Abstract
Thiazide diuretic (TZ) use is associated with higher bone mineral density, whereas loop diuretic (LD) use is associated with lower bone density and incident fracture. Dihydropyridine-sensitive calcium channels are expressed on parathyroid cells and may play a role in parathyroid hormone (PTH) regulation. The potential for diuretics and calcium-channel blockers (CCBs) to modulate PTH and calcium homeostasis may represent a mechanism by which they influence skeletal outcomes. We hypothesized that the use of LD and dihydropyridine CCBs is associated with higher PTH, and TZ use is associated with lower PTH. We conducted cross-sectional analyses of participants treated for hypertension in the Multi-Ethnic Study of Atherosclerosis who did not have primary hyperparathyroidism or chronic kidney disease (n =1888). We used adjusted regression models to evaluate the independent association between TZ, LD, and CCB medication classes and PTH. TZ use was associated with lower PTH when compared with non-TZ use (44.4 versus 46.9 pg/mL, p =0.02), whereas the use of LD and CCBs was associated with higher PTH when compared with non-users of each medication class (LD: 60.7 versus 45.5 pg/mL, p <0.0001; CCB: 49.5 versus. 44.4 pg/mL, p <0.0001). Adjusted regression models confirmed independent associations between TZ use and lower PTH (β =−3.2 pg/mL, p =0.0007), and LD or CCB use and higher PTH (LD: β =+12.0 pg/mL, p <0.0001; CCB: +3.7 pg/mL, p <0.0001). Among CCB users, the use of dihydropyridines was independently associated with higher PTH (β =+5.0 pg/mL, p <0.0001), whereas non-dihydropyridine use was not (β =+0.58 pg/mL, p =0.68). We conclude that in a large community-based cohort with normal kidney function, TZ use is associated with lower PTH, whereas LD and dihydropyridine CCB use is associated with higher PTH. These associations may provide a mechanistic explanation linking use of these medications to the development of skeletal outcomes.
Keywords: PARATHYROID HORMONE, PARATHYROID, HYPERTENSION, DIURETICS, CALCIUM-CHANNEL BLOCKERS, EPIDEMIOLOGY
Introduction
Parathyroid hormone (PTH) plays an important role in calcium and skeletal homeostasis. Prior studies have shown that having both hypertension and the use of antihypertensive medications may influence skeletal health and parathyroid function.(1–4) In this regard, loop diuretic use is associated with a decrease in bone mineral density(5) and increased fracture risk with long-term use.(6,7) In contrast, the use of thiazide diuretics is associated with more favorable skeletal outcomes, including a decrease in cortical bone loss,(8,9) increased bone mineral density (BMD), (10) and a reduction in fracture risk.(11,12) On the other hand, calcium-channel blockers have shown mixed effects on bone mineral density and fracture outcomes, likely because of the heterogeneous nature of the medication class, which is subdivided into dihydropyridine and non-dihydropyridine subclasses. For example, amlodipine (a dihydropyridine) use has been shown to preserve bone density in rats,(13) whereas in humans, chronic nifedipine use (a dihydropyridine) did not change bone metabolism in men.(14) The use of calcium-channel blockers has been associated with a reduced risk of fracture; however, it is the use of non-dihydropyridine calcium-channel blockers that appears to drive this observation.(15)
In those with chronic kidney disease (CKD), the use of loop diuretics has been associated with higher PTH levels,(16) whereas in individuals without CKD, loop diuretics have been associated with a higher risk of developing primary hyperparathyroidism.(1) Notably, human parathyroid cells express dihydropyridine-sensitive L-type calcium channels(17,18) involved in calcium signaling,(19) and calcium-channel blockers in general have been associated with inconsistent modulations of PTH in human interventional studies.(14,20–23)
Given how frequently these medications are used, and because the aforementioned associations may influence skeletal integrity and the risk for fracture, understanding how these antihypertensive medications may modulate PTH levels has public health relevance. We hypothesized that use of thiazide diuretics could lower PTH levels and use of loop diuretics and calcium-channel blockers (specifically the dihydropyridine class of calcium-channel blockers) could raise PTH levels. We tested these hypotheses by conducting a cross-sectional study to examine the independent association between each of these antihypertensive medication classes and PTH levels in a large community-based cohort of hypertensive participants without chronic kidney disease or apparent primary hyperparathyroidism.
Materials and Methods
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of 6814 community-living adults aged 45 to 84 years, which was established to study subclinical cardiovascular disease risk factors.(24) Subjects without cardiovascular disease were recruited from six centers across the US (New York, NY; Baltimore, MD; Forsyth County, NC; Chicago, IL; St. Paul, MN; and Los Angeles, CA). Institutional board review approved the study at all sites, and participants provided informed consent. Further details of the study have been described previously.(24)
For the current analysis, data from the baseline examination was used, which was conducted over a 24-month period from 2000 to 2002, and included demographic variables, laboratory data, and medication reporting. Of the 6814 participants, we restricted our analyses to only those using antihypertensive medications for the indication of hypertension because hypertension in itself is known to influence parathyroid function and is associated with higher PTH levels(1,2) (Supplemental Fig. S1). We excluded those with missing PTH values and those with potentially unrecognized primary hyperparathyroidism (Ca >10.2 mg/dL and PTH >65 pg/mL). To restrict our investigation to a population without secondary hyperparathyroidism due to chronic kidney disease, we also excluded those with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (Supplemental Fig. S1).
Assessment of demographic variables
All demographic, historical, and laboratory data used were collected at the baseline examination 1, during years 2000 to 2002. Questionnaires and standardized interviews administered by study coordinators were completed by all participants in order to obtain demographic, medical history, and medication use data.
Assessment of antihypertensive medication use
Antihypertensive medication use was determined by a medication inventory approach.(25) Participants were asked to bring all of their medication containers for medications used during the 2 weeks before the visit. The study interviewer recorded the name of each medication, the prescribed dose, and the frequency of administration directly from the containers. Participants were also asked about how many pills they had taken over the past 2 weeks on average per day/week/month. Antihypertensive medication use was categorized by drug class to include thiazide diuretics, loop diuretics, calcium-channel blockers, renin-angiotensin-aldosterone system inhibitors, beta-blockers, and alpha-blockers. Calcium-channel blockers were further subclassified into dihydropyridines (amlodipine or nifedipine) and non-dihydropyridines (verapamil or diltiazem).
Laboratory measurements
All serum and urine samples were collected after an overnight fast, in the morning before 10 a.m. and stored at −80°C before analysis. Intact serum PTH concentrations were measured at the University of Washington using the Beckman-Coulter Dx1 automated two-site immunoassay (Beckman-Coulter, Inc., Brea, CA, USA). Between-lot variations in PTH were adjusted using repeated measurements of aliquoted serum samples from 20 normal controls. The resulting interassay coefficient of variation was 6.1% at 30.1 pg/mL and 3.4% at 94.5 pg/mL, comparable to other validated PTH assays.(26) Annualized mean 25OHD concentrations were estimated from single 25OHD measurements as previously described.(27) Serum creatinine was used to estimate eGFR by the CKD-EPI equation.(28) Serum and urinary calcium, serum and urinary phosphorous, and FGF-23 were all measured as previously described.(29)
Statistical analysis
To evaluate the univariate associations between each medication and PTH, we first conducted t tests comparing mean PTH levels among users of each medication class (thiazides, loop diuretics, and calcium-channel blockers) to non-users of each medication class. Non-users of each medication class were by definition hypertensives using at least one or more other antihypertensive medication. We then used multivariable linear regression to examine the association between each individual medication class (thiazides, loop diuretics, and calcium-channel blockers) and PTH. Medication use was categorized as yes/no; therein, each categorical medication variable permitted comparison between users of that medication with all non-users of that medication that were using other antihypertensives. We adjusted for known and potential confounders of PTH in our regression models. Model A included adjustment for age, race, sex, body-mass index (BMI), eGFR, 25OHD, diabetes status (defined as normal, impaired fasting glucose, or diabetes based on the 2003 American Diabetes Association fasting criteria), smoking history (never, former, current), level of education (no schooling through grade 11, or completed high school/GED through some college completed, or technical school certificate through graduate/professional school degree), and physical activity (reported physical activity hours per week). Model B included adjustment for variables in model A plus systolic blood pressure and use of other antihypertensive medications (thiazides, loop diuretics, calcium-channel blockers, renin-angiotensin-aldosterone system inhibitors, beta-blockers, and alpha-blockers) in order to reduce confounding by other antihypertensive medication use. Model C was an exploratory model that included adjustment for variables in models A and B, as well as for biochemical parameters that may be responsible for influencing PTH: serum calcium, serum phosphate, urinary calcium-creatinine ratio, urinary phosphate-creatinine ratio, and serum fibroblast growth factor 23 (FGF-23). We considered model C to be exploratory because many of the biochemical parameters included as confounders in this model may actually be physiologic intermediates in the relationship between the antihypertensive medication classes of interest and PTH levels.
We used logistic regression to assess the odds of having a PTH level greater than the upper limit of the reference range (>65 pg/mL) for each antihypertensive medication class. In addition, we performed adjusted linear regression analyses after assigning treated hypertensives who used neither thiazides, loop diuretics, nor calcium-channel blockers (ie, users of RAAS inhibitors, beta-blockers, and/or alpha-blockers only) as the collective reference group. This referent group was chosen in order to interpret the magnitude of association effect between our antihypertensive classes of interest against a single and constant referent category. This model was adjusted for age, race, sex, BMI, eGFR, 25OHD, diabetes status, systolic blood pressure, education level, cigarette smoking, activity level, serum calcium, serum phosphate, urinary calcium-creatinine ratio, urinary phosphate-creatinine ratio, and serum FGF-23. We further restricted this analyses to participants using only a single antihypertensive medication (n =1167), given the concern for confounding by use of multiple medications in the cohort.
SAS version 9.3 (SAS Institute, Cary, NC, USA) statistical software was used for all analyses. A two-tailed p value <0.05 was used to indicate statistical significance.
Results
Study population
The mean age of the study population was 64.1 years, with 54.5% being women, and with a mean BMI of 29.7 kg/m2 (Table 1). The mean PTH concentration was 46.1 pg/mL (standard deviation [SD] 19.3 pg/mL), mean 25OHD of 24.6 ng/mL (SD 12.2 ng/mL), and the mean eGFR was 79.9 mL/min/1.73 m2 (SD 13.0 mL/min/1.73m2). The majority of the study population (61.8%) used only a single antihypertensive medication (Table 1).
Table 1.
Demographic and Biochemical Characteristics of the Study Population
| Characteristic | N =1888 |
|---|---|
| Age (years) | 64.1 (9.2) |
| Female (no. [%]) | 1029 (54.5) |
| Race/ethnicity (no. [%]) | |
| White | 616 (32.6) |
| Chinese-American | 170 (9.0) |
| African-American | 731 (38.7) |
| Hispanic | 371 (19.7) |
| BMI (kg/m2) | 29.7 (5.7) |
| Systolic blood pressure (mmHg) | 133.8 (21.4) |
| Diastolic blood pressure (mmHg) | 73.9 (10.5) |
| Antihypertensive medications (no. [%]) | |
| Thiazide | 518 (27.4) |
| Loop diuretic | 82 (4.3) |
| Calcium-channel blocker | 637 (33.7) |
| Dihydropyridine CCB | 448 (23.7) |
| Non-dihydropyridine CCB | 189 (10) |
| Beta-blocker | 472 (25) |
| RAAS-inhibitor | 891 (47.2) |
| Alpha-blocker | 194 (10.3) |
| No. of antihypertensive medications | |
| 1 | 1167 (61.8) |
| 2 | 523 (27.7) |
| 3 | 158 (8.4) |
| 3+ | 26 (1.4) |
| Education (highest level completed) (no. [%]) | |
| No schooling to some high school | 353 (18.8) |
| Completed high school to some college | 690 (36.7) |
| Completed post-secondary school degree | 838 (44.6) |
| Smoking status (no. [%]) | |
| Never smoker | 925 (49.2) |
| Former | 741 (39.4) |
| Current | 215 (11.4) |
| Physical activity (mean hr/d) | 12.4 (6.1) |
| Diabetes status (no. [%]) | |
| Normal | 1178 (32.4) |
| Impaired fasting glucose | 308 (16.3) |
| Diabetes | 402 (21.3) |
| PTH (pg/mL) | 46.1 (19.3) |
| eGFR (mL/min/1.73m2) | 79.9 (13.0) |
| Serum calcium (mg/dL) | 9.65 (0.38) |
| Urine calcium/Cr ratio (mg/g) | 0.09 (0.07) |
| Serum phosphate (mg/dL) | 3.6 (0.5) |
| Urine phosphate/Cr ratio (mg/g) | 0.48 (0.2) |
| Annualized 25OHD (ng/mL) | 24.6 (11.2) |
| FGF23 (pg/mL) | 40.4 (18.9) |
Values are mean (SD) unless otherwise specified.
BMI =body mass index; CCB =calcium-channel blocker; PTH =parathyroid hormone; eGFR =estimated glomerular filtration rate; Cr =creatinine; 25OHD =25-hydroxyvitamin D; FGF-23 =fibroblast growth factor 23.
Mean PTH levels with diuretic and calcium-channel blocker use
There were 518 participants who used thiazide diuretics, 82 participants who used loop diuretics, and 637 participants who used calcium-channel blockers. Among calcium-channel blocker (CCB) users, 448 participants used dihydropyridines and 189 used non-dihydropyridines (Table 2). Participants using thiazide diuretics had lower PTH levels, higher serum calcium levels, and a lower urine calcium/creatinine ratio, when compared with non-users of thiazides (Table 2). Loop-diuretic users had higher PTH levels, as well as lower serum calcium and higher urine calcium/creatinine ratio, when compared with non-users of loop diuretics (Table 2). The use of any calcium-channel blocker was associated with higher PTH levels and a higher urine calcium/creatinine ratio when compared with non-users of calcium-channel blockers. However, this difference appeared to be driven by the subclass of calcium-channel blocker used. Users of dihydropyridines had a higher PTH, lower serum calcium, and higher urinary calcium/creatinine ratio, whereas the use of non-dihydropyridines was not associated with any difference in PTH when compared with non-users. The percent of participants using each medication class who had a PTH value greater than the upper limit of the reference range paralleled the aforementioned trends (Table 2).
Table 2.
Relevant Characteristics by Individual Antihypertensive Class
| Characteristic | Thiazide
|
Loop diuretic
|
CCB
|
Dihydropyridine-CCB
|
Non-dihydropyridine-CCB
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Y (n =518) | N (n =1370) | Y (n =82) | N (n = 1806) | Y (n =637) | N (n =1251) | Y (n =448) | N (n =1440) | Y (n =189) | N (n =1699) | |
| Age (years) | 63.8 (9.3) | 64.3 (9.2) | 65.0 (8.4) | 64.1 (9.2) | 64.6 (9.0) | 63.9 (9.3) | 64.5 (9.1) | 64.0 (9.3) | 64.9 (9.0) | 64.0 (9.2) |
| Female (no. [%]) | 330 (63.7)*** | 699 (51.0) | 57 (69.5)** | 972 (53.8) | 357 (56.0) | 672 (53.7) | 238 (53.1) | 791 (54.9) | 119 (63.0)* | 910 (53.6) |
| Ethnicity (no. [%]) | ||||||||||
| White | 173 (33.4) | 443 (32.3) | 27 (32.9) | 589 (32.6) | 145 (22.8)*** | 471 (37.7) | 94 (21.0)*** | 522 (36.3) | 51 (27.0) | 565 (33.3) |
| Chinese-American | 17 (3.3)*** | 153 (11.2) | 2 (2.4)* | 168 (9.3) | 58 (9.1) | 112 (9.0) | 50 (11.2) | 120 (8.3) | 8 (4.2)* | 162 (9.5) |
| African-American | 260 (50.2)*** | 471 (34.4) | 35 (42.7) | 696 (38.5) | 314 (49.3)*** | 417 (33.3) | 217 (48.4)*** | 514 (35.7) | 97 (51.3)*** | 634 (37.3) |
| Hispanic | 68 (13.1)*** | 303(22.1) | 18 (22.0) | 353 (19.6) | 120 (18.8) | 251 (20.1) | 87 (19.4) | 284 (19.7) | 33 (17.5) | 338 (19.9) |
| BMI (kg/m2) | 30.7 (5.9)*** | 29.4 (5.6) | 34.5 (7.6)*** | 29.5 (5.5) | 29.9 (5.8) | 29.6 (5.6) | 29.8 (5.8) | 29.7 (5.7) | 30.3 (5.9) | 29.7 (5.7) |
| Systolic blood pressure (mmHg) | 133.0 (18.7) | 134.2 (22.4) | 132.8 (22.6) | 133.9 (21.4) | 135.4 (19.3)* | 133.0 (22.4) | 134.6 (19.8) | 133.6 (21.9) | 137.5 (17.9)* | 133.4 (21.8) |
| Diastolic blood pressure (mmHg) | 73.1 (10.2) | 74.2 (10.6) | 68.9 (10.3) | 74.1 (10.4) | 74.6 (9.7) | 73.5 (10.8) | 74.9 (9.7) | 73.6 (10.7) | 74.2 (9.7) | 73.9 (10.6) |
| PTH (pg/mL) | 44.4 (18.5)* | 46.8 (19.5) | 60.7 (22.4)*** | 45.5 (18.9) | 49.5 (20.9)*** | 44.4 (18.2) | 50.4 (20.8)*** | 44.8 (18.6) | 47.5 (21.3) | 46.0 (19.1) |
| PTH >65 pg/mL (%) | 10.8 | 13.9 | 29.3*** | 12.3 | 17.6*** | 10.7 | 19.4*** | 11.0 | 13.2 | 13.0 |
| eGFR (mL/min/1.73m2) | 79.0 (12.3)* | 80.3 (13.3) | 80.1 (12.3) | 79.9 (13.0) | 81.3 (13.1)** | 79.3 (12.9) | 81.6 (13.4)** | 79.4 (12.8) | 80.6 (12.1) | 79.9 (13.1) |
| Serum calcium, (mg/dL) | 9.7 (0.4)** | 9.6 (0.4) | 9.6 (0.36)* | 9.7 (0.38) | 9.6 (0.4) | 9.7 (0.4) | 9.6 (0.4) | 9.7 (0.4) | 9.6 (0.4) | 9.7 (0.4) |
| Urine calcium/Cr ratio (mg/g) | 0.07 (0.08)*** | 0.10 (0.06) | 0.13 (0.1)*** | 0.09 (0.07) | 0.11 (0.1)** | 0.09 (0.07) | 0.11 (0.1)*** | 0.09 (0.07) | 0.11 (0.08)* | 0.09 (0.07) |
| Serum phosphate (mg/dL) | 3.7 (0.5)* | 3.6 (0.5) | 3.7 (0.5) | 3.6 (0.5) | 3.6 (0.5)** | 3.7 (0.5) | 3.6 (0.5)* | 3.7 (0.5) | 3.6 (0.5) | 3.7 (0.5) |
| Urine phosphate/Cr ratio (mg/g) | 0.47 (0.5) | 0.48 (0.5) | 0.54 (0.2)** | 0.48 (0.2) | 0.47 (0.2) | 0.48 (0.2) | 0.49 (0.2) | 0.48 (0.2) | 0.43 (0.2)** | 0.48 (0.2) |
| Annualized 25(OH)D (ng/mL) | 23.3 (10.0)** | 25.1 (11.6) | 22.0 (10.6)* | 24.7 (11.2) | 24.0 (13.1) | 24.9 (10.1) | 24.6 (14.0) | 24.6 (10.1) | 22.7 (10.4)* | 24.8 (11.3) |
| FGF23 (pg/dL) | 41.3 (14.5) | 40.0 (20.3) | 41.5 (12.2) | 40.3 (19.1) | 40.0 (25.4) | 40.6 (14.4) | 38.9 (13.2) | 40.8 (20.3) | 42.7 (42.0) | 40.12 (14.1) |
Values are mean (SD) unless otherwise specified.
Y =user of medication in question; N =non-user of medication in question; CCB =calcium-channel blocker; BMI =body mass index; PTH =parathyroid hormone; eGFR =estimated glomerular filtration rate; Cr =creatinine; 25(OH)D, 25hydroxyvitamin D; FGF-23 =fibroblast growth factor 23.
p <0.05
p <0.01
p <0.001.
Independent associations between PTH and use of diuretics and calcium-channel blockers
The independent association between diuretic and calcium-channel blocker medications and PTH concentrations were evaluated using adjusted regression models. Thiazide diuretics were associated with lower PTH levels (−3.2 pg/mL [−5.1, −1.4], p =0.0007), whereas loop diuretics (+12.0 pg/mL [8.1, 16.0], p <0.0001) and calcium-channel blockers (+3.7 pg/mL [1.9, 5.4], p <0.0001) were associated with higher PTH levels (Table 3). The effect estimates of these relationships remained stable and significant after adjustment for variables in models A and B, which included known and potential confounders including demographic variables, systolic blood pressure, kidney function and vitamin D levels, and use of other antihypertensive medications (Table 3). Further adjustment with biochemical measurements known to influence PTH that are changed by diuretics in model C did not appreciably change the effect sizes (Table 3).
Table 3.
The Association (With 95% Confidence Intervals) Between Antihypertensive Medication Class and PTH Level
| Model | Thiazide
|
Loop Diuretic
|
CCB
|
|||
|---|---|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | |
| Univariate | −2.3 (−4.3, −0.4) | 0.02 | +15.2 (11.0, 19.4) | <0.0001 | +5.1 (3.3, 7.0) | <0.0001 |
| Multivariate A | −4.2 (−6.1, −2.3) | <0.0001 | +12.2 (8.1, 16.3) | <0.0001 | +4.3 (2.6, 6.1) | <0.0001 |
| Multivariate B | −3.3 (−5.2, −1.4) | 0.0007 | +11.4 (7.3, 15.4) | <0.0001 | +3.7 (1.9, 5.5) | <0.0001 |
| Multivariate C | −3.2 (−5.1, −1.4) | 0.0007 | +12.0 (8.1, 16.0) | <0.0001 | +3.7 (1.9, 5.4) | <0.0001 |
PTH =parathyroid hormone; CCB =calcium-channel blocker.
Units for β are pg/mL of PTH per use of antihypertensive drug.
Multivariate A: adjusted for age, ethnicity, sex, body mass index (BMI), vitamin D25OH, estimated glomerular filtration rate (eGFR), diabetes status, education level, cigarette smoking, and physical activity.
Multivariate B: adjusted for age, ethnicity, sex, BMI, vitamin D25OH, eGFR, systolic blood pressure, diabetes status, education level, cigarette smoking, physical activity, and other antihypertensive medication use (HCTZ: adjusted for use of loop diuretics, calcium-channel blockers, RAAS-inhibitor, beta-blocker, and alpha-blocker use; loop diuretics: adjusted for use of thiazide, calcium-channel blockers, RAAS-inhibitor, beta-blocker, and alpha-blocker use; CCB: adjusted for use of thiazide, loop diuretics, RAAS-inhibitor, beta-blocker, and alpha-blocker use).
Multivariate C: adjusted for age, ethnicity, sex, BMI, vitamin D25OH, eGFR, systolic blood pressure, diabetes status, education level, cigarette smoking, physical activity, other antihypertensive medication class use, serum calcium, serum phosphate, urinary calcium/cr, urinary phosphate/cr, and FGF-23.
PTH and the use of specific calcium-channel blocker subclasses
We repeated our regression models after subclassifying calcium channel blockers as dihydropyridines or non-dihydropyridines. Adjusted models revealed a positive and significant association between use of dihydropyridine calcium-channel blockers and PTH (+5.0 pg/mL [3.0, 6.9], p <0.0001), whereas non-dihydropyridine use was not associated with any significant difference in PTH (Table 4).
Table 4.
The Association (With 95% Confidence Intervals) Between Calcium-Channel Blocker Subclasses and PTH Level
| Model | Dihydropyridine-CCB
|
Non-dihydropyridine-CCB
|
||
|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | |
| Univariate | +5.6 (3.6, 7.6) | <0.0001 | +1.49 (−1.4, 4.4) | 0.31 |
| Multivariate A | +5.6 (3.6, 7.5) | <0.0001 | −0.54 (−3.3, 2.2) | 0.50 |
| Multivariate B | +5.2 (3.2, 7.2) | <0.0001 | +0.27 (−2.5, 3.1) | 0.85 |
| Multivariate C | +5.0 (3.0, 6.9) | <0.0001 | +0.58 (−2.1, 3.3) | 0.68 |
PTH =parathyroid hormone; CCB =calcium-channel blocker.
Units for β are pg/mL of PTH per use of antihypertensive drug.
Multivariate A: adjusted for age, ethnicity, sex, body mass index (BMI), vitamin D25OH, estimated glomerular filtration rate (eGFR), diabetes status, education level, cigarette smoking, and physical activity.
Multivariate B: adjusted for age, ethnicity, sex, BMI, vitamin D25OH, eGFR, systolic blood pressure, diabetes status, education level, cigarette smoking, physical activity, and other antihypertensive medication class use (dihydropyridine-CCB: adjusted for use of thiazide, loop diuretic, non-dihydropyridine-CCB, RAAS-inhibitor, beta-blocker, and alpha-blocker; non-dihydropyridine-CCB: adjusted for use of thiazide, loop diuretic, dihydropyridine-CCB, RAAS-inhibitor, beta-blocker, and alpha-blocker).
Multivariate C: adjusted for age, ethnicity, sex, BMI, vitamin D25OH, eGFR, systolic blood pressure, diabetes status, education level, cigarette smoking, physical activity, other antihypertensive medication class use, serum calcium, serum phosphate, urinary calcium/cr, urinary phosphate/creatinine, and FGF-23.
Medication classes and the odds of having a PTH value > 65 pg/mL
We used logistic regression to evaluate the odds of having a PTH level greater than the upper limit of the reference range (>65 pg/mL) for diuretics and calcium-channel blockers. Thiazide use was associated with having a PTH within the reference range, whereas the use of loop diuretics and dihydropyridine calcium-channel blockers was associated with higher odds for having a PTH greater than the reference range (Supplemental Table S1).
Non-users of diuretics and calcium-channel blockers as the reference group
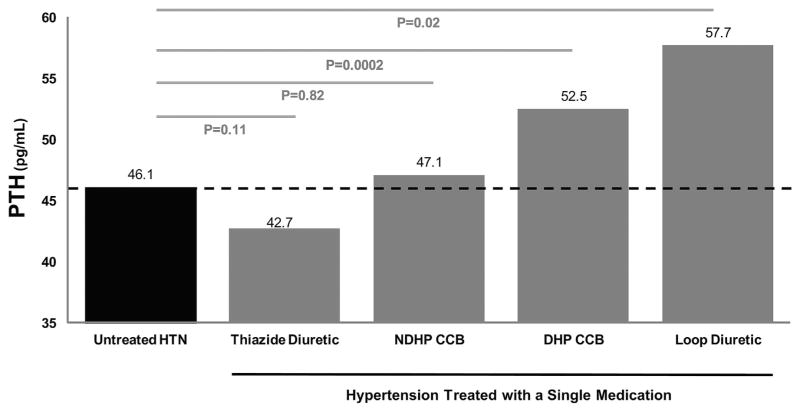
To compare the effect estimates of diuretic and calcium-channel blocker use to a uniform reference group, we conducted additional regression models after assigning non-users of thiazides, loop diuretics, and calcium-channel blockers as the reference category. We again observed the same pattern; both loop-diuretic and dihydropyridine CCB use were associated with higher PTH levels, whereas thiazide use was associated with lower PTH levels. Non-dihydropyridine CCB use was not associated with a significant difference in PTH levels (Supplemental Table S2). We further restricted this analyses to those using only a single antihypertensive medication (n =1167) to ensure the multivariable regression results were not confounded by use of multiple antihypertensive medications. In this smaller and restricted analysis, we observed a relative recapitulation of our findings in the total study population (Supplemental Table S2). Comparisons between users of these medications and untreated hypertensives are presented graphically in Fig. 1 and demonstrate a similar trend found in above analyses.
Fig. 1.
Mean PTH concentrations among hypertensive participants taking only one single antihypertensive medication (gray bars), compared with untreated hypertensives (black bar). PTH =parathyroid hormone; HTN =hypertension; NDHP =non-dihydropyridine; CCB =calcium-channel blocker; DHP =dihydropyridine.
Discussion
Antihypertensive medication use has been associated with skeletal and parathyroid outcomes, including changes in BMD, risk for fracture, and risk for developing primary hyperparathyroidism; however, the mechanisms between these exposures and outcomes have not been well investigated. We hypothesized that individual antihypertensive medication classes may modulate PTH and that the direction of these effects may provide some insight into prior links between antihypertensive medication use and skeletal outcomes. Herein, we conducted a cross-sectional study in a large community-living sample of individuals without renal disease or overt primary hyperparathyroidism and observed that the use of loop diuretics and dihydropyridine calcium-channel blockers was associated with higher PTH levels (approximately +12 pg/mL and +5 pg/mL, respectively), and the use of a thiazide diuretic was associated with lower PTH levels (approximately −3 pg/mL), when compared with participants not using these medications. These observations were independent of many potential confounders and remained consistent in more stringent subanalyses.
Many prior studies have shown links between diuretics and skeletal outcomes. Loop diuretics are associated with decreases in BMD(6) and with an increased risk for fracture.(7) An indication that the link between loop diuretics and these skeletal events may be mediated by PTH is suggested in prior prospective studies that showed the use of loop diuretics associated with an increased risk for developing primary hyperparathyroidism.(1) Loop diuretics can indirectly stimulate PTH secretion by increasing calciuria (potentially inducing a negative calcium balance), which may cause chronic parathyroid stimulation. Indeed, the maintenance of a very low calcium intake has been shown to increase the risk of primary hyperparathyroidism.(30) However, a recent study suggests that loop diuretics may also be capable of directly stimulating PTH secretion, independent from calciuric effects, via loop diuretic-responsive NKCC1 receptors in parathyroid tissue.(31) Our observation of higher PTH levels with loop diuretics is echoed by studies in cohorts with chronic kidney disease who have higher PTH levels(16) and more recently in a cohort without kidney disease.(32) Thiazides decrease calciuria (potentially inducing positive calcium balance) and have been shown to increase bone density and are associated with a lower risk for fracture.(33) The few interventional studies that evaluated PTH levels have shown a trend toward lower PTH levels;(8,10,33) however, these may have been underpowered to find a statistically significant effect. In contrast, our study, with a large sample size of thiazide users among users of other antihypertensives, was able to detect a significantly lower PTH.
Few studies have evaluated whether calcium-channel blockers have any impact on skeletal health; however, studies have suggested that they may associate with a decreased rate of fracture(15) and that L-type voltage gated calcium channels are expressed on parathyroid tissue.(18,19) To our knowledge, there are no randomized studies evaluating the effect of calcium-channel blockers on skeletal or parathyroid outcomes. Small interventional studies evaluating for a possible calcimimetic effect of calcium-channel blockers have yielded inconsistent results; some studies have shown that CCBs (diltiazem,(20) nifedipine(34)) may lower PTH, raise PTH (diltiazem,(23) nifedipine(22)), or have no effect on PTH (diltiazem,(21) nicardipine,(35) nifedipine(14)). Many of these studies focused on diltiazem (a non-dihydropyridine calcium-channel blocker), as well as older, less commonly prescribed dihydropyridine calcium-channel blockers such as nifedipine and nicardipine. Our analyses included amlodipine, a relatively newer and more widely prescribed dihydropyridine calcium-channel blocker for essential hypertension. We found a significant disparity between the association of dihydropyridine and non-dihydropyridine calcium-channel blockers with PTH; dihydropyridine calcium-channel blocker use was associated with a significantly higher PTH than non-users, whereas non-dihydropyridine use did not display any association with PTH concentrations. Our findings also demonstrate that dihydropyridine calcium-channel blocker use was associated with significantly higher urinary calcium excretion, similar to loop-diuretic use, whereas non-dihydropyridine calcium-channel blocker use was not associated with significant differences in calciuria.
Although our analyses was not specifically designed to investigate how individual medications could cause these associations with PTH, there are at least three potential mechanisms to consider as possible explanations for our results: 1) increasing or decreasing calciuria leading to compensatory changes in PTH; 2) a direct stimulation of the parathyroid glands; and/or 3) volume depletion with loop diuretics resulting in enhanced renin-angiotensin-aldosterone system activity, which can increase PTH secretion. Renal calcium handling is a noteworthy consideration because we observed that loop diuretic users had significantly lower serum calcium and higher urinary calcium-creatinine ratio, and the inverse finding with thiazide use. The accompanying higher and lower PTH levels may, therefore, be a consequence of these renal effects. Our observation that dihydropyridine, but not non-dihydropyridine, calcium-channel blockers displayed a pattern of serum and urinary calcium as well as PTH levels similar to loop diuretics is unique. Dihydropyridines do not have a well-described hypercalciuric effect but may in fact be inducing a mild hypercalciuria that induces a secondary PTH hypersecretion. In this regard, future studies should consider whether specifically dihydropyridine calcium-channel blockers influence renal calcium handling and subsequent skeletal outcomes. Some studies have provided support for a direct stimulatory effect on parathyroid tissue by antihypertensive agents. Even after adjustment for serum and urinary calcium and phosphorous, the association of antihypertensive medications with PTH did not appreciably change. It may be that one-time measurement of these electrolytes did not fully capture time-averaged levels and, therefore, did not fully attenuate the components of the pathway leading to changes in PTH through calcium and phosphate. However, this finding also raises the possibility of mechanisms independent from a compensatory PTH response reactive to electrolyte changes. Recent studies have shown that dihydropyridine L-type calcium channels, loop-diuretic-responsive NKCC1 receptors, and angiotensin type 1 and mineralocorticoid receptors are all present in parathyroid tissue.(19,31,36)
Strengths and limitations
Our study had a large sample size, with comprehensive and systematically collected demographic and laboratory variables. We were able to recapitulate our findings even after restricting the study population to hypertensive participants using only a single antihypertensive medication, thereby reducing confounding attributable to multiple medication use. Further, all of our findings were robust and stable after extensive multivariable adjustments. Our study also has important limitations. Given the cross-sectional nature of this study, we cannot comment on temporal relationships between antihypertensive medication use and PTH. The cumulative and chronic duration of medication use was not assessed; participants were only confirmed to have taken the antihypertensive medications for 2 weeks before the assessment. Because we restricted our study population to hypertensive participants without CKD, the number of participants using loop diuretics was relatively low (n =82); however, the statistical significance of our findings remained robust even with this limitation. We lacked information regarding dietary calcium and vitamin D supplementation, which are known to influence PTH; however, given that all laboratory collection was performed while fasting, the acute effect of calcium intake on PTH is expected to be minimal. All measurements were made at one point in time, thus we do not have the ability to determine diurnal changes or changes over time within individuals. In addition, we did not have measures of ionized calcium, and the serum calcium levels used in our models were not corrected for serum albumin.
Lastly, we acknowledge that the reference group in our main analyses differed by each antihypertensive medication class; this makes comparison of the magnitude of effect estimates between classes challenging. However, we showed that the directionality and relative magnitude of our effect estimates remained consistent when we conducted an analyses whereby we assigned a consistent reference group of all non-users of diuretics and CCBs (reference group used only RAAS inhibitors, beta-blockers, and alpha-blockers).
In summary, we observed that the use of loop diuretics and dihydropyridine calcium-channel blockers was independently associated with higher PTH levels and that use of thiazide diuretics was independently associated with lower PTH levels. Whether these findings may explain prior observations of skeletal outcomes associated with the use of these antihypertensive medications and implicate PTH regulation as a possible mechanism for these observations warrants further study. Future studies to evaluate whether dihydropyridine calcium-channel blockers increase calciuria and negatively impact skeletal health may provide further insights. How these observations could impact the selection of antihypertensive medications in patients at higher risk for adverse skeletal and parathyroid outcomes also warrants further study.
Supplementary Material
Acknowledgments
The authors thank the investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institute of Health under award numbers R01 HL096875, N01 HC95159, N01 HC95166, N01 HC95169, R01 HL071739, R01 HL072403, and T32 HL00760929 (SZ). AV was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL111771, by the National Institutes of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under award number R01 DK107407, and by grant 2015085 from the Doris Duke Charitable Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Authors’ roles: SZ, AV, and JB developed the study concept and design, performed the statistical analyses, drafted the manuscript, and take responsibility for the data integrity. IB, MA, BP, CR, JI, BK, and DS participated in the study design, reviewed and interpreted the data, and drafted the manuscript.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Vaidya A, Curhan GC, Paik JM, Kronenberg H, Taylor EN. Hypertension, antihypertensive medications, and risk of incident primary hyperparathyroidism. J Clin Endocrinol Metab. 2015 Jun;100( 6):2396–404. doi: 10.1210/jc.2015-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown J, de Boer IH, Robinson-Cohen C, et al. Aldosterone, parathyroid hormone, and the use of renin-angiotensin-aldosterone system inhibitors: the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2015 Feb;100(2):490–9. doi: 10.1210/jc.2014-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brickman AS, Nyby MD, von Hungen K, Eggena P, Tuck ML. Calcitropic hormones, platelet calcium, and blood pressure in essential hypertension. Hypertension. 1990 Nov;16(5):515–22. doi: 10.1161/01.hyp.16.5.515. [DOI] [PubMed] [Google Scholar]
- 4.van Ballegooijen AJ, Kestenbaum B, Sachs MC, et al. Association of 25-hydroxyvitamin D and parathyroid hormone with incident hypertension: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014 Apr 1;63(12):1214–22. doi: 10.1016/j.jacc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim LS, Fink HA, Blackwell T, Taylor BC, Ensrud KE. Loop diuretic use and rates of hip bone loss and risk of falls and fractures in older women. J Am Geriatr Soc. 2009 May;57(5):855–62. doi: 10.1111/j.1532-5415.2009.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Loop diuretics increase bone turnover and decrease BMD in osteopenic postmenopausal women: results from a randomized controlled study with bumetanide. J Bone Miner Res. 2006 Jan;21( 1):163–70. doi: 10.1359/JBMR.051003. [DOI] [PubMed] [Google Scholar]
- 7.Carbone LD, Johnson KC, Bush AJ, et al. Loop diuretic use and fracture in postmenopausal women: findings from the Women’s Health Initiative. Arch Int Med. 2009 Jan 26;169(2):132–40. doi: 10.1001/archinternmed.2008.526. [DOI] [PubMed] [Google Scholar]
- 8.Reid IR, Ames RW, Orr-Walker BJ, et al. Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: a randomized controlled trial. Am J Med. 2000 Oct 1;109(5):362–70. doi: 10.1016/s0002-9343(00)00510-6. [DOI] [PubMed] [Google Scholar]
- 9.Wasnich R, Davis J, Ross P, Vogel J. Effect of thiazide on rates of bone mineral loss: a longitudinal study. BMJ. 1990 Dec 8;301( 6764):1303–5. doi: 10.1136/bmj.301.6764.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE. Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000 Oct 3;133(7):516–26. doi: 10.7326/0003-4819-133-7-200010030-00010. [DOI] [PubMed] [Google Scholar]
- 11.Feskanich D, Willett WC, Stampfer MJ, Colditz GA. A prospective study of thiazide use and fractures in women. Osteoporos Int. 1997;7(1):79–84. doi: 10.1007/BF01623465. [DOI] [PubMed] [Google Scholar]
- 12.Aung K, Htay T. Thiazide diuretics and the risk of hip fracture. Cochrane Database Syst Rev. 2011;(10):CD005185. doi: 10.1002/14651858.CD005185.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Ushijima K, Liu Y, Maekawa T, et al. Protective effect of amlodipine against osteoporosis in stroke-prone spontaneously hypertensive rats. Eur J Pharmacol. 2010 Jun 10;635(1–3):227–30. doi: 10.1016/j.ejphar.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 14.Albers MM, Johnson W, Vivian V, Jackson RD. Chronic use of the calcium channel blocker nifedipine has no significant effect on bone metabolism in men. Bone. 1991;12(1):39–42. doi: 10.1016/8756-3282(91)90053-l. [DOI] [PubMed] [Google Scholar]
- 15.Rejnmark L, Vestergaard P, Mosekilde L. Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case-control study. J Hypertens. 2006 Mar;24(3):581–9. doi: 10.1097/01.hjh.0000203845.26690.cb. [DOI] [PubMed] [Google Scholar]
- 16.Isakova T, Anderson CA, Leonard MB, et al. Diuretics, calciuria and secondary hyperparathyroidism in the Chronic Renal Insufficiency Cohort. Nephrol Dial Transplant. 2011 Apr;26(4):1258–65. doi: 10.1093/ndt/gfr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang W, Pratt SA, Chen TH, et al. Parathyroid cells express dihydropyridine-sensitive cation currents and L-type calcium channel subunits. Am J Physiol Endocrinol Metab. 2001 Jul;281(1):E180–9. doi: 10.1152/ajpendo.2001.281.1.E180. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama K, Matsuba D, Adachi-Akahane S, et al. Dihydropyridine-and voltage-sensitive Ca2+ entry in human parathyroid cells. Exp Physiol. 2009 Jul;94(7):847–55. doi: 10.1113/expphysiol.2009.046813. [DOI] [PubMed] [Google Scholar]
- 19.Iida R, Yokoyama K, Ohkido I, et al. Detection of dihydropyridine- and voltage-sensitive intracellular Ca(2+) signals in normal human parathyroid cells. J Physiol Sci. 2013 Jul;63(4):235–40. doi: 10.1007/s12576-013-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seely EW, LeBoff MS, Brown EM, et al. The calcium channel blocker diltiazem lowers serum parathyroid hormone levels in vivo and in vitro. J Clin Endocrinol Metab. 1989 Jun;68(6):1007–12. doi: 10.1210/jcem-68-6-1007. [DOI] [PubMed] [Google Scholar]
- 21.Townsend R, DiPette DJ, Evans RR, et al. Effects of calcium channel blockade on calcium homeostasis in mild to moderate essential hypertension. Am J Med Sci. 1990 Sep;300(3):133–7. doi: 10.1097/00000441-199009000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Wynne AG, Romanski SA, Klee GG, Ory SJ, O’Fallon WM, Fitzpatrick LA. Nifedipine, but not verapamil, acutely elevates parathyroid hormone levels in premenopausal women. Clin Endocrinol. 1995 Jan;42(1):9–15. doi: 10.1111/j.1365-2265.1995.tb02592.x. [DOI] [PubMed] [Google Scholar]
- 23.Villiger L, Casez JP, Takkinen R, Jaeger P. Diltiazem stimulates parathyroid hormone secretion in vivo whereas felodipine does not. J Clin Endocrinol Metab. 1993 Apr;76(4):890–4. doi: 10.1210/jcem.76.4.8473401. [DOI] [PubMed] [Google Scholar]
- 24.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002 Nov 1;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 25.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992 Jun;45(6):683–92. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 26.Cavalier E, Delanaye P, Vranken L, et al. Interpretation of serum PTH concentrations with different kits in dialysis patients according to the KDIGO guidelines: importance of the reference (normal) values. Nephrol Dial Transplant. 2012 May;27(5):1950–6. doi: 10.1093/ndt/gfr535. [DOI] [PubMed] [Google Scholar]
- 27.Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013 Jun;97( 6):1243–51. doi: 10.3945/ajcn.112.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Int Med. 2009 May 5;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bansal N, Katz R, de Boer IH, et al. Influence of estrogen therapy on calcium, phosphorus, and other regulatory hormones in postmenopausal women: the MESA study. J Clin Endocrinol Metab. 2013 Dec;98(12):4890–8. doi: 10.1210/jc.2013-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paik JM, Curhan GC, Taylor EN. Calcium intake and risk of primary hyperparathyroidism in women: prospective cohort study. BMJ. 2012;345:e6390. doi: 10.1136/bmj.e6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller ME, Forni Ogna V, Maillard M, et al. Furosemide stimulation of parathormone in humans: role of the calcium-sensing receptor and the renin-angiotensin system. Pflugers Arch. 2015 Jun 20; doi: 10.1007/s00424-015-1714-4. [DOI] [PubMed] [Google Scholar]
- 32.Corapi KM, McMahon GM, Wenger JB, Seifter JL, Bhan I. Association of loop diuretic use with higher parathyroid hormone levels in patients with normal renal function. JAMA Intern Med. 2015 Jan;175( 1):137–8. doi: 10.1001/jamainternmed.2014.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ott SM, LaCroix AZ, Scholes D, Ichikawa LE, Wu K. Effects of three years of low-dose thiazides on mineral metabolism in healthy elderly persons. Osteoporos Int. 2008 Sep;19(9):1315–22. doi: 10.1007/s00198-008-0612-4. [DOI] [PubMed] [Google Scholar]
- 34.Zofkova I, Kancheva RL. The effect of nifedipine on serum parathyroid hormone and calcitonin in postmenopausal women. Life Sci. 1995;57(11):1087–96. doi: 10.1016/0024-3205(95)02054-m. [DOI] [PubMed] [Google Scholar]
- 35.Soro S, Cocca A, Pasanisi F, et al. The effects of nicardipine on sodium and calcium metabolism in hypertensive patients: a chronic study. J Clin Pharmacol. 1990 Feb;30(2):133–7. doi: 10.1002/j.1552-4604.1990.tb03451.x. [DOI] [PubMed] [Google Scholar]
- 36.Brown JM, Williams JS, Luther JM, et al. Human interventions to characterize novel relationships between the renin-angiotensin-aldosterone system and parathyroid hormone. Hypertension. 2014 Feb;63(2):273–80. doi: 10.1161/HYPERTENSIONAHA.113.01910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.