The authors report no declarations of interest.
Contributor Information
William Goh, Hawaii Permanente Medical Group, Department of Ob/Gyn, 3299 Moanalua Road, Honolulu, HI 96819.
Ivica Zalud, Chairman, Department of Ob/Gyn and Women’s Health, John A. Burns School of Medicine, University of Hawaii, 1319 Punahou Street #824, Honolulu, HI 96822.
References
- 1.Belfort M. Placenta accreta. Am J Obstet Gynecol. 2010;203(5):430–9. doi: 10.1016/j.ajog.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997 Jul;177(1):210–4. doi: 10.1016/s0002-9378(97)70463-0. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005 May;192(5):1458–61. doi: 10.1016/j.ajog.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 4.Silver RM, Landon MB, Rouse DJ, Leveno KJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006 Jun;107(6):1226–32. doi: 10.1097/01.AOG.0000219750.79480.84. [DOI] [PubMed] [Google Scholar]
- 5.Comstock C. Antenatal diagnosis of placenta accreta: a review. Ultrasound Obstet Gynecol. 2005;26:89–96. doi: 10.1002/uog.1926. [DOI] [PubMed] [Google Scholar]
- 6.Comstock C, Love J, Bronsteen R, Lee W, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190(4):1135–40. doi: 10.1016/j.ajog.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Lim Y, KIm H, Chang K, et al. Sonographic findings of placenta lacunae and the prediction of adherent placenta in women with placenta previa totalis and prior Cesarean section. Ultrasound Obstet Gynecol. 2006;28(2):178–82. doi: 10.1002/uog.2797. [DOI] [PubMed] [Google Scholar]
- 8.Twickler D, Lucas M, Balls A, Santos-Ramos R, et al. Color flow mapping for myometrial invasion in women with a prior cesarean delivery. J Matern Fetal Med. 2002;9(6):330–5. doi: 10.1002/1520-6661(200011/12)9:6<330::AID-MFM1002>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Lerner J, Deane S, Timor-Tritsch I. Characterization of placenta accreta using transvaginal sonography and color Ddoppler imaging. Ultrasound Obstet Gynecol. 1995;5:198–201. doi: 10.1046/j.1469-0705.1995.05030198.x. [DOI] [PubMed] [Google Scholar]
- 10.Rac M, Dashe J, Wells E, Moschos E, et al. Ultrasound predictors of placental invasion: the Placenta Accreta Index. Am J Obstet Gynecol. 2014;14 doi: 10.1016/j.ajog.2014.10.022. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 11.Cali G, Giambanco L, Puccio G, Forlani F. Morbidly adherent placenta: evaluation of ultrasound diagnostic criteria and differentiation of placenta accreta from percreta. Ultrasound Obstet Gynecol. 2013;41(4):406–12. doi: 10.1002/uog.12385. [DOI] [PubMed] [Google Scholar]
- 12.Chalubinski K, Pils S, Klein K, Seeman R, et al. Prenatal sonography can predict degree of placental invasion. Ultrasound Obstet Gynecol. 2013;42(5):518–24. doi: 10.1002/uog.12451. [DOI] [PubMed] [Google Scholar]
- 13.D’Antonio F, Iacovell C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42(5):509–17. doi: 10.1002/uog.13194. [DOI] [PubMed] [Google Scholar]
- 14.Lax A, Prince M, Mennitt K, Schwebach J, et al. The value of specific MRI features in the evaluation of suspected placental invasion. Magn Reson Imaging. 2007;25(1):87–93. doi: 10.1016/j.mri.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Warshak C, Eskander R, Hull A, Scioscia A, et al. Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of placenta accreta. Obstet Gynecol. 2006;108(3 Pt 1):573–81. doi: 10.1097/01.AOG.0000233155.62906.6d. [DOI] [PubMed] [Google Scholar]
- 16.Riteau A, Tassin M, Chambon G, Vaillant C, et al. Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of placenta accreta. PloS. 2014;9(4):e94866. doi: 10.1371/journal.pone.0094866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warshak CR, Ramos GA, Eskander R, Benirschke K, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol. 2010 Jan;115(1):65–9. doi: 10.1097/AOG.0b013e3181c4f12a. [DOI] [PubMed] [Google Scholar]
- 18.Eller AG, Porter TF, Soisson P, Silver RM. Optimal management strategies for placenta accreta. BJOG. 2009 Apr;116(5):648–54. doi: 10.1111/j.1471-0528.2008.02037.x. [DOI] [PubMed] [Google Scholar]
- 19.Warshak C, Ramos G, Eskander R, Benirschke K, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol. 2010;115(1):65–9. doi: 10.1097/AOG.0b013e3181c4f12a. [DOI] [PubMed] [Google Scholar]
- 20.Bowman Z, Eller A, Kennedy A, Richards D, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211(1):177.e1–7. doi: 10.1016/j.ajog.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Sentilhes L, Ambroselli C, Kayem G, Provansal M, et al. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol. 2010 Mar;115(3):526–34. doi: 10.1097/AOG.0b013e3181d066d4. [DOI] [PubMed] [Google Scholar]
- 22.Sentilhes L, Kayem G, Ambroselli C, Provansal M, et al. Fertility and pregnancy outcomes following conservative treatment for placenta accreta. Hum Reprod. 2010 Nov;25(11):2803–10. doi: 10.1093/humrep/deq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angstmann T, Gard G, Harrington T, Ward E, et al. Surgical management of placenta accreta: a cohort series and suggested approach. Am J Obstet Gynecol. 2010;202(1):38.e1–9. doi: 10.1016/j.ajog.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 24.Elgamy A, Abdelazid A, Ellaithy M. The use of cell salvage in women undergoing cesarean hysterectomy for abnormal placentation. Int J Obstet Anes. 2013;22:289–93. doi: 10.1016/j.ijoa.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Tam Tam K, Dozier J, Martin J. Approaches to reduce urinary tract injury during management of placenta accreta, increta, and percreta: a systematic review. J Matern Fetal Neonatal Med. 2012 Apr;25(4):329–34. doi: 10.3109/14767058.2011.576720. [DOI] [PubMed] [Google Scholar]
- 26.Craven C, Chedwick L, Ward K. Placental basal plate formation is associated with fibrin deposition in decidual veins at sites of trophoblastic cell invasion. Am J Obstet Gyneceol. 2002 Feb;186(2):291–6. doi: 10.1067/mob.2002.119717. [DOI] [PubMed] [Google Scholar]
- 27.Tseng J, Chou M. Differential expression of growth-, angiogenesis - and invasion - related factors in the development of placenta accreta. Taiwanese J Obstet Gynecol. 2006 Jun;45(2):100–5. doi: 10.1016/S1028-4559(09)60205-9. [DOI] [PubMed] [Google Scholar]
- 28.Laban M, Ibrahim E, Elsafty M, Hassanin A. Placenta accreta is associated with decreased natural killer (dNK) cells population: a comparative pilot study. Eur J Obstet Gynecol Reprod Bio. 2014;181:284–8. doi: 10.1016/j.ejogrb.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Bryant-Greenwood G, Yamamoto S, Lowndes K, et al. Human decidual relaxin and preterm birth. Ann N Y Acad Sci. 2005;1041:338–44. doi: 10.1196/annals.1282.054. [DOI] [PubMed] [Google Scholar]
- 30.Millar L, Streiner N, Webster L, et al. Early placental insulin-like protein (INSL4 or EPIL) in placental and fetal membrane growth. Biol Reprod. 2005;73:695–702. doi: 10.1095/biolreprod.105.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goh W, Yamamoto S, Thompson K, Bryant-Greenwood G. Relaxin, its receptor (RXFP1), and insulin-like peptide 4 expression through gestation and in placenta accreta. Reprod Sci. 2013;20(8):968–80. doi: 10.1177/1933719112472735. [DOI] [PMC free article] [PubMed] [Google Scholar]