Abstract
It has been reported that CXCR4-overexpressing Mesenchymal stem cells (MSCCX4) can repair heart tissue post myocardial infarction (MI). This study aims to investigate the MSCCX4-derived paracrine cardio-protective signaling in the presence of MI. MSCs were divided into 3 groups: MSC only, MSCCX4, and CXCR4 gene-specific siRNA-transduced MSC (MSCsiR). MSCs were exposed to hypoxia, and then MSCs-conditioned culture medium was incubated with neonatal and adult cardiomyocytes respectively. Cell proliferation-regulating genes were assessed by RT-PCR. In vitro: The number of cardiomyocytes undergoing DNA synthesis, cytokinesis, and mitosis was increased to a greater extent in MSCCX4 medium-treated group than control group, while this pro-proliferative effect was reduced in MSCsiR-treated cells. Accordingly, the maximal enhancement of VEGF, cyclin 2, and TGF-β2 was observed in hypoxia-exposed MSCCX4. In vivo: MSCs were labeled with EGFP and engrafted into injured-myocardium in rats. The number of EGFP and CD31 positive cells in the MSCCX4 group were significantly increased than other 2 groups, associated with the reduced left ventricular (LV) fibrosis, the increased LV free wall thickness, the enhanced angiogenesis, and the improved contractile function. CXCR4 overexpression can mobilize MSCs into ischemic area, whereby these cells can promoted angiogenesis and alleviate left ventricular remodeling via paracrine signaling mechanism.
Keywords: Stem cells, Myocardial infarction, Proliferation, CXCR4 receptor
Introduction
Adult mammalian cardiomyocytes are considered terminally differentiated and incapable of proliferation, irreversibly withdrawn from the cell cycle soon after birth.1,2 However, recent reports have shown that adult cardiac myocytes can be induced to reenter the cell cycle with periostin,3 p38 MAP kinase inhibitor,4 cyclin D1/CDK4,5 cyclin A2,6 and transforming growth factor-β (TGF-β).7 Although the adult heart has limited capacity for myocyte proliferation, these new discoveries raise the possibility that increasing the number of the existing cardiomyocytes by activating their proliferative potential can replace damaged myocardium following infarction. Therefore, genetic manipulations may be important in enhancing cardiomyocyte proliferation and differentiation from MSC or native cardiomyocytes for cardiac repair.
CXCR4 is a major regulator of stem/progenitor cell activities.8 The importance of SDF-1α/CXCR4 signaling is documented in CXCR4 knockout mice which die in utero, thereby indicating a fundamental developmental role for this receptor-ligand axis.9 Pretreatment of cells with small molecules, such as statins, p38 MAP kinase inhibitors, or endothelial nitric oxide synthase enhancers, have been used to augment cell proliferation and functional recovery after induction of ischemia.10 In this study, we hypothesize that CXCR4 overexpression enhances cell proliferation and releasing paracrine factors from MSC. Our data shows that MSCCX4 secrete multiple cytokines such as VEGF, cyclin A2 and TGF-β2 in response to hypoxia, augmenting endogenous regenerative processes and cell cycle reentry. The observation that conditioned medium from MSCCX4 induces differentiation of MSC into endothelial cells and alleviates heart remodeling provides mechanistic support for the superior regenerative capacity of MSCCX4 that may provide an improved alternative to future therapies following MI.
Materials and Methods
Animal experiments
All animal work was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23, revised 1996) and the Institutional Animal Care and Use guidelines at the University of Cincinnati. Adenovirus vector propagation and transfection of small interfering RNA (siRNA) in MSC. Adenoviral vectors carrying CXCR4/EGFP gene (Ad-CXCR4+/EGFP) and without CXCR4 gene (Ad-Null/EGFP) were prepared as described [11]. The vectors were propagated in 293 cells and purified on a CsCl2 gradient. For gene transduction, MSC were exposed to the respective virus (1 × 109 particles/ml) for 8 hours and kept in culture for an additional 24-hour in DMEM medium without the viral vector. The transduction procedure was repeated three times to achieve optimal transduction efficiency. We chose adenovirus-based siRNA constructs to express siRNA to knock down CXCR4. The method for transfection of siRNA in MSC has been described previously in detail.11
In vitro studies
Preparation of Conditioned Medium
We used MSCCX4 with MSCNull as a control to study the effects of CXCR4 overexpression. Parent MSC (MSCPar) served as an additional control to rule out confounding effects of GFP transduction and expression. To block the effects of endogenous CXCR4, an additional group with adenovirus containing siRNA targeting the CXCR4 gene (MSCsiR) was used. Conditioned medium was collected from each group of MSC grown under normal or hypoxic conditions for 8 hours. As a negative control, fresh culture medium (not exposed to cells) was used. FGF (100 ng/ml; R&D systems) was used as a positive control. FGF plus MSCCX4 conditioned medium was used to study their additive effect. Conditioned medium was concentrated with Millipore Solvent Resistant Cell (Millipore) and then added to the neonatal cardiomyocytes in different dilutions (1/1000, 1/100 and 1/10) for dose-dependent effects.
Cardiomyocyte Isolation and Culture
For positive control, primary cultures of the neonatal rat cardiomyocytes were prepared as we described previously.12 Briefly, ventricular cardiomyocytes from 2-day-old rat neonatal hearts and adult hearts (200–250g) were isolated as described with minor modifications.12 After digestion of rat neonatal hearts (0.14 mg/mL collagenase II [Invitrogen], 0.55 mg/mL pancreatin [Sigma]), cells were cultured in DMEM/F12 (GIBCO) containing 3 mM Na-pyruvate, 0.2% BSA, 0.1 mM ascorbic acid (Sigma), 0.5% Insulin-Transferrin-Selenium (100×), penicillin (100 U/mL), streptomycin (100 μg/mL), and 2 mM L-glutamine (GIBCO). Adult cardiomyocytes were cultured for 1 day in standard medium (DMEM, 25 mM HEPES, 5 mM taurine, 5 mM creatine, 2 mM L-carnitine [Sigma], 20 U/ml insulin [GIBCO], 0.2% BSA, penicillin [100 U/mL], and streptomycin [100 μg/mL]). Cells were stimulated in culture medium without BSA containing 2 mM L-glutamine. Neonatal and adult cardiomyocytes were initially cultured for 48 h in the presence of 20 μM cytosine β-D-arabinofuranoside (araC; Sigma) and 5% horse serum before treatment to prevent proliferation of non-cardiomyocytes. Adult cardiomyocytes were incubated for another 3 days with araC during treatment. BrdU (30 μM) was added for the last 3 days. Cells were then divided into the various treatment groups mentioned above. To determine the effects of conditioned medium on proliferation of neonatal and adult cardiomyocytes in vitro, concentrated control medium or conditioned medium from hypoxic and normoxic MSCNull, MSCCX4 or MSCPar was added to the dishes (n=6 per group) at the dilutions described above. To determine their additive effect on cell proliferation, neonatal and adult rat cardiomyocytes were also treated with FGF (100 ng/ml; R&D systems), which is known to induce cardiomyocyte division,13 combined with MSCCX4 conditioned medium. Primary neonatal and adult rat cardiomyocytes were examined and quantified with a hemocytometer after 4 and 7 days of treatment with conditioned medium. Corresponding samples were stained for the cardiomyocyte marker troponin I to determine the percentage of differentiated cardiomyocytes. To count cardiomyocyte number, cardiomyocytes were incubated with 0.2 μg/ml of Hoechst 33342 (Invitrogen) for 10 minutes and nuclei were then counted in four random ×20 microscopic fields.3
Preparation of MSC and RT-PCR Analysis
MSC primary culture: Bone marrow cells (BMC) were extracted from 8-week-old SD male rats. The animals were killed by cervical dislocation and BMCs were flushed out of tibias and femurs. The method for preparation of MSC has been previously described in detail.10 Passage 2–4 MSC were used in the study. To mimic the ischemic injury in vitro, the culture medium was then immediately changed to hypoxic buffer (in mmol/L): 118 NaCl, 24 NaHCO3, 1.0 NaH2PO4, 2.5 CaCl2-2H2O, 1.2 MgCl2, 20 sodium lactate, 16 KCl, 10 2-deoxyglucose (pH adjusted to 6.2), and dishes were incubated under hypoxic conditions of 1% O2, 5% CO2, and 94% N2 at 37°C in a hypoxic incubator (Sanyo, O2/CO2 incubator-MCO-18M) for 8 hours. Normal culture (serum free regular medium under 21% O2 and 5% CO2) served as a control. Cells were re-oxygenated by replacing ischemic buffer with culture medium at 5% CO2 and 37°C for another 18 hours as previously described.14
RNA of MSC was extracted after various treatments under 8 hours of hypoxic conditions using the Trizol reagent (Invitrogen, Carlsbad, Calif, U. S. A.). PCR was performed with a DNA cycler (Perkin-Elmer, Wellesley, Mass, United States), using primers for VEGF, cycline A2, TGF-β2 and GAPDH. Rat sequence-specific oligonucleotide primers designed from published rat mRNA sequences (GenBank accession nos. NM_0031836, NM_053702 and X71904, respectively), were as follows: VEGF, sense: 5′-CAATGATGAAGCCCTGGAGT -3′, antisense: 5′-TTTCTTGCGCTTTCGTTTTT -3′. Cycline A2, sense: 5′-ATGTCAACCCCGAAAA AGTG-3′, antisense: 5′-GGGACGTGCTCATCGTTTAT-3′. TGF-β2, sense: 5′-CTCCACATATGCCAGTGGTG-3′, antisense: 5′-CTAAAGCAATAGGCGGCATC -3′. GAPDH, sense: 5′ATGGGAAGCTGGTCATCAAC-3′, antisense: 5′-GTGGTTCACACCCATCACAA-3′. The amplification profile consisted of sequential steps of denaturing, annealing, and extending with a final 7-min extend cycle: for VEGF, cycline A2 and TGF-β2, 35 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 1 min. The resulting PCR products were analyzed by 1% agarose gel electrophoresis stained with ethidium bromide.15 We repeated individual experiments at least 3 times, and calculated the ratio of the density of target cDNA to that of GAPDH in each experiment.
Immunofluorescence (IF) Microscopy
BrdU (30 μM, Sigma) was added to the medium of cardiomyocytes to examine DNA synthesis. To inhibit DNA polymerization, cytosine-D-arabinofuranoside (araC) (10 μM, Sigma) was added to the dishes. Mitosis was determined by visualization of nuclei stained positive for an antibody to phosphorylated histone H3 (H3P). An antibody against aurora B kinase (Sigma-Aldrich, Cat.# A5102) was used to visualize cytokinesis. Signals were visualized with secondary antibodies conjugated to donkey anti-rabbit (FITC, Jackson Immunoresearch). BrdU was stained with a 5-Bromo-2′-deoxy-uridine Labeling and Detection Kit I (Roche Applied Science) and nuclei were visualized with 4′,6′-diamidino-2-phenylindole (DAPI, Invitrogen). Fluorescence imaging was performed with an Olympus BX41 (Olympus America Inc., Melville, NY, U.S.A.) equipped with epiflouresence microscopy, and images were recorded using a digital camera with MagnaFire™ 2.1 software.
In vitro tube formation assay
A tube formation assay was performed with a tube formation assay kit (Chemicon) according to the manufacturer’s instructions. Briefly, the solution of ECMatrix was thawed on ice overnight, mixed with 10 diluents of ECMatrix, and placed in a 96-well tissue culture plate at 37°C for 1 hour to allow the matrix solution to solidify. MSCNull or MSCCX, or MSCsiR were exposed to hypoxic conditions for 48 hours. They were then seeded (5×103cells/well) on top of the solidified matrigel and incubated at 37°C for 4 hours. After cellular network structures were fully developed, the total capillary tube number was measured under an inverted light microscope (Olympus America, Melville, NY) at 200-magnification. Five independent fields were assessed for each well, and the average number of tubes was determined. Networks of tube-like structures were measured using Image Pro Plus software 6.0 (MediaCybernetic, Silver Spring, Mass., USA).
In vivo studies
Surgical procedures for left anterior descending artery (LAD) ligation and experimental groups
A myocardial infarction (MI) model was developed in female Sprague-Dawley (SD) rats, 200–250g (n = 18/group), as we previously described.15 Rats were anesthetized with isoflurane and a midline cervical skin incision was performed for intubation. The animals were mechanically ventilated with room air supplemented with oxygen (1.5L/min) using a rodent ventilator (Model 683, Harvard Apparatus, South Natick, MA). Body temperature was carefully monitored with a probe (Cole-Parmer Instrument, Vernon Hill, IL) and maintained at 37°C throughout the surgical procedure. The heart was exposed by left side limited thoracotomy and LAD was ligated with a 6-0 polyester suture 1 mm from the tip of the normally positioned left auricle. We identified transferred MSC by simultaneous presence of GFP from genetically engineered rat MSC previously treated with an ex vivo adenoviral transduction method for Null/GFP (MSCNull), or overexpressing CXCR4/GFP (MSCCX4), or siRNA targeting CXCR4 (MSCsiR) (11). Thirty minutes after LAD ligation, the animals were divided into three groups and 2×106 MSCNull served as control, MSCCX4, or MSCsiR were injected into at risk areas. A sham group (chest opened without LAD ligation) served as an additional control. Heart tissue was harvested for immunohistochemical studies.
MSC Homing Following MSC Infusion
One month post-MI, tissue was prepared as described above for immunostaining.11 The number of GFP+ cells was quantified in the infarct zone by independent blinded researchers across a minimum of 4 sections from the mid-LV per animal.
Immunohistochemistry for Detection of Angiogenesis
One month post-MI, the immunohistochemical studies were performed as described previously.11 Endothelial cells were identified using CD31 antibody (PECAM-1, Santa Cruz Biotechnology). Cardiomyocytes were identified with α-sarcomeric actin antibody (Sigma) and 4′, 6-diamino-2-phenylindole (DAPI, Sigma) was used to stain.11 Five to 10 randomly selected fields for each sample were imaged by use of an Olympus BX41 microscope (Olympus America Inc., Melville, NY, USA) equipped with epiflouresence attachment and images were recorded using a digital camera with MagnaFire™ 2.1 software. Vessel density within the infarct border zone was measured. All quantitative evaluations were performed with ImagePro Plus software (Media Cybernetics).
Measurement of Fibrotic Size and Left Ventricle (LV) Wall Thickness
Fixed hearts were embedded in paraffin and sections from the mid-LV were stained with Masson-Trichrome. Images of LV area from each slide were taken by Olympus BX41 with CCD (MagnaFire™, Olympus) camera. The thickness of the LV free wall and septum were analyzed by direct measurements. Fibrosis and total LV area of each image were measured using the Image-Pro Plus (Media Cybernetics Inc., Carlsbad, CA, USA), and the percentage of the fibrotic area was calculated as previously described: (Fibrosis area/total LV area) × 100.16
Assessment of Heart Function
Rats were anesthetized with sodium pentobarbital (40 mg/kg) by intraperitoneal injection and the right carotid artery was cannulated with a micro tip pressure transducer catheter (SPR-839, Millar Instruments) connected to MPCU-200 P-V signal conditioning hardware which provided analog outputs of the time-varying ventricular pressure and volume signals for data acquisition. The inferior vena cava (IVC) was exposed and IVC occlusion (IVCO) was performed by external compression. Hemodynamic and PV loops were recorded during steady state. LV systolic function was evaluated by maximum dP/dt (dP/dtmax), minimum dP/dt (dP/dtmin), end-systolic elastance (Ees), ejection fraction (EF) and Tau as previously described.17 Hemodynamic parameter analysis was carried out using Millar’s PVAN software (Version 3.2).
Results
Conditioned medium from MSCCX4 induces proliferation of neonatal and adult cardiomyocytes
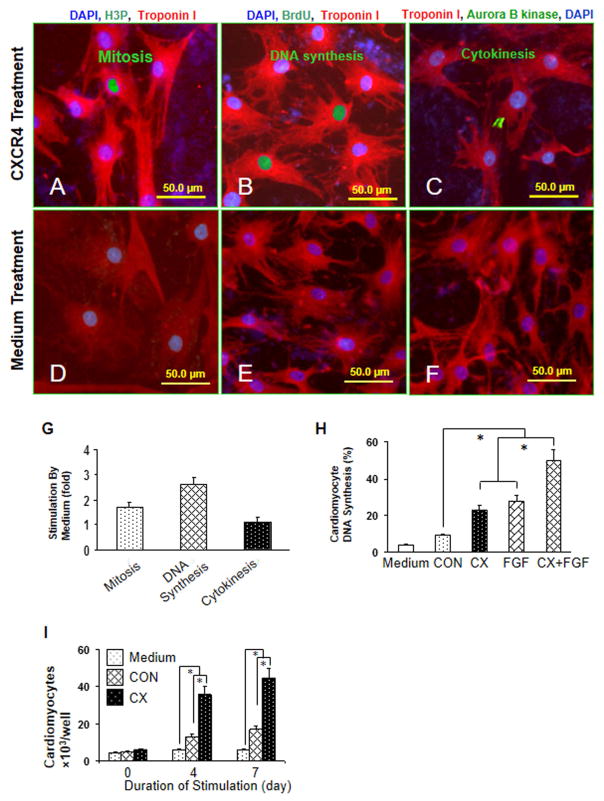
Primary neonatal rat ventricular cardiomyocytes were treated with MSCCX4 conditioned medium to see its effects on mitosis (Fig. 1A), DNA synthesis (Fig. 1B) and cytokinesis (Fig. 1C), as compared with the effects of MSCNull conditioned medium on mitosis (Fig. 1D), DNA synthesis (Fig. 1E) and cytokinesis (Fig. 1F) at day 4. Neonatal cardiomyocyte mitosis (1.7 ± 0.2 fold), DNA synthesis (2.6 ± 0.3 fold) and cytokinesis (1.1 ± 0.2 fold) in MSCCX4 group were significant higher as compared with the MSCNull group (Fig. 1G). MSCCX4 conditioned medium induced approximately a 2.6-fold increase to 23.6 ± 2.7% in neonatal cardiomyocyte DNA synthesis (Fig. 1H), similar to the effect of FGF (28.4 ± 2.6%). The additive effect of MSCCX4 conditioned medium and FGF on cardiomyocyte DNA synthesis was the highest (51.3 ± 4.1%, Fig. 1H). The proliferation of neonatal cardiomyocytes in the MSCCX4 group was significantly higher as compared with control groups (Fig. 1I).
Fig. 1. MSCCX4 conditioned medium induces proliferation of neonatal cardiomyocytes.
Primary neonatal rat ventricular cardiomyocytes were treated with concentrated media from MSCCX4. Cell cycle activity was detected with an antibody directed against phosphorylated histone H3 (H3P) for mitosis (A, D), BrdU for DNA synthesis (B, E), and aurora B kinase for cytokinesis (C, F) and were quantified by immunofluorescence microscopy (G). The additive effect of media from MSCCX4 plus FGF (100ng/ml) on DNA synthesis was observed (H). Neonatal cardiomyocyte proliferation was determined by cell counting (I). Color codes are given at the top. Data are means ± SEM of 20 independent experiments. *p<0.05; CX indicates medium from MSCCX4 group; CON, medium from MSCNull group.
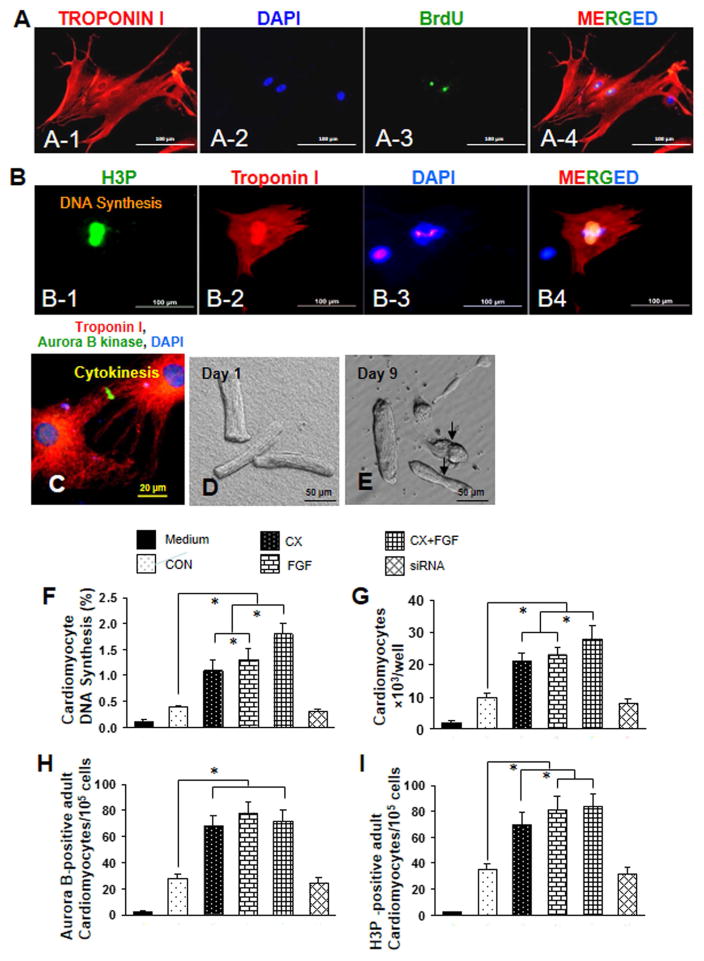
MSCCX4 conditioned medium also induced proliferation of primary adult rat cardiomyocytes, (Fig. 2). Cell cycle activity was determined by immunofluorescence (IF) microscopy 10 days after incubation with conditioned medium. MSCCX4 conditioned medium induced DNA synthesis (Fig. 2: A-1 to A-4), mitosis (Fig. 2: B-1 to B-4) and cytokinesis (Fig. 2C). Primary adult rat cardiomyocytes in culture were photographed in phase contrast after attachment on day 1 (Fig. 2D). After treatment with MSCCX4 conditioned medium and exposure to BrdU for 5 days, several dividing adult cardiomyocytes were observed (Fig. 2E). DNA synthesis was quantified by counting BrdU positive nuclei under IF microscopy (Fig. 2F). MSCCX4 conditioned medium induced approximately a 3-fold increase to 1.2 ± 0.2% in adult cardiomyocyte DNA synthesis compared to MSCNull, similar to the effect of FGF (1.3 ± 0.2%). The additive effect of MSCCX4 conditioned media and FGF on DNA synthesis was the highest compared to MSCNull (1.8 ± 0.3% vs. 0.4 ± 0.02%, p<0.01). Similarly, cardiomyocyte proliferation was increased by approximately 2-fold in treatment with MSCCX4 conditioned medium as compared with the control group, p<0.01 (Fig. 2G), and the additive effect of MSCCX4 conditioned media and FGF was the highest (27.4 ×103) compared to the control group (Fig. 2 G).
Fig. 2. MSCCX4 conditioned medium stimulates cytokinesis, mitosis and DNA synthesis of differentiated mononucleated cardiomyocytes.
(A) Cell cycle activity was determined by immunofluorescence microscopy after 10 days and detected by an antibody directed against BrdU for DNA synthesis, (B) phosphorylated histone H3 (H3P) for mitosis (B-1 to B-4), and (C) aurora B kinase for cytokinesis. A-1, A-2, A-3 and A-4 who the same cells however “A-1” was stained with troponin I antibody, “A-2” stained with DAPI, “A-3” stained with “BrdU” antibody and “A-4” colocalized with troponin I, DAPI and BrdU. Color codes are given at the top (A~C). (D) Primary adult rat ventricular cardiomyocytes in culture were photographed in phase contrast after attachment on day 1. (E) These cells were then treated with concentrated media from MSCCX4 and labeled with BrdU for the last 5 days; at day 9, several dividing adult myocytes were observed. (F) The additive effect of MSCCX4 conditioned medium and FGF on DNA synthesis is shown. (G) Cardiomyocyte proliferation determined by cell counting is shown. (H, I) Cell cycle activity (cytokinesis) was detected with an antibody against aurora B kinase (H) and phosphorylated histone H3 (H3P) was used for mitosis (I); signals were quantified by immunofluorescence microscopy. Data are means ± SEM of 20 independent experiments. CON indicates medium from MSCs group (control); CX, medium from MSCCX4 group; C+F, medium from MSCCX4 plus FGF; siRNA, medium from MSCsiR group. Data are means ± SEM of 5 independent experiments. *p<0.05.
To determine whether adult cardiomyocytes underwent cytokinesis, we used aurora B. MSCCX4 conditioned medium significantly increased cytokinesis as compared with MSCNull group (68.4/105 vs. 29.1/105 cells, p<0.01) (Fig. 2H). The maximum effect was observed with MSCCX4 conditioned medium combined with FGF. However, siRNA completely blocked cytokinesis, indicating that MSCCX4 conditioned medium stimulates adult cardiomyocyte division.
To determine whether adult cardiomyocytes undergo karyokinesis, we performed H3P analysis. MSCCX4 conditioned medium increased H3P-positive cardiomyocytes approximately 1.8-fold as compared with MSCNull (p<0.05), with an additional increase, approximately 2.4-fold, observed with FGF and MSCCX4 conditioned medium together (p<0.05). There was no difference, however, between control and siRNA groups (Fig. 2I), suggesting the important role of MSCCX4 conditioned medium in cardiomyocyte proliferation.
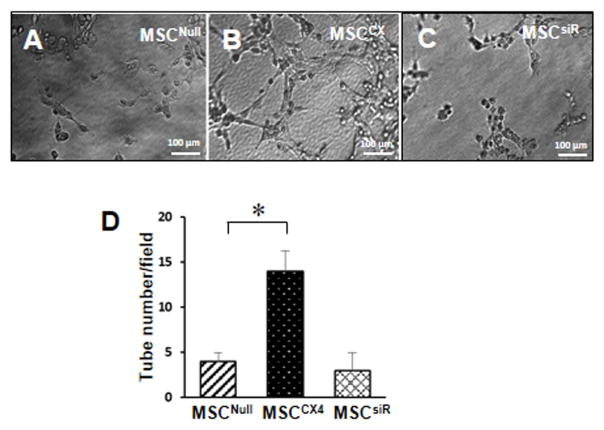
Overexpression of CXCR4 enhanced the angiogenesis in MSCs
The tube formation assay was performed on matrigel-precoated wells. The MSCNull exhibited small round shapes, isolated cells, and minimal migration under in hypoxia (Fig. 3A). However, MSCCX led to the development of capillary tubes, sprouting of new capillaries, and finally the formation of cellular networks (Fig. 3B). The number of this increased tube-like structures was abolished in MSCsiR group (Fig. 3C). The number of tube-like structures was significantly increased in MSCCX4 as compared to MSCNull or MSCsiR under hypoxia (p<0.05, Fig. 3D).
Fig. 3. Tube formation of MSC in matrigel.
(A) MSCNull, (B) MSCCX4, and (C) MSCsiR were seeded on the matrigel respectively, and the tubewere observed using optical microscope. (D) Quantitative analysis of total branch points to indicate tube formation. Data are means ± SEM of 5 replicate. *p<0.05 vs. MSCNull.
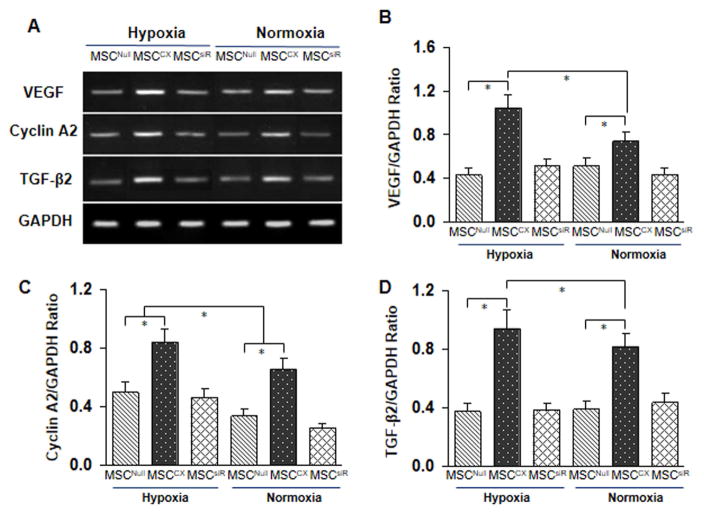
Genetically modified MSCCX4 expresses cytoprotective and proliferative genes
CXCR4 overexpression mobilizes MSC toward the ischemic area, a process that may be influenced by paracrine mechanisms. To determine the effect of paracrine factors released by MSCCX4 that contribute to cell homing, proliferation and angiogenesis, we examined the expression of angiogenic and growth factors by quantitative RT-PCR using RNA extracted from MSCNull, or MSCCX4, or MSCsiR after 8 hours of exposure to normoxia or hypoxia (Fig. 4A). The following genes were significantly upregulated in the MSCCX4 in both normoxia (p<0.05 vs. MSCNull) as well as hypoxia (p<0.05 vs. MSCNull): Vascular endothelial growth factor (VEGF), an angiogenic factor (Fig. 4B), Cyclin A2, a cell-cycle-perpetuating factor (Fig. 4C), and Transforming growth factor-β2 (TGFβ2), a mediator of growth and differentiation (Fig. 4D). Our RT-PCR data also showed that VEGF, cyclin A2 and TGFβ2 were significantly upregulated in the MSCCX4 group as compared with MSCNull group, p<0.05. However, these upregulated genes (VEGF, Cyclin A2 and TGFβ2) in the MSCCX4 group were completely suppressed by siRNA targeting CXCR4, suggesting that CXCR4 plays an important role in their expression in MSC (Fig. 4).
Fig. 4. MSCCX4 conditioned medium modulates expression of specific genes in differentiated cardiomyocytes.
To determine the effect of MSCCX4 preconditioned media on cardiomyocyte differentiation and proliferation, RT-PCR was performed. (A) RNA was extracted from both MSCNull (CON) and MSCCX4 after 8 hours of exposure to normoxia or hypoxia, and gel electrophoresis indicated the RNAs encoding vascular endothelial growth factor (VEGF); Cycline A2, and transforming growth factor- β2 (TGF-2). (B, C and D)The corresponding quantitative analysis was were performed determined by real time-PCR. MSCCX, medium from MSCCX4 group. Data are means ± SEM of 5 independent experiments. *p<0.05 vs. control.
Immunohistochemical evidence for cell mobilization
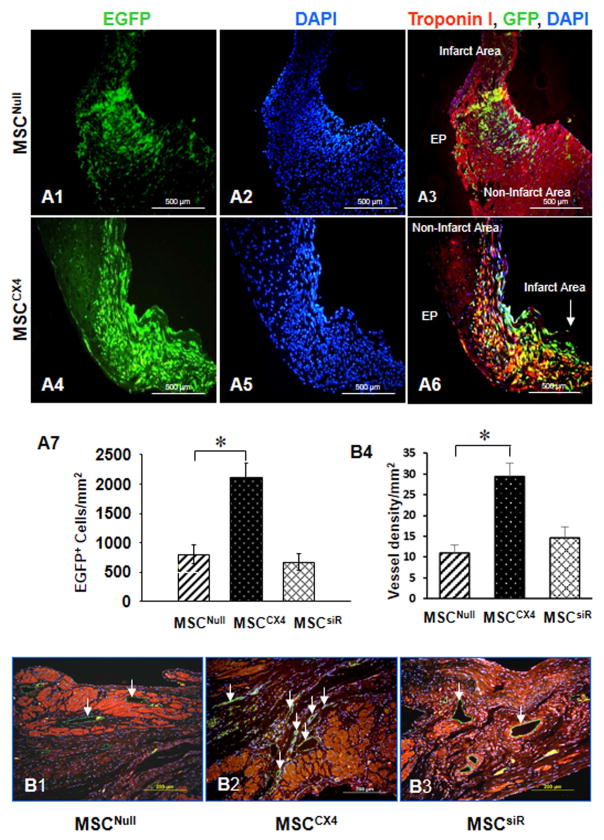
Transplanted cells were identified by EGFP (green) (Fig. 5). MSCs migrated into the MI scar and per infarcted area (Figs. 4A1–6). As compared with MSCNull group (Fig. A1–A3), homing of MSCs to damaged cardiac area was the highest in MSCCX4 group (Fig. A4–A6 and Fig. A-7). However, MSC homing was blocked by siRNA against CXCR4 in MSCsiR group (picture panel not shown, which is similar to MSCNull group) and the number of EGFP positive cells per randomly chosen field did not differ between MSCNull (Fig. 5A-7).
Fig. 5. Effects of MSCCX4 transplantation in the infarct area at 4 weeks post MI.
(A) Migration of the EGFP positive cells was observed in MSCNull group (A1–A3) and in MSCCX4 (A4–A6). Color codes given at the top. Original magnification: ×100. EGFP migration was estimated quantitatively in infarcted myocardium from various treatment groups (A7). (B) MSCCX4 group promotes vessel formation by significant expression of CD31 (green color (white arrow) (B2) in comparison with MSCNull (B1) or MSCsiR group (B3), (magnification×200). Quantitative analysis of capillary density (anti-CD31 antibody staining) was determined by various treatments (B4). Data are means ± SEM of 6 independent experiments. *p<0.05 vs. MSCNull.
Immunohistochemical evidence for angiogenesis in vivo studies
To investigate if CXCR4 overexpressing MSC may affect vascular density post-MI, vascular density after one month MI was evaluated. PECAM-1(CD31) was used as a marker for endothelial cells. Quantitative analysis demonstrated that the number of capillary density was significantly increased in MSCCX4 group (Fig. 5B-2) compared with MSCNull (Fig. 5B-1) or MSCsiR groups (Fig. B-3). The number of endothelial cells per randomly chosen field did not differ between MSCNull (12.6 ± 3.4) and MSCsiR (14.2 ± 4.7). However, the number of CD31 positive cells was the highest as compare with any other groups (p<0.01; Fig. 5B-4).
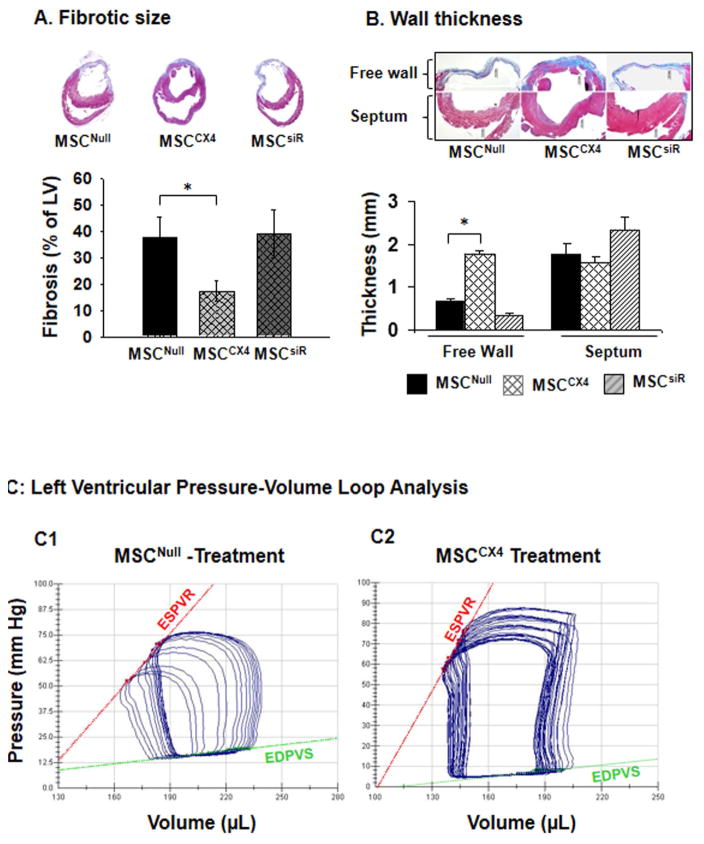
MSCCX4 reduces fibrosis and improves cardiac function after MI
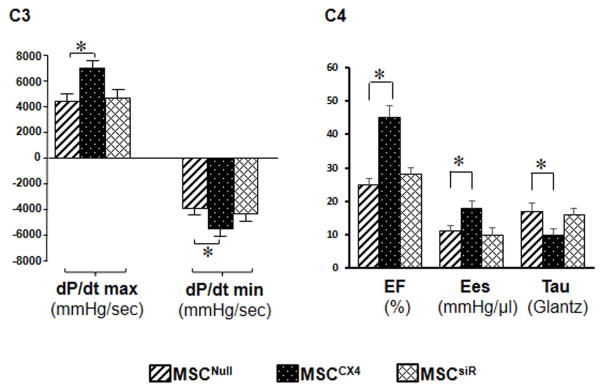
Four weeks after MI, the percentage of fibrosis in the LV wall in MSCCX4 treated hearts was significantly reduced as compared with the MSCNull or siRNA groups (Fig. 6A). We also analyzed ventricular remodeling by measuring the thickness of the LV free wall and the interventricular septum at 4 weeks after MI (Fig. 6B). The LV free wall was significantly thicker while that of the LV septum was no different in the MSCCX4 group as compared to the MSCCX4 or MSCsiR groups (Fig, 6B). These results indicate that transplantation of MSCCX4 reduced infarct size and improved ventricular remodeling. We analyzed cardiac function at 4 weeks after MI by LV catheterization during preload reduction (Fig. 6C). Administration of MSCCX4 improved myocardial function, as indicated by a steeper slope of the end-systolic pressure-volume relationship. The slope of the regression line is ventricular end-systolic elastance (Ees) (red line), which has been used as an index of cardiac contractility. Ees of MSCCX4 group was approximately 2-fold greater (17.4 ± 1.1 mmHg/μl) than that of the MSCNull group (9.3 ± 0.4 mmHg/μl) implying that the LV contractility of the MSCCX4 group was significantly increased as compared with the MSCNull group (Fig. 6C). LV-systolic function as determined by maximum dP/dt in the MSCCX4 group was improved significantly as compared with MSCNull group (Fig. 6, C3). Load-dependent parameters (EF) revealed marked increases in MSCCX4 as compared with MSCNull, p<0.05. LV diastolic function as indicated by dP/dtmin and tau-(Glantz) was also significantly improved in MSCCX4 as compared with MSCNull (Fig. 6, C4). However, there was no significant difference in these parameters between MSCNull and MSCsiR groups. Thus, myocardial delivery of MSCCX4 improved LV remodeling and cardiac function after MI.
Fig. 6. MSCCX4 promotes the recovery of heart performance and attenuates myocardial infarction-induced cardiac remodeling.
(A) Masson’s trichrome staining in various treatments allowed quantification the infarct scar size 4 weeks after administration of cells in various treatment groups. (B) Cardiac remodeling was measured by the thickness of the left ventricular free wall in the infarct area and septum. (C) Representative pressure–volume (P–V) loops were obtained with a P–V conductance catheter system at different preloads after vena cava occlusion, showing differences in the end-systolic P–V relation (ESPVR) between rats pretreated with MSCNull (C1) and MSCCX4 (C2). Left ventricular pressure-volume loop analysis is performed to indicate dP/dt maximum (max), dP/dt minimum (min) (C3), and EF, Ees, Tau (C4). EF indicates ejection fraction; Ees, the slope of the regression line, is ventricular end-systolic elastance; Tau (Glantz), Tau–Glantz method; * p<0.05 vs. MSCNull. Data are means ± SEM of 6 independent experiments.
Discussion
In this study, we showed that MSCCX4 secreted a number of cytokines such as VEGF, cyclin A2 and TGFβ2 in response to hypoxia. The observation that conditioned medium from MSCCX4 enhanced the proliferation of neonatal and adult cardiomyocytes in vitro provides mechanistic support for the regenerative capacity of these MSC. Particularly, employment of neonatal cardiomyocytes allowed the DNA synthesis, cytokinesis, and mitosis to be investigated upon the treatment of MSCCX4-derived conditioned culture medium. Although mammalian cardiomyocytes become terminally differentiated early in the development of the heart, the adult cardiomyocytes still exhibit, albeit limited, a certain amount of proliferative capability, especially in the presence of certain growth factors (i.e., insulin-like growth factor, epidermal growth factor, and fibroblast growth factor). Such a feature enable us to track the cell division in adult cardiomyocytes response to the treatment of MSCCX4-derived conditioned culture medium. Since adult cardiomyocytes withdraw from the cell cycle, strategies to stimulate proliferation of differentiating cardiomyocytes from conditional medium derived from MSCCX4 could be important in repopulation. Cardiac specific overexpression of D-type cyclins can induce DNA synthesis in adult myocytes.21 Kuhn et al showed that adult cardiomyocytes can be stimulated to reenter the cell cycle with periostin, a ligand for αVβ3 and αVβ5 integrins.3 Singla DK et al demonstrated that treatment of mouse ES cells with TGF-β2, but not TGF-β1 or TGF-β3, induced significant increased embryoid body (EB) proliferation as well as an increase in the number of beating cardiac myocytes.22 Additionally, targeted expression of cyclin A2 led to augmented endogenous regenerative mechanisms via induction of enhanced proliferative capacity in side population cells.23 Thus, genetic manipulations may enhance the proliferation of differentiating cardiomyocytes for cardiac repair. Trans-differentiation of MSC is controlled by factors in the micro-environment, or supplied in the culture media of ex vivo cultivated cells. MSC do not differentiate spontaneously but do so in the presence of growth factors and cytokines.24,25 CXCR4 is a major regulator of stem/progenitor cell activities.11 Our data demonstrates that MSCCX4 release paracrine factors, such as VEGF, cyclin A2 and TGFβ2, that contribute to cell survival, proliferation and angiogenesis (Fig. 3). MSCCX4 transplanted into the border zone migrate specifically toward the SDF-1 gradient in the ischemic myocardium in where they may release of certain cytokines to stimulate native cardiomyocyte proliferation. The cellular responses seem to work only in the microenvironment of the ischemic myocardium because they are not activated after transplantation into uninjured myocardium.26 Mobilization and proliferation in a given tissue are dependent on the specific signals present in the local microenvironment of the damaged heart. Therefore, the ischemic micro-environment has critical patho-biological functions that are essential for seeding and proliferation of MSC in remodeling of damaged hearts. Our data also demonstrate that transplantation of MSCCX4 exerts a marked inhibitory effect on pathological myocardial remodeling that may be important in mediating therapeutic benefit. Whether the effect on remodeling is due to mechanical improvement resulting from cell transplantation, angiogenesis, or paracrine mediators released by MSCCX4 still requires further investigation. A previous study has revealed a crosstalk between MSCs and cardiomyocytes, contributing to the inhibition of cardiac hypertrophy through synergistic VEGF release and via suppressing Ca2+/calcineurin/NFATc3 hypertrophic signals in cardiomyocytes.27 Although our data did not indicate whether such an anti-hypertrophic response can be further tuned up in MSCCX4-engrafted hearts, the MSCCX4-induced paracrine responses actually enhanced the proliferation of neonatal and adult cardiomyocytes in vitro, which supports the regenerative capacity of these MSC.
We have demonstrated that targeted genetic expression of CXCR4 in MSCs leads to enhance in vivo mobilization and engraftment of MSCs into ischemic area where these cells promoted angiogenesis and alleviated early signs of left ventricular remodeling. The end result of MSCCX4 transplantation was a significant recovery from MI in a serial manner. Future therapies for acute myocardial infarction may involve transplantation of MSCCX4 to augment endogenous regenerative processes and facilitate repair of the damaged heart.
Acknowledgments
We thank Ruilian Ma for providing technical assistance.
Funding Sources:
This work was supported by NIH grants, HL110740 and HL107957 (Y. Wang); Research supported by the National Natural Sciences Foundation of China (#81370230 and #81570279); the Natural Science Foundation of Guangdong, China (#2014A030311041), and the Science and Technology Program of Guangzhou China (#201508020107); the Natural Science Foundation of Qinghai (Innovation Team Project, #2013-Z-921).
Footnotes and abbreviations
- MSC
mesenchymal stem cells
- MSCCX4
MSC overexpressing CXCR4
- MSCsiR
MSC expressing siRNA targeting CXCR4 gene
- BMC
bone marrow cells
- araC
cytosine-D-arabinofuranoside
- H3P
phosphorylated histone H3
- LAD
left anterior descending coronary artery
- MI
myocardial infarction
- TGF-β
transforming growth factor-β
- IF
immunofluorescence
- LV
left ventricle
- SD
Sprague-Dawley
- IVC
inferior vena cava
- IVCO
inferior vena cava occlusion
- Ees
end-systolic elastance
- EF
ejection fraction
Footnotes
Author Disclosure Statement:
The authors declare no conflicts of interest.
References
- 1.Ueno H, Perryman MB, Roberts R, Schneider MD. Differentiation of cardiac myocytes after mitogen withdrawal exhibits three sequential states of the ventricular growth response. J Cell Biol. 1988;107:1911–1918. doi: 10.1083/jcb.107.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamamori-Adachi M1, Goto I, Yamada K, Kitajima S. Differential regulation of cyclin D1 and D2 in protecting against cardiomyocyte proliferation. Cell Cycle. 2008;7:3768–3774. doi: 10.4161/cc.7.23.7239. [DOI] [PubMed] [Google Scholar]
- 3.Kühn B, del Monte F, Hajjar RJ, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–9. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 4.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:15546–11551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamamori-Adachi M, Ito H, Sumrejkanchanakij P, Adachi S, Hiroe M, Shimizu M, Kawauchi J, Sunamori M, Marumo F, Kitajima S, Ikeda MA. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ Res. 2003;92:e12–19. doi: 10.1161/01.res.0000049105.15329.1c. [DOI] [PubMed] [Google Scholar]
- 6.Cheng RK, Asai T, Tang H, et al. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res. 2007;100:1741–1748. doi: 10.1161/CIRCRESAHA.107.153544. [DOI] [PubMed] [Google Scholar]
- 7.Dai P, Nakagami T, Tanaka H, Hitomi T, Takamatsu T. Cx43 mediates TGF-beta signaling through competitive Smads binding to microtubules. Mol Biol Cell. 2007;18:2264–2273. doi: 10.1091/mbc.E06-12-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 9.Kucia M, Ratajczak J, Ratajczak MZ. Bone marrow as a source of circulating CXCR4+ tissue-committed stem cells. Biol Cell. 2005;97:133–146. doi: 10.1042/BC20040069. [DOI] [PubMed] [Google Scholar]
- 10.Ma FX, Han ZC. Statins, nitric oxide and neovascularization. Cardiovasc Drug Rev. 2005;23:281–292. doi: 10.1111/j.1527-3466.2005.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Zhang D, Millard RW, et al. Gene manipulated peritoneal cell patch repairs infarcted myocardium. J Mol Cell Cardiol. 2010;48:702–712. doi: 10.1016/j.yjmcc.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M, Wang Y, Hirai K, Ayub A, Ashraf M. Calcium preconditioning inhibits mitochondrial permeability transition and apoptosis. Am J Physiol Heart Circ Physiol. 2001;280:H899–908. doi: 10.1152/ajpheart.2001.280.2.H899. [DOI] [PubMed] [Google Scholar]
- 13.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–1054. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 14.Dai Y, Ashraf M, Zuo S, et al. Mobilized bone marrow progenitor cells serve as donors of cytoprotective genes for cardiac repair. J Mol Cell Cardiol. 2008;44:607–617. doi: 10.1016/j.yjmcc.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–42. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Ahmad N, Wang B, Ashraf M. Chronic Preconditioning: A Novel Approach For Cardiac Protection. Am J Physiol Heart Circ Physiol. 2007;292:H2300–2305. doi: 10.1152/ajpheart.01163.2006. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Ahmad N, Wani MA, Ashraf M. Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J Mol Cell Cardiol. 2004;37:1041–1052. doi: 10.1016/j.yjmcc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Haider HKh, Ahmad N, Zhang D, Ashraf M. Evidence for Ischemia Induced Host Derived Bone Marrow Cell Mobilization into Cardiac Allografts. J Mol Cell Cardiol. 2006;41:478–487. doi: 10.1016/j.yjmcc.2006.06.074. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda K, Yuasa S. Stem cells as a source of regenerative cardiomyocytes. Circ Res. 2006;98:1002–1013. doi: 10.1161/01.RES.0000218272.18669.6e. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Haider HK, Ahmad N, et al. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol. 2006;40:736–745. doi: 10.1016/j.yjmcc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Busk PK, Hinrichsen R, Bartkova J, et al. Cyclin D2 induces proliferation of cardiac myocytes and represses hypertrophy. Exp Cell Res. 2005;304:149–161. doi: 10.1016/j.yexcr.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Singla DK, Sun B. Transforming growth factor-beta 2 enhances differentiation of cardiac myocytes from embryonic stem cells. Biochem Biophys Res Commun. 2005;332:135–141. doi: 10.1016/j.bbrc.2005.04.098. [DOI] [PubMed] [Google Scholar]
- 23.Cheng RK, Asai T, Tang H, et al. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res. 2007;100:1741–1748. doi: 10.1161/CIRCRESAHA.107.153544. [DOI] [PubMed] [Google Scholar]
- 24.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 26.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 27.Cai B, Tan X, Zhang Y, et al. Mesenchymal Stem Cells and Cardiomyocytes Interplay to Prevent Myocardial Hypertrophy. Stem Cells Transl Med. 2015;4:1425–35. doi: 10.5966/sctm.2015-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]