Abstract
Transient Receptor Potential Vanilloid 1 (TRPV1) and Transient Receptor Potential Ankyrin 1 (TRPA1) expressed mainly by primary sensory neurons function as major nociceptive integrators. They are also present on the rat endometrium in an oestrogen-regulated manner. TRPV1 is upregulated in peritoneal and ovarian endometriosis patients, but there is no information about TRPA1 and their pathophysiological significances. In this study, patients undergoing laparoscopic surgery were investigated: severe dysmenorrhoea due to rectosigmoid deep infiltrating endometriosis (n = 15), uterine fibroid-induced moderate dysmenorrhoea (n = 7) and tubal infertility with no pain (n = 6). TRPA1 and TRPV1 mRNA and protein expressions were determined by quantitative polymerase chain reaction and semi-quantitative immunohistochemistry from the endometrium samples taken by curettage. Results were correlated with the clinical characteristics including pain intensity. TRPA1 and TRPV1 receptors were expressed in the healthy human endometrium at mRNA and protein levels. Sparse, scattered cytoplasmic TRPA1 and TRPV1 immunopositivities were found in the stroma and epithelial layers. We detected upregulated mRNA levels in deep infiltrating endometriosis lesions, and TRPV1 gene expression was also elevated in autocontrol endometrium of deep infiltrating endometriosis patients. Histological scoring revealed significant TRPA1 and TRPV1 difference between deep infiltrating endometriosis stroma and epithelium, and in deep infiltrating endometriosis epithelium compared to control samples. Besides, we measured elevated stromal TRPV1 immunopositivity in deep infiltrating endometriosis. Stromal TRPA1 and TRPV1 immunoreactivities strongly correlated with dysmenorrhoea severity, as well TRPV1 expression on ectopic epithelial cells and macrophages with dyspareunia. Epithelial TRPA1 and stromal TRPV1 immunopositivity also positively correlated with dyschezia severity. We provide the first evidence for the presence of non-neuronal TRPA1 receptor in the healthy human endometrium and confirm the expression of TRPV1 channels. Their upregulations in rectosigmoid deep infiltrating endometriosis lesions and correlations with pain intensity suggest potential roles in pathophysiological mechanisms of the disease.
Keywords: Dysmenorrhoea, endometriosis, pain, transient receptor potential ankyrin 1, transient receptor potential vanilloid 1
Introduction
Chronic pelvic pain is a challenging clinical problem exposing 10% of all gynaecological visits in developed countries. Meanwhile, more than one third of women undergoing surgery due to pelvic pain are diagnosed with endometriosis. This disease often co-occurs with other painful conditions like irritable bowel syndrome (IBS), interstitial cystitis/painful bladder syndrome (IC/PBS) and vulvodynia.1–3
Endometriosis is a complex oestrogen-dependent inflammatory condition defined as the presence of endometrium-like tissue at ectopic sites, most commonly on the peritoneum and ovaries. The disease affects around 10% of women in reproductive age generally emerging in the context of severe pain and infertility.4 It comprises three main pathological entities, such as peritoneal endometriosis (pEL), ovarian endometriosis (EM) and deep infiltrating endometriosis (DIE) distinguished by etiological, morphological and histochemical aspects.5 DIE is the most severe form of endometriosis with the invasion of endometrial tissue into organs deeper than 5 mm. The gastrointestinal tract is a common localization of DIE with the rectum and sigmoid being involved in 90% of these cases. Large bowel DIE is associated with a constellation of severe pain symptoms including non-specific gynaecological complaints (dysmenorrhoea (DM), dyspareunia) and organ-specific manifestations (non-menstrual chronic pelvic pain (CPP), dyschezia, dysuria) dramatically impairing the life quality.4,5
The failure of etiologic treatment shifted the focus on a symptom-guided individualized approach targeting less aggressive surgical strategy and more effective pharmacological therapy.6,7 Thus, chronic pain alleviation, fertility sparing, quality of life and avoidance of dysfunction have become the main goals in the therapy.6,8 However, currently available tools do not meet the requirements of this complex strategy. Combined oral contraceptives are the first line therapy inducing a hypo-estrogenic milieu resulting in lesion regression and preventing ‘early’ postoperative recurrence.8 However, hormonal suppression is not an option for those who plan pregnancy. Although disputed in its extent, adequate surgery can result in pain relief by reducing inflammation and restoring pelvic anatomy.6 Novel non-hormonal therapeutic agents with an effective anti-inflammatory and analgesic profile could provide promising perspectives for this disease. Therefore, identification of the molecular mechanisms and targets involved in the pathophysiology of endometriosis and related pain is very important.
Although the relationship between lesions and pain is unclear, emerging evidence suggests the role of local neuro-inflammatory interactions.3,9,10 DIE nodules have dense sensory innervations providing complex neurotrophic, inflammatory and nociceptive functions.11,12 Interstitial macrophages, mast cells and adjacent neural elements are also related to the severe pain, and their mutual proximity draws attention to their interactions.12–14 Besides immune cells, ectopic endometrial cells are also capable of potentiating this milieu by secreting pro-inflammatory mediators that are involved in sensitization mechanisms leading to increased pain.15–18
The eutopic endometrium of endometriosis patients is also different from the normal tissue. Clinical observations suggested a role for the eutopic endometrium in pain, since hysterectomized patients experienced better outcomes.19 Ultrastructural and molecular differences have been found in the eutopic endometrium.20
The Transient Receptor Potential Vanilloid 1 (TRPV1) and Transient Receptor Potential Ankyrin 1 (TRPA1) receptors are structurally related non-selective cation channels predominantly localized on capsaicin-sensitive peptidergic sensory neurons.21 The most well-known exogenous activator of TRPV1 is capsaicin, while those of TRPA1 are allyl-isothiocyanate and cinnamaldehyde, but physical stimuli are also able to activate both receptors, such as heat (T > 43℃ for TRPV1) or cold (T < 17℃) and mechanical stretching also for TRPA1. They are important molecular integrators of a broad range of inflammatory stimuli (protons, bradykinin, prostaglandins, lipoxygenase products, anandamide, nitric oxide (NO), hydrogen peroxide, formaldehyde, methylglyoxal, acrolein and reactive oxygen species (ROS)) and play crucial roles in pain and inflammation. Thus, TRPV1 and TRPA1 antagonists became attractive candidates for the development of analgesic drugs.22–24
The presence of TRPV1 and TRPA1 on a range of non-neuronal cell types has been described, such as the skin, lung, kidney, pancreas, spleen, cornea, testis and the human endometrium, but their physiological and pathophysiological significance remains enigmatic.25 In human keratinocytes, the activation of TRPV1 and TRPA1 promotes inflammation by prostanoid and cytokine release. In contrast, in human mononuclear cells, their stimulation induces anti-inflammatory effects.26 These findings lead to speculations about a potential interaction between adjacent neuronal and non-neuronal receptors, capable to trigger sensory receptor sensitization and pain.27,28
The expression of TRPV1 at protein level has been shown in the intact human endometrium at both neuronal and non-neuronal sites.28,29 Although the non-neuronal receptor expression was steady during the menstrual cycle, neuronal TRPV1 expression presumably has an oestrogen-dependent regulation.30,31 In contrast, we have recently confirmed the oestrogen-dependent upregulation of both TRPV1 and TRPA1 in the rat endometrium.31 The consistent upregulation of TRPV1 in the peritoneal and endometrial tissues of women with chronic pelvic pain suggests its potential significance in various gynaecological pain symptoms.29 Further research revealed increased TRPV1 expression at both neuronal and non-neuronal sites in the pEL lesions and EM.28,32,33 In the ectopic endometrium, the density of TRPV1-expressing nerve fibres was higher and correlated positively with the severity of CPP and DM. TRPA1 mRNA upregulation has been observed only in the autologous unaffected peritoneal tissue of women with endometriosis.30 Furthermore, incubation of ectopic endometrial stromal cells with pro-inflammatory mediators promoted TRPV1 mRNA upregulation and its selective pharmacological stimulation elicited nitrogen monoxide (NO) and interleukin-1β (IL-1β) release.28 Neuronal TRPV1 expression in the eutopic endometrium of women with endometriosis did not differ from that of healthy controls.34 In contrast, non-neuronal TRPV1 immunoreactivity was significantly higher in both ectopic and autologous eutopic endometrium of women with adenomyosis as compared to controls.29
Despite these data on TRPV1 expression in the human endometrium and association with constant severe pelvic pain, there are no data about its expression in DIE. Furthermore, there is no information about TRPA1 expression in the human endometrium at all.
Therefore, our goal was to describe the expression of TRPV1 and TRPA1 receptor at mRNA and protein levels in rectosigmoid DIE lesions in comparison with the eutopic and intact human endometrium, as well as to find potential correlations with the clinical symptoms.
Methods
Study participants and tissue
Twenty-seven women, aged between 18 and 45 years, underwent laparoscopic surgery due to chronic DM or subfertility with no history of pain and were grouped as follows: Group 1 (n = 15), severe DM was found in conjunction with rectosigmoid DIE. Group 2 served as controls, patients with uterine fibroid-induced moderate DM (n = 7), and Group 3 created from patients with tubal infertility with no pain (n = 6). Patients were operated in the Department of Obstetrics and Gynaecology, University Hospital of Pécs, Hungary between 2013 and 2014. Exclusion criteria were as follows: pregnancy,1 menopause,2 recent hormonal contraception or intrauterine device use (within three months),3 coexistence of endometriosis with uterine fibroids,4 diffuse adenomyosis,5 clinical evidence of chronic medical disease or malignancy6 and clinical or laboratory evidence of acute inflammatory processes.7 Autologous eutopic endometrium (n = 6), ectopic endometrium from rectosigmoid DIE nodules (n = 15) and healthy rectosigmoid bowel wall samples (n = 15) from intact resection margins were matched with endometrial samples of women diagnosed with uterine fibroids (n = 7), marked as negative controls. Endometrium of patients with tubal infertility but with no detectable gynaecological pathology at laparoscopic inspection and no history of pain or endometriosis were evaluated as control samples (n = 6). Since TRPV1 receptor expression in human endometrium is steady during the menstrual cycle, we predominantly used proliferative phased endometrium as control at molecular processing.28,29 Endometrial sampling was made by curettage immediately prior surgery in all groups. The menstrual phase was calculated by the days elapsed from the first day of the last period whereas histologic dating of the endometrium was performed in conformity with Noyes criteria.35 Diagnosis of certainty and the depth of DIE lesion infiltration into colon layers were defined by histopathology with a simple scoring system (1: serosa, 2: subserosa, 3: muscularis, 4: submucosa, 5: mucosa). The stage and severity of endometriosis were determined using the revised American Fertility Society (rAFS) Scoring system.36 Common gastrointestinal and genitourinary tract symptoms such as abdominal discomfort, persistent change in bowel habits, anal mucus discharge, rectal bleeding, discomfort at bladder filling, urinary urgency, haematuria (frequent urination), resembling IBS or IC/PBS were evaluated jointly in a qualitative manner. IBS and IC/PBS were only considered when the other differential diagnostic options were excluded.
We created a complex data matrix including endometriosis-related pain history, demographic variables, spectrum and severity of subjective pain sensation and DIE lesion-related morphologic data. These variables were determined by hospital record analysis, interviewing and pain scale assessment, numerical rating scale where the given scores were mean as follows: 0: no pain, 1–3: mild pain, 4–6: moderate pain, 7–10: severe pain.37 Thus, we investigated the potential relationships between the clinical symptoms and the molecular findings.
RNA isolation and quantitative real-time polymerase chain reaction
Total RNA was extracted using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA) and Direct-Zol™ RNA isolation kit (Zymo Research, Irvine, CA, USA) following the manufacturer’s instructions. RNA samples were treated with DNase I (Zymo Research, Irvine, CA, USA), to remove contaminating genomic DNA, and quantified with NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies. Wilmington, Delaware USA). One microgram of total RNA was reverse transcribed with Maxima™ First Strand cDNA Synthesis Kit for reverse transcription-quantitative polymerase chain reaction (Thermo Scientific, Waltham, MA, USA). Reactions were performed on a Stratagene Mx3000P QPCR System (Agilent Technologies, Santa Clara, CA, USA) using ribosomal protein L29 (RPL29) mRNA levels as endogenous control. Each reaction contained 20 ng of cDNA, 1X Luminaris Color HiGreen Low ROX qPCR Master Mix (Thermo Scientific, Waltham, MA, USA), 0.3 µM of each primers and 6.8 µl water. The following primer pairs were used to amplify the target loci: RPL29 sense: 5′-GGCGTTGTTGACCCTATTTCC-3′ and antisense: 5′-TGTGTGTGGTGTGGTTCTTGG-3′; TRPA1 sense: 5′-ATGGACAGCTTGGTTACCTCCAC-3′ and antisense: 5′- CAGCACTCTGCTGGTTTGTATG.
AA-3′ TRPV1 sense: 5′-CAGCTCAATTGCTGTGCAGGTTA-3′ and antisense: 5′-TGCCAGTATGGATGGAGTGGAA-3′. The amplification efficiencies were the following: RPL29: 118.6%, TRPA1: 74.8%, TRPV1: 96.8% (Supplementary material, Figure 2). PCR amplification was performed under the following conditions: 95℃ for 10 min, followed by 40 cycles of 95℃ for 30 sec, 60℃ for 30 sec and 72℃ for 1 min. All real-time PCR reactions were carried out in a triplicate and included a melt curve analysis to ensure specificity of signal. Relative expression ratios were calculated using the MxPro QPCR Software (Agilent Technologies, Santa Clara, CA, USA) with the ΔΔCt method using samples of patients with tubal infertility as non-endometriosis controls.38 The sizes of the products were routinely controlled by agarose gel electrophoresis (2.5% agarose gel containing 0.01% GelRed (Biotium, Harward, CA, USA)) at 70 V for 40 min, using human TRPA1 and TRPV1 expressing CHO cells as positive controls (Supplementary material, Figure 3). RNA samples without reverse transcription did not provide any amplification products with the applied primers indicating that genomic DNA contamination was not present. Vilber-Lourmat Bio-Profil (version 97) gel documentation system with Bio-Capt Software (version 12.6) was used for image acquisition.
Immunohistochemistry and image analysis
Serial 4 µm sections were made from 4% formaldehyde fixed, paraffin embedded tissue sample blocks. Some slides were stained with haematoxylin and eosin and used for histopathological evaluation, others of the same blocks were stained with primary rabbit polyclonal antibody against the TRPA1 (ab68847, Abcam, Cambridge, UK) and guinea pig polyclonal antibody against the TRPV1 receptor (GP14100, Neuromics, Edina, MN,) diluted to 1:300 and 1:100, respectively. The antibody specificities for human tissue have been previously validated by us on human colon biopsy samples by preadsorption with the immunizing peptides.39 After routine deparrafinization and rehydration procedures, antigen retrieval was performed by heating the slides at 98℃ in ethylenediaminetetraacetic acid buffer (pH 9.0) for 30 min and left to cool naturally at room temperature. Overnight incubation of sections with the primary antibody performed at 4℃ temperature. After appropriate washing, slides were further incubated with the EnVision system anti-rabbit secondary antibody conjugated with horseradish peroxidase (DakoCytomation, Carpinteria, CA, USA) or VECTASTAIN® ABC-Peroxidase Kit- Guinea Pig IgG (PK-4007, BioMarker Ltd., Budapest, Hungary) for 30 min at room temperature. Bound antibody complexes were stained for 3–5 min or until appropriate for microscopic examination with 3.3-diaminobenzidine tetrachloride containing 0.01% hydrogen peroxide. The processing was ended by counterstaining with haematoxylin dye. Standardization was made using routinely performed positive and negative controls for each staining parameter. Negative control slides were reached incubating normal endometrium with tris-buffered saline instead of primary antibody. Staining of human myenteric ganglia slides served as positive control. Slides were scanned using an automatic digital slide scanner (Pannoramic Midi II, 3DHistech, Hungary) yielding high-quality digital images of the entire samples. Anti-TRPA1 and anti-TRPV1 staining intensity of individual cells was quantified from 0 to 3 (0: no staining, 1: weak staining, 2: moderate staining, 3: strong staining). By adding the scores of 50 analysed cells, the histology score (H-score) was calculated ranging from 0 to 150, as established in the literature.40 The glandular epithelium and the endometriosis stroma were evaluated separately, thus, 100 cells were analysed for each slide. For each staining parameter, double-inspection was made with a two-week interval by two independent pathologists who were blinded to the patients’ clinicopathological data.
Ethical approval
All patients signed a written informed consent prior their inclusion into the study. The research project was approved by the institutional ethics committee of University of Pécs Medical School, Hungary with a registration number of 5816.
Statistical analysis
The distribution of the data in each group was determined by the Kolmogorov-Smirnov normality test. Statistical analysis of two unmatched groups was performed by the student’s t-test (pSt) for unpaired comparison in case of normal distribution and the nonparametric Mann-Whitney U-test (pMW) if the data were not normally distributed. Correlation between the severity of clinical symptoms and TRPA1, TRPV1 immunopositivity in DIE samples was detected by the Pearson (DM, dyschezia) or Spearman (dysuria, dyspareunia, IBS, IC) rank correlation coefficients, in cases of normal and non-normal distributions, respectively. P value of less than 0.05 was considered statistically significant. All calculations were made with a licensed copy of GraphPad Prism 6.0 Software (http://www.graphpad.com/scientific-software/prism/).
Results
General information
The general information about the patients is summarized in Table 1.
Table 1.
Demographics and pain parameters of the study participants.
| Characteristic | Group 1 (n = 15) | Group 2 (n = 6) | Group 3 (n = 6) | P |
|---|---|---|---|---|
| Age at tissue harvesting (years) | 33.5 ± 4.2 | 36.7 ± 6.2 | 31.4 ± 6.7 | |
| BMI | 21.4 ± 3.3 | 21.8 ± 2.5 | 26.0 ± 7.3 | |
| Age at menarche (years) | 12.9 ± 1.0 | 13.0 ± 1.3 | 13.6 ± 0.8 | |
| Duration of menstrual cycle (days) | 29.3 ± 1.8 | 28.4 ± 2.4 | 29.6 ± 2.8 | |
| Duration of menses (days) | 5.0 ± 0.9 | 5.4 ± 1.3 | 4.0 ± 1.0 | *pMW = 0.0485 #pSt = 0.0377 |
| Menstrual phase | ||||
| Proliferative (1–14 days) | 7 (46.7%) | 6 (85.7%) | 4 (57.0%) | |
| Secretory (15–28 days) | 4 (26.7%) | 1 (14.3%) | 2 (29.0%) | |
| Data not available | 4 (26.7%) | 0 (0.0%) | 1 (14.0%) | |
| Gravidity | ||||
| 0 | 9 (60.0%) | 2 (28.6%) | 6 (86.0%) | |
| 1 | 6 (40.0%) | 1 (14.3%) | 1 (14.0%) | #pMW = 0.0373 |
| ≥2 | 0 (0.0%) | 4 (57.1%) | 0 (0.0%) | |
| Parity | ||||
| 0 | 11 (73.3%) | 2 (28.6%) | 6 (86.0%) | |
| 1 | 4 (26.7%) | 1 (14.3%) | 1 (14.0%) | #pMW = 0.0373 |
| ≥2 | 0 (0.00%) | 4 (57.14%) | 0 (0.0%) | |
| Severity of dysmenorrhoea at tissue harvesting (NRS) | 8.7 ± 1.2 | 4.3 ± 0.8 | 0.0 | ****pMW < 0.0001 ###pMW = 0.0006 |
| Severity of dyschezia at tissue harvesting (NRS) | 6.2 ± 3.7 | 0.0 | 0.0 | |
| Severity of dysuria at tissue harvesting (NRS) | 4.0 ± 4.6 | 0.0 | 0.0 | |
| Severity of dyspareunia at tissue harvesting (NRS) | 2.4 ± 2.8 | 0.0 | 0.0 |
Note: DIE: deep infiltrating endometriosis; DM: dysmenorrhoea; BMI: body mass index, NRS: numeric rating scale.
All patients were operated in the Department of Obstetrics and Gynaecology, Medical School, Clinical Centre, University of Pécs, Hungary. The samples were taken by curettage immediately prior to surgery in all groups. Group 1: patients with rectosigmoid DIE suffering of DM, Group 2: patients with DM but without endometriosis, Group 3: healthy controls (patients with tubal infertility with no pain). Statistical analysis was performed using Kolmogorov-Smirnov normality test followed by student’s t-test (pSt) (#P < 0.05, ###P < 0.001 Group 2 vs. Group 3) in case of normal distribution, or Mann-Whitney U test (pMW) (*P < 0.05, ****P < 0.0001 Group 1 vs. Group 3) if the data were not normally distributed. Data are presented as means ± SD.
All three groups were similar in terms of the demographic parameters; however, the duration of the menstruation cycles differed significantly in Group 1 (5.0 ± 0.9 days) and Group 2 (5.4 ± 1.3 days) compared to controls in Group 3 (4.0 ± 1.0 days). In addition, a significantly higher gravidity/parity index was recorded in Group 2 (0.04 both). The clinicopathologic background and detailed pain spectrum of women with DIE are described in Table 2. We processed 15 cases presenting all three (i.e., pEL, EM, DIE) main pathologic entities of endometriosis; superficial lesions resembled moderate to severe disease according to the rAFS Scoring system. Occasional findings of coexisting DIE lesions besides rectosigmoid presentation were made (number of DIE lesions/woman: 1.5 ± 0.6). Nodules were mostly localized in the muscular layer, submucosal or mucosal involvement was exceptional (1 case, 6.7% of bowel nodules). Longitudinal nodule size varied between 0.8 and 2.5 cm. The total number of previous surgeries for endometriosis in the DIE group was 26, since all the patients underwent at least one surgery for endometriosis, but 73% of them had two surgeries (1.7 ± 0.7 surgery per patient).
Table 2.
Summary of the occurrence and severity disease-related clinical and histopathological parameters of the patients with rectosigmoid deep infiltrating endometriosis involved in this study.
| Characteristic | Total number | Mean ± SD | % of all investigated patients |
|---|---|---|---|
| Previous surgery for endometriosis | 15 | 1.73 ± 0.70 | 100a |
| DIE lesions | 22 | 1.46 ± 0.56 | |
| Longitudinal diameter of the rectosigmoid DIE nodule, cm | 1.66 ± 0.38 | ||
| <1 | 1 | 6.66 | |
| 1–3 | 15 | 93.33 | |
| >3 | 0 | 0 | |
| Infiltration of the nodules in the colonic wall | |||
| Muscular layer | 12 | 80 | |
| Submucosal layer | 2 | 13.33 | |
| Mucosal layer | 1 | 6.66 | |
| DIE lesions removed | |||
| Rectosigmoid nodule | 15 | 68.18 | |
| Vesicouterine excavation lesionb | 2 | 9.09 | |
| USL lesion | 4 | 18.18 | |
| Otherc | 1 | 4.54 | |
| Total | 22 | 100 | |
| Associated endometriomas | 19 | 1.26 ± 0.45 | 100 |
| Associated superficial peritoneal endometriosis | 15 | 1.00 ± 0.00 | 100 |
| Main indication for surgery | |||
| Dysmenorrhoea | 10 | 66.66 | |
| Dyschezia | 4 | 26.66 | |
| Dysuria | 1 | 6.66 | |
| Associated painful symptoms | |||
| Dysmenorrhoea | 15 | 100 | |
| Dyschezia | 12 | 80.00 | |
| Deep dyspareunia | 7 | 50.00 | |
| Dysuria | 6 | 40.00 | |
| IBS | 10 | 53.33 | |
| IC/PBS | 5 | 46.66 | |
| Migraine | 7 | 46.66 | |
| Age at onset of severe dysmenorrhoea (years) | 27.27 ± 2.43 | ||
| Duration of severe dysmenorrhoea (years) | 5.40 ± 1.95 | ||
| Duration of continuous COC usage (years) | 8.30 ± 1.84 | ||
| Mean rAFS score | 56.20 ± 13.90 | ||
| rAFS stage | |||
| III | 2 | 35.00 ± 1.41 | 13.33 |
| IV | 13 | 59.46 ± 11.78 | 86.66 |
Note: DIE: deep infiltrating endometriosis; USL: uterosacral ligament; IBS: irritable bowel syndrome; IC/PBS: interstitial cystitis or painful bladder syndrome; COC: combined oral contraception therapy; rAFS: retrospective American Fertility Society.
All patients (100%) underwent at least one surgery for endometriosis; however, 73% of them had two surgeries.
Two subserosal vesical DIE lesions were removed by vesical shaving.
Intraoperative discovery of an intestinal DIE nodule in one patient.
In the vast majority of cases, severe DM served as main operative indication (66.7%). Other painful complaints were dyschezia, deep dyspareunia and dysuria; 53.3% of patients suffered from symptoms resembling IBS, while 46.7% of them had IC/PBS.
Patients benefited from a multidisciplinary management and a macroscopically complete surgery was performed in all cases. Rectosigmoid segment resection was the main surgical procedure performed. Fertility sparing approach was achieved in all cases.
We found no correlation between the severity of symptoms and the extent of endometriosis in terms of the mean rAFS score, size and depth of the DIE lesions. In addition, the duration of severe pain symptoms was not related to the intensity of pain, size and depth of the DIE nodules. Longitudinal nodule size proved to be independent of the depth of lesion (Table 2).
TRPA1 and TRPV1 mRNA is increased in the ectopic endometrium of DIE patients
Both TRPA1 and TRPV1 were detected at the mRNA level in the normal endometrium, reaching the threshold cycle between 28 and 36 cycles (Supplementary material, Figure 1). This clearly shows their local, not sensory neuronal expressions. Quantitative real-time polymerase chain reaction revealed differences in ectopic (rectosigmoid DIE nodule) and autologous eutopic endometrial samples (auto control endometrium) compared to normal endometrium (control). As shown in Figure 1, there was a remarkable 4.0–5.0 fold elevation of TRPA1 mRNA expression in the ectopic endometrium of rectosigmoid DIE lesions (Figure 1(a)). We detected significantly elevated (1.5–2.0 fold) TRPV1 receptor mRNA level in both ectopic and autologous eutopic endometrium (P = 0.0038) of women with endometriosis (Figure 1(b)). However, the relative TRPA1 and TRPV1 expressions did not differ in the endometrium of women with sole DM or intact sigmoid bowel wall of DIE patients.
Figure 1.
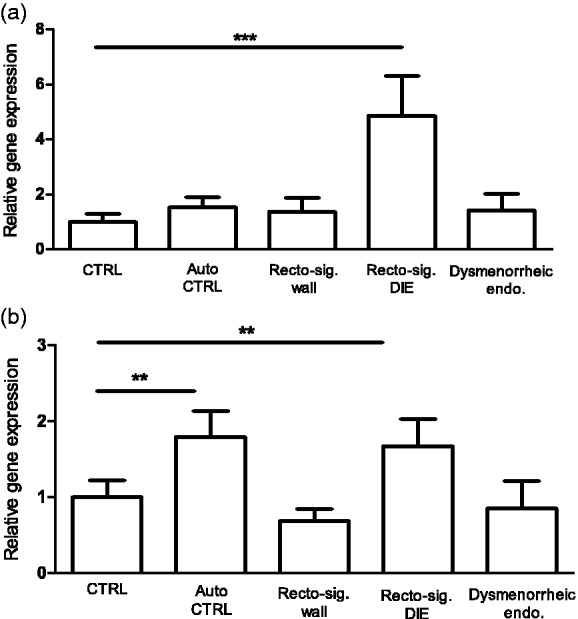
Relative gene expressions of TRPA1 (a) and TRPV1 (b) receptors. Columns represent the relative gene expression ratios normalised to RPL29 reference gene with qRT-PCR in the healthy control endometrium (n = 6), compared to autologous eutopic endometrium as autocontrol (n = 6), intact autologous rectosigmoid wall (n = 15), rectosigmoid DIE nodule (n = 15) and dysmenorrhoeic endometrium (n = 7) of women without endometriosis. Data are presented as mean ± SEM. (**P < 0.005, ***P < 0.001, Mann-Whitney U test).
TRPA1: transient receptor potential ankyrin 1; TRPV1: transient receptor potential vanilloid 1; RPL29: ribosomal protein L29; qRT-PCR: quantitative real-time polymerase chain reaction; CTRL: healthy control endometrium; Auto CTRL: autologous eutopic endometrium; DIE: deep infiltrating endometriosis.
TRPA1 and TRPV1 immunoreactivity is upregulated in the ectopic endometrium of DIE patients
Scattered cytoplasmic TRPA1 and TRPV1 receptor immunostaining was detected in stromal and epithelial cells of the normal endometrium (Figure 2(c) and Figure 3(c)). TRPV1 labelling was sparser compared to TRPA1. Remarkable intracellular TRPA1 and TRPV1 positivity was identified in both tissue compartments of the DIE samples (Figure 2(d) to (f) and Figure 3(d) to (f)). Similarly to the normal endometrium, here the glandular epithelial layer was stained more vigorously. In some ectopic endometrial sections, macrophages and endothelial cells were intensely positive for both receptors, while myenteric intramural ganglia and plasmocytes of the colonic stroma showed more intensive immunoreactivity for TRPA1 than for TRPV1. Significantly increased epithelial TRPA1 protein expression was found in the DIE samples compared to the control group. Moreover, 50% increase was detected in DIE epithelium compared to DIE stroma (Figure 4(a)). The TRPV1 protein expression was significantly higher both in the epithelium and stroma of the DIE patients compared to the control samples and also showed significantly increased immunopositivity (>50%) in the DIE epithelium (Figure 4(b)).
Figure 2.
Immunohistochemical staining of the TRPA1 receptor in healthy eutopic endometrium and in rectosigmoid DIE nodule. (a) Negative control using tris-buffered saline instead of the primary antibody in normal endometrial tissue. (b) Rectal myenteric ganglia, serving as positive control for TRPA1 expression. (c) Healthy eutopic endometrial tissue. (d) Rectosigmoid DIE nodule. (e) Rectosigmoid DIE nodule, glandular component. (f) Rectosigmoid DIE nodule, stromal component. (d) and (f) Sections shown on panels were taken from the same DIE patient who experienced severe, endometriosis-associated pain. Background staining was performed with haematoxylin and eosin to reveal the tissue structure. Black arrow heads denote TRPA1 receptor labelling. Magnification is X400, except panel (d) where it is X100. Scale bars: 50 µm, except panel (d) where it is 200 µm.
TRPA1: transient receptor potential ankyrin 1; DIE: deep infiltrating endometriosis.
Figure 3.
Immunohistochemical staining of TRPV1 receptor in healthy eutopic endometrium and in rectosigmoid DIE nodules. (a) Negative control using tris-buffered saline instead of the primary antibody in normal endometrial tissue. (b) Rectal myenteric ganglia, serving as positive control for TRPA1 expression. (c) Healthy eutopic endometrial tissue. (d) Rectosigmoid DIE nodule. (e) Rectosigmoid DIE nodule, glandular component. (f) Rectosigmoid DIE nodule, stromal component. (d) and (f) Sections shown on panels were taken from the same DIE patient who experienced severe, endometriosis associated pain. Background staining was performed with hematoxylin and eosin to reveal the tissue structure. Black arrow heads denote TRPV1 receptor labelling. Magnification is X400, except panel (d) where it is X100. Scale bars: 50 µm, except panel (d) where it is 200 µm.
TRPV1: transient receptor potential vanilloid 1; DIE: deep infiltrating endometriosis.
Figure 4.
Histology scores of TRPA1 (a) and TRPV1 (b) receptors in healthy control endometrium (n = 6) and rectosigmoid DIE nodule (n = 6) epithelium and stroma. Box plots with the whiskers represent the medians ± 25–75 percentiles of the histology score values (*P < 0.05, ***P < 0.001, one-way ANOVA, Bonferroni’s multiple comparison test).
TRPA1: transient receptor potential ankyrin 1; TRPV1: transient receptor potential vanilloid 1; DIE: deep infiltrating endometriosis.
Correlation of TRPA1 and TRPV1 immunopositivity in the ectopic endometrium of DIE patients with the clinical severity
There was strong positive correlation between DM severity and stromal TRPA1 (rp = 0.85) and TRPV1 (rp = 0.96) immunoreactivities, the severity of dyspareunia and TRPV1 expression on ectopic epithelial cells (rS = 0.88) and macrophages (rp = 0.89). Epithelial TRPA1 (rp = 0.82) and stromal TRPV1 (rp = 0.88) immunopositivity significantly correlated with the severity of dyschezia. We did not detect any correlation between DIE-associated painful symptoms and endothelial TRPA1 and TRPV1 immunopositivity (Table 3).
Table 3.
Correlation between TRPA1, TRPV1 immunopositivity and DIE-associated painful symptoms.
| Stroma |
Epithelium |
Macrophage |
Endothelium |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| (a) | ||||||||
| Dysmenorrhoea# | 0.85 | 0.03 | 0.77 | ns | 0.44 | ns | 0.47 | ns |
| Dyschezia# | 0.72 | ns | 0.82 | 0.04 | 0.54 | ns | 0.41 | ns |
| Dysuria | 0.3 | ns | 0.42 | ns | −0.67 | ns | −0.21 | ns |
| Dyspareunia | 0.56 | ns | 0.42 | ns | 0.22 | ns | −0.1 | ns |
| IBS | 0.8 | ns | 0.43 | ns | 0.71 | ns | 0.33 | ns |
| Interstitial cystitis | −0.1 | ns | 0.45 | ns | −0.71 | ns | −0.33 | ns |
| (b) | ||||||||
| Dysmenorrhoea# | 0.96 | 0.002 | 0.56 | ns | 0.56 | ns | 0.49 | ns |
| Dyschezia# | 0.88 | 0.02 | 0.71 | ns | 0.61 | ns | 0.41 | ns |
| Dysuria | −0.19 | ns | −0.3 | ns | 0.43 | ns | −0.083 | ns |
| Dyspareunia | 0.64 | ns | 0.88 | 0.03 | 0.89 | 0.03 | 0.03 | ns |
| IBS | 0.82 | ns | 0.8 | ns | 0.21 | ns | 0.21 | ns |
| Interstitial cystitis | 0 | ns | −0.1 | ns | 0.53 | ns | −0.21 | ns |
Note: TRPA1: transient receptor potential ankyrin 1; TRPV1: transient receptor potential vanilloid 1; DIE: deep infiltrating endometriosis; IBS: irritable bowel syndrome; NRS: numeric rating scale; ns: non significant.
Relationship between TRPA1 (a) and TRPV1 (b) immunopositivity quantified by histological score and DIE-associated painful conditions evaluated using NRS in DIE patients. Statistical analysis was performed using Kolmogorov-Smirnov normality test followed by parametric Person (# in the case of dysmenorrhoea and dyschezia) or nonparametric Spearman rank correlation. Statistically significant values (P ≤ 0.05; P < 0.005) are in bold.
Discussion
We provide here the first evidence on the presence of TRPA1 receptor at mRNA and protein levels in the human endometrium and its upregulation, alongside with the TRPV1 receptor in DIE nodules of the rectum and sigmoid colon. More interestingly, TRPA1 and TRPV1 expressions show correlations with the severity of many DIE-related pain symptoms, including DM, dyspareunia and dyschezia.
Local inflammation and sensory neuronal sprouting play a key role in the pathogenesis of endometriosis-related pain, which is mediated by a broad range of pro-inflammatory molecules.
These stimulate TRPV1/TRPA1 activity both on sensory nerve terminals and non-neuronal structures, which in turn further trigger the pain.
Despite ubiquitous TRPA1 and TRPV1 mRNA expressions in all the investigated tissues, significant receptor upregulation is limited to the DIE samples. Similarly, we observed elevated TRPV1 mRNA in the eutopic endometrium of endometriosis patients as compared to the endometrium of healthy women. These observations are in agreement with recent findings showing elevated TRPV1 mRNA expression in endometriosis lesions.28,30,33
We believe that the increased TRPA1 and TRPV1 immunoreactivity in the stromal and most epithelial cells of the rectosigmoid DIE samples, as well as the positive correlation between their expression and the severity of painful symptoms suggests a TRPA1/TRPV1-driven sensory function for these non-neuronal cells.
Growing evidence supports the role of the TRPV1-expressing nerves in endometriosis-related pain. It has been suggested that non-neuronal TRPV1 receptors in pEL might interfere with the inflammatory peritoneal environment and nociception in women with CPP.32 Despite a higher non-neuronal TRPV1 immunoreactivity in pEL samples of women with stronger pain symptoms, direct correlation between receptor expression and pain intensity was not found.33 Liu et al.28 detected functional TRPV1 receptors on cultured human ectopic endometrial stromal cells derived from EM cyst wall, which responded to prostaglandin-E2 (PGE2) and tumor necrosis factor alpha (TNFα) exposure by increased TRPV1 mRNA transcription. Furthermore, non-neuronal TRPV1 receptors had a lower stimulation threshold and their selective pharmacological activation provoked increased NO and IL-1β release.28 Therefore, it is also possible in vivo that TRPV1 activation on ectopic endometrial cells by a variety of inflammatory stimuli results in TNFα and IL-1β release in DIE samples. Besides inducing the pro-inflammatory cytokine cascade, TNFα and IL-1β are able to sensitize both neuronal and non-neuronal TRPV1 receptors28,41 triggering a vicious circle. TRPV1 on ectopic endometrial cells can be activated by mild stimuli to initiate Ca2+-dependent signalling pathways, pro-inflammatory cytokine release, cyclooxygenase-2 (COX-2), nerve growth factor (NGF) and ROS production.39,53–55 COX-2 catalyses the conversion of arachidonic acid into PGE2, PGF2α and PGI2 which are also potent TRPV1 sensitizers.42 High COX-2 levels were found in both ectopic and eutopic endometrium of women with endometriosis and are associated with hyperalgesia and DM.43,44 This may explain the effectiveness of several non-steroid anti-inflammatory drugs in the alleviation of endometriosis-related pain. Furthermore, elevated COX-2 and subsequent PGE2 production might induce TRPV1 mRNA upregulation in the eutopic endometrium of women with DIE.
NGF is a key molecule in neuronal sprouting, but its role in endometriosis-related pain has not been fully established.45 In DIE nodules, the close spatial relationship between the endometriosis foci and locally densified sensory nerve endings might facilitate the TNFα and NGF binding to their neuronal receptors and subsequent stimulation of the neuronal TRPV1 receptors.46 The cross-sensitization of the sensory TRPA1 and TRPV1 receptors through non-neuronal TRPA1/TRPV1 activation promotes peripheral sensitization and nociceptive pain.10,25,46 Sustained peripheral sensitization elicits permanent changes in the central nervous system explaining individual variances in pain perception and the presence of pain independently of endometriosis.2,47 Furthermore, TRPV1-positive nerves induce neurogenic inflammation by the release of neuropeptides with inflammatory and nociceptive functions, such as substance P and calcitonin gene-related peptide.48 A similar sensory role for non-neuronal TRPV1 receptors has been described in the urothelium, gustatory epithelium and auditory hair cells as well.49–51
In the present study, the non-neuronal TRPA1 expression was more pronounced than TRPV1 in both the endometriosis tissue and healthy control endometrium. Despite a great deal of recent attention, there is little evidence about TRPA1 in painful gynaecological conditions. Except the unaffected peritoneum adjacent to pEL lesions, TRPA1 mRNA was similar in the ectopic endometrium of pEL and the peritoneal tissue of healthy controls.30 Elevated TRPA1 protein expression increased in tissues with increased mechanical stress.25 Therefore, distortions of bowel anatomy through adhesions might contribute to the local upregulation of TRPA1 in DIE samples. ROS, such as NO, inflammatory and hypoxic conditions found in DIE nodules are also able to activate and/or upregulate TRPA1.25,52 NO has a role in angiogenesis, inflammation and nociception, its levels are elevated in endometriosis,53,54 and its reduction alleviated CPP.54 Inflammatory stimulation of TRPV1 receptors on endothelia and human ectopic endometrial stromal cells from EM samples trigger NO release which in turn might act on proximal TRPA1 receptors in a paracrine/autocrine way.28,55 ROS facilitates TRPA1 upregulation and subsequent interleukin 8 production of epithelial cells.56 Thus, as a ROS-sensor, non-neuronal TRPA1 receptors might operate synergistically with the non-neuronal TRPV1 to create a strong in situ nociceptive milieu.
Stromal TRPA1 and TRPV1 immunoreactivities strongly correlated with DM severity, as well TRPV1 expression on ectopic epithelial cells and macrophages with dyspareunia. Epithelial TRPA1 and stromal TRPV1 immunopositivity also positively correlated with dyschezia severity. Accumulating evidence supports a potential causal relationship between endometriosis and these functional pain symptoms, viscero-visceral sensitization and neurogenic inflammation might be a plausible explanation for these frequently coexisting conditions.47,57,58 The uterus shares the same visceral innervation with the rectum and urinary bladder and a neural cross-talk among these organs is required for their physiological activities.59,60 In fact, animal studies revealed that a subpopulation of TRPV1/TRPA1 expressing sensory neurons in the dorsal root ganglia collect nociceptive input from adjacent organs including mutual uterine-rectal, uterine-vesical and rectal-vesical neurons.61,62 Alterations in these intricate neural pathways might evoke overlapping pain symptoms (DM, dyschezia, dyspareunia, etc.) through spinal convergence and viscero-visceral sensitization.58,60 In agreement with this finding, intrauterine capsaicin instillation triggered lower urinary tract sensitization, and mustard oil sensitized the distal colon in rat.63,64 Neuronal TRPV1 increase in the rectum of patients with rectal hypersensitivity showed positive correlation with the symptoms. We hypothesize that DIE is an initial irritating event which sensitizes TRPA1/TRPV1-expressing colonic afferents to mechanical and chemical stimuli and induces local neuropeptide release (substance P, calcitonin gene-related peptide) resulting in neurogenic inflammation and persistent endometriosis-related pain. In our patient population, the self-reported alleviation of IBS after the rectosigmoid segment resection for DIE also supports this theory.
The major limitation of the present work is its mostly descriptive nature and relatively small sample size that we tried to balance with an extensive data collection and accurate statistical analysis. However, due to the importance of this topic, dramatically increasing interest in this area and novelty of our findings, these exploratory investigations have also got a great scientific value. The use of adequate control samples (intact endometrium, autologous eutopic endometrium) minimizes research bias.
Little is known about the presence, distribution and function of other TRP channels in different endometrial cells, but they are likely to be important molecules in intracellular signal transduction between epithelial and stromal cells of the uterus.65 The expression of TRPC3 and TRPM7 is stable during the menstrual cycle. In the beginning of the luteal phase, TRPM4 and TRPM6 elevate which is followed by TRPV2, TRPC4 and TRPC6 increase.66 These results suggest that specific up- and down-regulation of several TRP channels may play a role in physiological endometrial receptivity.
In summary, this is the first evidence for TRPA1 expression in the healthy human endometrium and its upregulation – alongside with that of TRPV1 – in rectosigmoid DIE nodules. Their expression increase positively correlates with the severity of different related pain symptoms suggesting their roles in the pathophysiological mechanisms.
Supplementary Material
Author Contributions
Noémi Bohonyi and Krisztina Pohóczky should be regarded as joint first authors. Zsuzsanna Helyes and Miklós Koppán contributed equally to this paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Hungarian grant KTIA_NAP_13-2014-0022 (MTA-PTE NAP B Pain Research Group, identification number: 888819), EFOP-3.6.1-16-2016-00004, GINOP-2.3.2-15-2016-00021 (The use of chip-technology in increasing the effectiveness of human in vitro fertilization), GINOP-2.3.2-15-2016-00050 – PEPSYS and GINOP-2.3.2 STAY ALIVE grants. The present scientific contribution is dedicated to the 650th anniversary of the foundation of the University of Pécs, Hungary.
References
- 1.Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol 1996; 87: 55–58. [DOI] [PubMed] [Google Scholar]
- 2.Berkley KJ. A life of pelvic pain. Physiol Behav 2005; 86: 272–280. [DOI] [PubMed] [Google Scholar]
- 3.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update 2011; 17: 327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011; 96: 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril 2016; 5: 585–596. [DOI] [PubMed] [Google Scholar]
- 6.Roman H, Vassilieff M, Gourcerol G, et al. Surgical management of deep infiltrating endometriosis of the rectum: pleading for a symptom-guided approach. Hum Reprod 2011; 1: 274–281. [DOI] [PubMed] [Google Scholar]
- 7.Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update 2015; 1: 329–339. [DOI] [PubMed] [Google Scholar]
- 8.Soares SR, Martínez-Varea A, Hidalgo-Mora JJ, et al. Pharmacologic therapies in endometriosis: a systematic review. Fertil Steril 2016; 5: 529–555. [DOI] [PubMed] [Google Scholar]
- 9.Arnold J, Vercellino G.F, Chiantera V, et al. Neuroimmunomodulatory alterations in non-lesional peritoneum close to peritoneal endometriosis. Neuroimmunomodulation 2013; 20: 9–18. [DOI] [PubMed] [Google Scholar]
- 10.McKinnon BD, Bertschi D, Bersinger NA, et al. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab 2015; 26: 1–10. [DOI] [PubMed] [Google Scholar]
- 11.Anaf V, Simon P, El Nakadi I, et al. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod 2002; 1: 1895–1900. [DOI] [PubMed] [Google Scholar]
- 12.Anaf V, Chapron C, El Nakadi I, et al. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil Steril 2016; 5: 1336–1343. [DOI] [PubMed] [Google Scholar]
- 13.Tran LVP, Tokushige N, Berbic M, et al. Macrophages and nerve fibres in peritoneal endometriosis. Hum Reprod 2009; 1: 835–841. [DOI] [PubMed] [Google Scholar]
- 14.Greaves E, Temp J, Esnal-Zufiurre A, et al. Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis. Am J Pathol 2015; 16: 2286–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang RJ, Wild RA, Ojago JM. Effect of tumor necrosis factor-alpha on adhesion of human endometrial stromal cells to peritoneal mesothelial cells: an in vitro system. Fertil Steril 1993; 59: 1196–1201. [DOI] [PubMed] [Google Scholar]
- 16.Arici A, Tazuke SI, Kliman HJ, et al. Endocrinology and paracrinology: interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukin-8 expression in human mesothelial cells. Mol Hum Reprod 1996; 1: 40–45. [DOI] [PubMed] [Google Scholar]
- 17.Lebovic DI, Chao VA, Martini J-F, et al. IL-1β induction of RANTES (Regulated upon Activation, Normal T Cell Expressed and Secreted) chemokine gene expression in endometriotic stromal cells depends on a nuclear factor-κB site in the proximal promoter. J Clin Endocrinol Metab 2001; 1: 4759–4764. [DOI] [PubMed] [Google Scholar]
- 18.Martin DC. Hysterectomy for treatment of pain associated with endometriosis. J Minim Invasive Gynecol 2016; 13: 566–572. [DOI] [PubMed] [Google Scholar]
- 19.Vercellini P, Barbara G, Abbiati A, et al. Repetitive surgery for recurrent symptomatic endometriosis: what to do? Eur J Obstet Gynecol Reprod Biol 2009; 146: 15–21. [DOI] [PubMed] [Google Scholar]
- 20.Jones CJP, Inuwa IM, Nardo LG, et al. Eutopic endometrium from women with endometriosis shows altered ultrastructure and glycosylation compared to that from healthy controls—A pilot observational study. Reprod Sci 2009; 1: 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szállási Á, Cortright DN, Blum CA, et al. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 2007; 6: 357–372. [DOI] [PubMed] [Google Scholar]
- 22.Szolcsányi J. Antidromic vasodilatation and neurogenic inflammation. Agents Actions 1988; 23: 4–11. [DOI] [PubMed] [Google Scholar]
- 23.Mezey É, Tóth ZE, Cortright DN, et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A 2000; 28: 3655–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szolcsányi J, Pintér E. Transient receptor potential vanilloid 1 as a therapeutic target in analgesia. Expert Opin Ther Targets 2013; 1: 641–657. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol 2012; 166: 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Southall MD, Li T, Gharibova LS, et al. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J Pharmacol Exp Ther 2003; 304: 217–222. [DOI] [PubMed] [Google Scholar]
- 27.Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther 2010; 125: 181–195. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Liu X, Duan K, et al. The expression and functionality of transient receptor potential vanilloid 1 in ovarian endometriomas. Reprod Sci 2012; 19: 1110–1124. [DOI] [PubMed] [Google Scholar]
- 29.Nie J, Liu X, Guo SW. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am J Obstet Gynecol 2016; 202: 346.e1–346.e8. [DOI] [PubMed] [Google Scholar]
- 30.Greaves E, Grieve K, Horne AW, et al. Elevated peritoneal expression and estrogen regulation of nociceptive ion channels in endometriosis. J Clin Endocrinol Metab 2014; 99: E1738–E1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pohóczky K, Kun J, Szalontai B, et al. Estrogen-dependent up-regulation of TRPA1 and TRPV1 receptor proteins in the rat endometrium. J Mol Endocrinol 2016; 56: 135–149. [DOI] [PubMed] [Google Scholar]
- 32.Poli-Neto OB, Adnor Filho A, e Silva JCR, et al. Increased capsaicin receptor TRPV1 in the peritoneum of women with chronic pelvic pain. Clin J Pain 2009; 25: 218–222. [DOI] [PubMed] [Google Scholar]
- 33.Rocha MG, Silva JCR, Ribeiro da Silva A, et al. TRPV1 expression on peritoneal endometriosis foci is associated with chronic pelvic pain. Reprod Sci 2011; 18: 511–515. [DOI] [PubMed] [Google Scholar]
- 34.Newman TA, Bailey JL, Stocker LJ, et al. Expression of neuronal markers in the endometrium of women with and those without endometriosis. Hum Reprod 2013; 28: 2502–2510. [DOI] [PubMed] [Google Scholar]
- 35.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obs Gynecol 1975; 122: 262–263. [DOI] [PubMed] [Google Scholar]
- 36.Hornstein MD, Gleason RE, Orav J, et al. The reproducibility of the revised American fertility society classification of endometriosis. Fertil Steril 2016; 59: 1015–1021. [PubMed] [Google Scholar]
- 37.Gerlinger C, Schumacher U, Wentzeck R, et al. How can we measure endometriosis-associated pelvic pain? J Endometr Pelvic Pain Disord 2012; 4: 109–116. [Google Scholar]
- 38.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kun J, Szitter I, Kemény Á, et al. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. Plos One 2014; 9: e08164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirsch FR, Varella- Garcia M, Braun PA, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 2003; 21: 3798–3807. [DOI] [PubMed] [Google Scholar]
- 41.Mita S, Shimizu Y, Sato A, et al. Dienogest inhibits nerve growth factor expression induced by tumor necrosis factor-α or interleukin-1β. Fertil Steril 2016; 101: 595–601. [DOI] [PubMed] [Google Scholar]
- 42.Moriyama T, Higashi T, Togashi K, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain 2005; 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sales KJ, Jabbour HN. Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction 2003; 126: 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan KN, Kitajima M, Fujishita A, et al. Pelvic pain in women with ovarian endometrioma is mostly associated with coexisting peritoneal lesions. Hum Reprod 2013; 28: 109–118. [DOI] [PubMed] [Google Scholar]
- 45.Eskander MA, Ruparel S, Green DP, et al. Persistent nociception triggered by nerve growth factor (NGF) is mediated by TRPV1 and oxidative mechanisms. J Neurosci 2015; 35: 8593–8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laux-Biehlmann A, d’Hooghe T, Zollner TM. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol Sci 2016; 36: 270–276. [DOI] [PubMed] [Google Scholar]
- 47.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Updat 2011; 17: 327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pintér E, Pozsgai G, Hajna Z, et al. Neuropeptide receptors as potential drug targets in the treatment of inflammatory conditions. Br J Clin Pharmacol 2014; 77: 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oakley B. Neuronal-epithelial interactions in mammalian gustatory epithelium. Ciba Found Symp 1991; 160: 277–287. [DOI] [PubMed] [Google Scholar]
- 50.Birder LA, Kanai AJ, de Groat WC, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 2001; 98: 13396–13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takumida M, Kubo N, Ohtani M, et al. Transient receptor potential channels in the inner ear: presence of transient receptor potential channel subfamily 1 and 4 in the guinea pig inner ear. Acta Otolaryngol 2005; 125: 929–934. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu S, Takahashi N, Mori Y. TRPs as chemosensors (ROS, RNS, RCS, gasotransmitters). In: Nilius B, Flockerzi V. (eds). Mammalian transient receptor potential (TRP) cation channels: volume II, Cham: Springer International Publishing, 2014, pp. 767–794. [Google Scholar]
- 53.Luo ZD, Cizkova D. The role of nitric oxide in nociception. Curr Rev Pain 2000; 4: 459–466. [DOI] [PubMed] [Google Scholar]
- 54.Rocha MG, Gomes VA, Tanus-Santos JE, et al. Reduction of blood nitric oxide levels is associated with clinical improvement of the chronic pelvic pain related to endometriosis. Braz J Med Biol Res 2015; 48: 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poblete IM, Orliac ML, Briones R, et al. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J Physiol 2005; 568: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin AH, Liu MH, Ko HK, et al. Lung epithelial TRPA1 transduces the extracellular ROS into transcriptional regulation of lung inflammation induced by cigarette smoke: the role of influxed Ca2+. Mediators Inflamm 2015; 2015: 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zielińska M, Jarmuż A, Wasilewski A, et al. Role of transient receptor potential channels in intestinal inflammation and visceral pain. Inflamm Bowel Dis 2015; 21: 419–427. [DOI] [PubMed] [Google Scholar]
- 58.Issa B, Onon TS, Agrawal A, et al. Visceral hypersensitivity in endometriosis: a new target for treatment? Gut 2012; 61: 367–372. [DOI] [PubMed] [Google Scholar]
- 59.Qin C, Malykhina AP, Akbarali HI, et al. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology 2016; 129: 1967–1978. [DOI] [PubMed] [Google Scholar]
- 60.Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol 2006; 291: R1592–R1601. [DOI] [PubMed] [Google Scholar]
- 61.Floyd K, McMahon SB, Morrison JF. Inhibitory interactions between colonic and vesical afferents in the micturition reflex of the cat. J Physiol 1982; 322: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrison TC, Dmitrieva N, Winnard KP, et al. Opposing viscerovisceral effects of surgically induced endometriosis and a control abdominal surgery on the rat bladder. Fertil Steril 2016; 86: 1067–1073. [DOI] [PubMed] [Google Scholar]
- 63.Peng HY, Huang PC, Liao JM, et al. Estrous cycle variation of TRPV1-mediated cross-organ sensitization between uterus and NMDA-dependent pelvic-urethra reflex activity. Am J Physiol- Endocrinol Metab 2008; 295: E559–E568. [DOI] [PubMed] [Google Scholar]
- 64.Peng HY, Chen GD, Tung KC, et al. Colon mustard oil instillation induced cross-organ reflex sensitization on the pelvic–urethra reflex activity in rats. Pain 2009; 142: 75–88. [DOI] [PubMed] [Google Scholar]
- 65.Dorr J, Fecher-Trost C. TRP channels in female reproductive organs and placenta. Adv Exp Med Biol 2011; 704: 909–928. [DOI] [PubMed] [Google Scholar]
- 66.De Clercq K, Held K, Van Bree R, et al. Functional expression of transient receptor potential channels in human endometrial stromal cells during the luteal phase of the menstrual cycle. Hum Reprod 2015; 30: 1421–1436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





