Abstract
Dark green islands (DGIs) surrounded by light green tissues (LGTs) are common leaf symptoms of plants that are systemically infected by various viruses that induce leaf mosaic in infected plants. The inoculation of Cucumber mosaic virus (CMV) in Nicotiana tabacum produced a commonly occurring sequence of classic patterns of DGIs and LGTs. Previous studies confirmed that there are significant differences between DGIs and LGTs in terms of physiology, biochemistry and molecular biology, but the mechanisms by which DGIs form remain unclear. To investigate the global gene expression changes that occur in these special tissues, individual differential gene expression tag libraries were constructed from three total RNA samples isolated from DGIs, LGTs and control plants (CK) and were sequenced using an Illumina HiSeqTM 2000. An analysis of differentially expressed genes (DEGs) and Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed. These analyses revealed the differences between DGIs, LGTs and CK. GO enrichment and KEGG pathway analyses suggested that several pathways related to photosynthesis and chlorophyll metabolism were enriched in DGIs compared to LGTs and CK. Several pathways related to apoptosis were significantly up-regulated in LGTs compared to DGIs. Additionally, we identified sets of DEGs that may be related to the formation or development of DGIs and LGTs. Our systematic analyses provide comprehensive transcriptomic information regarding DGIs and LGTs in CMV-infected N. tabacum. These data will help characterize the detailed mechanisms of DGI and LGT formation.
Introduction
Viruses can cause a variety of diseases in plants, and virus infection results in a range of symptoms. Mosaic viruses are plant viruses that induce leaf mosaic in infected plants; this syndrome includes the formation of discrete regions of dark green tissues [1]. The dark green tissues are also called dark green islands (DGIs), which are surrounded by yellow or light green tissues (LGTs) [1–3]. DGIs, which are somewhat of a mystery in plants, have attracted the attention of many researchers [4]. Therefore, extensive literature is available concerning the comparison of DGIs and LGTs [5–7].
Previous studies indicated that DGIs typically possess more than one cell layer and can also contain multiple cells that they were produced by a single cell division [5]. The cells within the DGIs have been demonstrated to be free of viral RNAs and proteins. However, those cells are connected to infected cells in LGTs through plasmodesmata [5]. The formation of DGIs on leaves is complex and can occur randomly in any direction [8, 9]. Moreover, there is no definite causal agent for DGI formation, and no specific method defines the final size of DGIs [9]. DGIs can vary in size and position on systemically infected leaves [9]. Additionally, several factors, including the virus strain, the leaf age, the season of the year, and the time of infection, can influence the number of DGIs on systematic leaves [9]. Moreover, DGIs were found to be resistant to reinfection by the same or other strains of challenging viruses that induce mosaic symptoms but were susceptible to infection by dissimilar viruses [3, 10, 11]. Several studies also revealed that DGI cells exhibit cytology similar to that of healthy leaf cells [12–14]. In contrast, the cells within LGTs are usually deformed [5, 12, 15]. For instance, LGT cells are smaller than cells in healthy tissues or DGIs, and the arrangement of LGT cells is often irregular and disordered [12]. It has been shown that recovered tissues undergo posttranscriptional degradation of viral and transgene RNA [16]. Moore et al. (2001) proposed that similar to recovered tissues, a post-transcriptional gene silencing (PTGS) mechanism could be responsible for the maintenance of DGIs [14]. Our previous study also suggested that there are significant differences between DGIs and LGTs in terms of physiology, biochemistry and molecular biology [13]. Additionally, DGIs were more similar to recovered tissues and could resist viral infection better than LGTs, which is a prediction that is supported by most DGI characteristics [13].
Cucumber mosaic virus (CMV), which is a type of mosaic virus, has a worldwide distribution and a very wide host range [17]. The symptoms of CMV can vary greatly depending on the species and age of the plants [18]. The interaction between Nicotiana tabacum and CMV provides a commonly occurring sequence of classic patterns of DGIs and LGTs as well as special tissues in successive expanded leaves at 6–10 weeks post-inoculation. In recent years, the development of RNA-sequencing (RNA-seq) technology has provided a rapid and cost-effective platform for measuring differences in gene expression [19, 20]. This new technology revolutionized the procedures that are used to analyze gene expression levels [20–22], which are important for evaluating specific, different, and abundant genes from millions of sequencing tags in experimental models.
Digital gene expression (DGE) exploration of the special tissues formed on CMV-infected N. tabacum plants have rarely been reported. In the present study, an RNA-seq method was used in combination with DGE profile analysis to investigate the gene expression changes that occur in DGIs and LGTs. Our preliminary results provided new insights at the molecular and cellular levels and identified the biological events associated with the differences between DGIs and LGTs. This evidence may broaden our understanding of the mechanisms by which these tissues form.
Materials and methods
Plant cultivation and virus inoculation
N. tabacum cv. NC89 plants were grown in a temperature-controlled growth chamber at an irradiation dose of 60 μM m-2 s-1 on a 12-h light/12-h dark cycle with an average temperature of 23°C. After approximately 5 weeks of growth, two leaves from the bottom insertions were mechanically inoculated with CMV-AH, as described previously [23]. Corresponding leaves from the control plants were mock-inoculated with phosphate buffer. Overall, 160 and 100 tobacco plants were CMV-inoculated and mock-inoculated, respectively. Sample leaves were obtained from the mock-inoculated and CMV-infected plants at 20 days post-inoculation (dpi), and at least 80 DGIs and 80 LGTs regions from different plants were cut out with a sterile scalpel, frozen immediately in liquid nitrogen and stored at -80°C until RNA extraction for DGE sequencing.
RNA sequencing and transcriptome data analysis
Illumina sequencing was carried out at Novogene Bioinformatics Technology Co., Ltd., in Beijing, China. Equal quantities of total RNA from the three samples (DGIs, LGTs and CK) were mixed to prepare the pooled RNA sample for RNA-Seq. Total RNA was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendations. Then, RNA degradation and contamination were monitored on 1% agarose gels. We evaluated the RNA purity using a NanoPhotometer® spectrophotometer (IMPLEN, Westlake Village, CA, USA). Moreover, the Qubit® RNA Assay Kit was used in a Qubit® 2.0 Fluorometer (Life Technologies, CA, USA) to measure the RNA concentration. RNA integrity was assessed with the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA).
Sequencing libraries were generated using the NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA), following the manufacturer’s protocol. Index codes were added to attribute sequences to each sample. First, mRNA was purified from the total RNA with poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under an elevated temperature in NEBNext First Strand Synthesis Reaction Buffer (5X). Next, a random hexamer primer and M-MuLV Reverse Transcriptase (RNase H-) were used to synthesize the first strand cDNA. Then, second strand cDNA synthesis was performed using DNA Polymerase I and RNase H. Exonuclease/polymerase activities were used to convert the remaining overhangs into blunt ends. After adenylation of the 3’ ends of the DNA fragments, the NEBNext Adaptor with hairpin loop structures was ligated to prepare for hybridization. To select cDNA fragments of 150–200 bp in length, the AMPure XP system (Beckman Coulter, Beverly, USA) was used. Next, 3 μl of the USER Enzyme (NEB, USA) was used with size-selected, adaptor-ligated cDNA at 37°C for 15 min, followed by a 5 min incubation at 95°C before PCR. PCR was then performed with the Phusion High-Fidelity DNA polymerase, Universal PCR primers and the Index (X) Primer. Finally, the PCR products were purified (AMPure XP System), and the library quality was assessed using the Agilent Bioanalyzer 2100 system.
Clustering of the index-coded samples was performed using the cBot Cluster Generation System and the TruSeq PE Cluster Kit v3-cBot-HS (Illumina), according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Hiseq 2000 platform, and 125 bp paired-end reads were generated.
Raw data (raw reads) in fastq format were first processed through in-house Perl scripts. Clean data (clean reads) were obtained by removing reads containing adapter sequences, reads containing poly-N, and low-quality reads from raw data. The Q20, Q30, GC-content, and sequence duplication level of the clean data were calculated. All downstream analyses were based on high-quality clean data.
The left files (read1 files) from all libraries/samples were pooled into one large left.fq file. The right files (read2 files) were pooled into one large right.fq file. Transcriptome assembly was accomplished based on the left.fq and right.fq files using program Trinity with min_kmer_cov set to 2 by default and all other parameters set to the default values [24]. Gene functions were annotated based on the following databases: the non-redundant (NR, NCBI) protein database, the nucleotide sequence (NT, NCBI) database, the Protein family (PFAM), the Swiss-Prot database, the Kyoto Encyclopedia of Genes and Genomes Ortholog (KO) database, the Clusters of euKaryotic Ortholog Groups of proteins (KOG, NCBI) database and the Gene Ontology (GO) database.
Library preparation for DGE sequencing and DGE data analysis
Total RNA was isolated from the three samples (DGIs, LGTs and CK) stored at -80°C using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The methods used for RNA extraction, RNA quantification, RNA qualification, library preparation and clustering for DGE sequencing were the same as those described above for transcriptome sequencing. The library preparations were also sequenced on an Illumina HiSeqTM 2000 platform, and 50 bp single-end reads were generated. In addition, the quality control procedures were similar to the quality control procedures described above for transcriptome sequencing.
Gene expression levels were estimated via RSEM [25] for each sample. The bowtie parameter mismatch was 2. Clean data were mapped back onto the assembled transcriptome, and a read count for each gene was obtained from the mapping results.
In RNA-seq, FPKM (expected number of Fragments Per Kilobase of transcript sequence per Millions base pairs sequenced), considers the effects of sequencing depth and gene length for the reads count simultaneously. In currently, it is the most common method of for estimating levels of gene expression [26]. Therefore, we made a FPKM conversion for the read count.
Because there were no biological replicates, the read counts were adjusted for each sequenced library using the edgeR program package through one scaling normalized factor prior to differential gene expression analysis [27–29]. Differential expression analysis of two samples was performed using the DEGseq (2010) R package. The P-value was adjusted using the q-value [30]. Corrected P-values were also called q-values. A q-value<0.005 and log2(fold_change)|>1 were set as the thresholds for significant differential expression [31].
GO enrichment analysis of the differentially expressed genes (DEGs) was implemented using the GOseq R packages based on the Wallenius non-central hyper-geometric distribution [32, 33], which can adjust for gene length bias in DEGs.
The Kyoto Encyclopedia of Genes and Genomes (KEGG), which is a valuable database resource, was used to facilitate our understanding of the high-level functions and utilities of various levels of the biological system, including the cell, organism and ecosystem levels, from molecular-level information, especially large-scale molecular datasets generated by genome sequencing and other high-throughput experimental technologies (http://www.genome.jp/kegg/) [34]. The KOBAS (2.0) software was used to detect the statistical enrichment of DEGs in KEGG pathways [35]. In KEGG pathway analysis, the formula for calculating enriched P-values was:
Here, N is the total number of genes that had KEGG annotations, n is the number of DEGs in N, M is the total number of genes annotated to specific pathways, and m is number of DEGs in M. A P-value of 0.05 was selected as the threshold for deciding whether a gene set was significantly enriched.
Leaf-press blotting and paraffin sections
For the leaf-press blotting assays, systemic CMV-infected N. tabacum leaves with typical DGIs and LGTs were pressed onto nitrocellulose membranes, using the method described by Gal-On et al (1994), with some modifications [36]. The subsequent steps were the same as the steps used for protein blotting [37]. Paraffin sections were collected as described previously, with several modifications [38].
Measurement of chlorophyll fluorescence parameters and chlorophyll content
The content of chlorophylls (Chl) a and b was determined according to the methods described by Kichtenthaler and Wellburn [39]. The Chl fluorescence parameters of the leaves were measured using an FMS2 fluorescence meter (PAM-2100, Walz, Germany) according to previously described methods [13].
Determination of electrolyte leakage, methane dicarboxylic aldehyde and hydrogen peroxide
Electrolyte leakage and methane dicarboxylic aldehyde (MDA) content viassayed as described previously [37]. Endogenous concentrations of hydrogen peroxide (H2O2) were detected as described previously [40]. H2O2 concentrations were determined using the Amplex red hydrogen peroxide/peroxidase assay kit (Invitrogen).
Determination of cytokinin, abscisic acid and indole acetic acid
The endogenous level of cytokinin (CTK) was quantified via high-performance liquid chromatography-mass spectrometry (HPLC-MS), according to previously described methods [13]. The compound 6-benzylaminopurine (6-BA) was obtained from Sigma-Aldrich and used as an internal standard. Abscisic acid (ABA) and indole acetic acid (IAA) concentrations were measured via an enzyme-linked immunosorbent assay (ELISA), using the protocols described by Teng et al. (2006) and Chen et al. (2009), respectively [41, 42].
Quantitative real-time polymerase chain reaction validation
Validation of the RNA-seq data for 12 different genes was performed using quantitative real-time polymerase chain reaction (qRT-PCR) analysis (Bio-Rad Laboratories, Hercules, CA, USA). The qRT-PCR analysis was performed using 3 RNA samples: the RNA sample used in the DGE experiment and two other replicates from new plant materials. Primers for selected genes were designed using the Primer-Premier 5 software (PREMIER Biosoft, Palo Alto, CA, USA). The primer pairs are summarized in S1 Table. The cDNA was amplified using SYBR Premix Ex Taq (TaKaRa). The amplification of the target genes was monitored every cycle based on SYBRgreen I fluorescence. The relative quantification of the target gene expression level was achieved using the comparative Ct method [43]. Three technical replicates were performed for each experiment. The amplification of the elongation factor 1a gene (EF1a) was used as an internal control.
Statistical analyses
The results are expressed as the means of at least three independent measurements, and the average results are presented with standard deviations (n = 3). The student’s t-test was used for comparisons between different samples. Differences were considered to be statistically significant when P <0.05.
Results
Annotation of unigenes in N. tabacum
When N. tabacum was systemically infected with CMV, DGIs formed and gave rise to symptoms that included chlorosis of the leaves surrounding discrete regions of DGIs (Fig 1A). The appearance of DGIs and LGTs differed significantly. Several previous studies indicated that significant differences exist between DGIs and LGTs [2, 4, 6, 10, 13, 44]. One of the most significant differences between these types of tissues is virus accumulation. Leaf-press blotting showed directly that virus accumulation in LGTs was much higher than virus accumulation in DGIs (S1 Fig). To thoroughly analyze the differences between these special tissues, RNA-Seq was used to profile transcriptional changes.
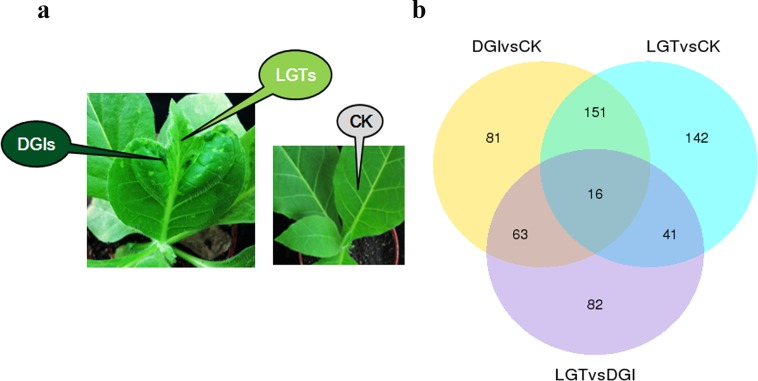
Fig 1.
(a) Symptoms of N. tabacum leaves systemically infected with CMV. CK, N. tabacum were mock inoculated with phosphate buffer; DGI, dark green tissues of CMV-infected N. tabacum; LGT, light green tissue of CMV- infected N. tabacum; (b) Venn diagram of DEGs between three comparisons (DGI vs. CK, LGT vs. CK and LGT vs. DGI). The sum of the numbers in each large circle represents total number of differentially expressed genes between combinations, the overlap part of the circles represents common differentially expressed genes between combinations.
Since the genome sequence of N. tabacum is not available, we first defined the transcriptome of N. tabacum using RNA-Seq. Equal quantities of total RNA from the three samples (DGIs, LGTs and CK) were pooled and used for library construction. The library was sequenced on an Illumina HiSeqTM 2000 platform. A total of 107,359, 006 clean reads (from a total of 110,411,642 raw reads) were generated using Illumina paired-end sequencing (S1 Data) and assembled into 156,074 transcripts. The 156,074 transcripts were assembled according to average and N50 lengths of 892 and 1585 bp, respectively (S2 Fig). After gap filling with paired-end reads, the transcripts were assembled into 106,429 unigenes. The unigene length distribution is displayed in S3 and S4 Figs. The N50 length of the unigenes was 1116 bp, and the N90 length was 269 bp. The BLAST results for the unigenes were compared against seven databases, including NR, NT, PFAM, the Swiss-Prot database, the KO database, the KOG database and the GO database. The results are shown in S2 Data.
The gene functions of the sequences with BLAST hits were annotated based on three databases: KOG, KO and KEGG pathway. KOG analysis indicated that 12,151 unigenes had KOG annotations and could be grouped into 26 clusters (S5 Fig). In contrast, 27,618 unigenes had GO annotations and were distributed among 48 functional classes (S6 Fig). 10132 unigenes with KEGG annotations were distributed in 272 KEGG pathways. Signal transduction, carbohydrate metabolism and translation were the dominant KEGG pathways (S7 Fig).
Identification of DEGs in DGIs, LGTs and CK
To better understand these special tissues, DEGs of transcripts in N. tabacum leaves after mock inoculation (CK) and CMV inoculation (DGIs and LGTs) were examined using an Illumina platform. A total of 13,810,892 (CK), 12,791,386 (DGIs), 12,910,775 (LGTs) sequence reads were generated. A sequencing summary is provided in Table 1 and S8 Fig.
Table 1. Statistics of DGE sequencing.
| Sample | Raw Reads | Clean Reads | Clean Bases | Error(%) | Q20(%) | Q30(%) | GC Content(%) |
|---|---|---|---|---|---|---|---|
| CK | 13810892 | 13748924 | 0.69G | 0.01 | 99.03 | 96.81 | 42.36 |
| DGI | 12791386 | 12739290 | 0.64G | 0.01 | 99.02 | 96.78 | 42.49 |
| LGT | 12910775 | 12855148 | 0.64G | 0.01 | 99 | 96.74 | 42.37 |
The transcriptome sequences assembled by Trinity were used as reference sequences [45, 46]. The clean data were mapped back onto the assembled transcriptome using the RSEM software [25]. The bowtie parameter mismatch was 2. In the CK, DGIs and LGTs, 92.57%, 92.14%, and 92.22% of the total reads from DGE were mapped to the reference sequences, respectively (Table 2).
Table 2. DGE reads mapped to the reference sequences.
| Sample name | Total reads | Total mapped |
|---|---|---|
| CK | 13748924 | 12727858 (92.57%) |
| DGI | 12739290 | 11738400 (92.14%) |
| LGT | 12855148 | 11855547 (92.22%) |
The normalized expression levels of control and CMV-infected plants were compared to detect differentially expressed genes. A Venn diagram showed the distribution of DEGs among the three comparisons (Fig 1B). A total of 311 DEGs were obtained after a comparison of DGIs to CK (DGIs vs. CK). Overall, 350 DEGs were identified after comparing LGTs to CK (LGTs vs. CK). In comparison, only 202 DEGs were identified after a comparison between LGTs and DGIs (LGTs vs. DGIs). In total, 81, 142 and 82 DEGs were specific for DGIs vs. CK, LGTs vs. CK and LGTs vs. DGIs, respectively. Curiously, the LGTs vs. CK comparison showed a greater number of total and specific differentially expressed genes in comparison to the DGIs vs. CK comparison, including the genes that encode cytochrome P450 (c47617_g1, c51201_g3, c44961_g1), probable WRKY transcription factor 40 (c46829_g1, c51556_g2, c40144_g1, c40144_g3) and probable xyloglucan endotransglucosylase (c47038_g4, c36196_g1, c19467_g2). In addition, in DGIs, 295 (95%) and 16 (5%) of the 311 genes were up-regulated and down-regulated, respectively, relative to CK (S3 and S4 Data). In LGTs, 301 (86%) and 49 (14%) of the 350 genes were up-regulated and down-regulated, respectively, relative to CK (S5 and S6 Data). However, only 201 DEGs were detected in the LGTs vs. CK comparison; of those DEGs, 39 (19%) were up-regulated and 163 (81%) were down-regulated (S7 and S8 Data).
Functional annotation of DEGs
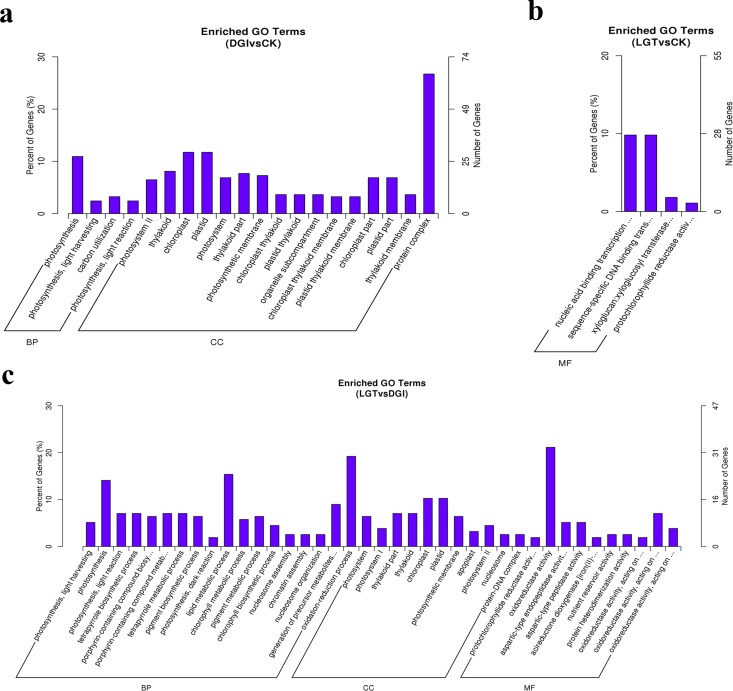
To obtain a functional categorization of the DEGs, GO analysis was used to classify the DEGs. Of the 311 total DEGs identified in the DGIs vs. CK analysis and the 350 total DEGs identified in the LGTs vs. CK analysis, 247 and 275 DEGs, respectively, were assigned one or more GO terms (S9 and S10 Data). Twenty significantly different GO annotations were obtained from the DGIs vs. CK analysis (Fig 2A), with four annotations belonging to biological processes and sixteen annotations belonging to cellular components. However, a GO analysis of these genes did not reveal significantly enriched groups of genes belonging to the molecular function categories. With 27 genes, photosynthesis (GO: 0015979) was dominant in the main category of biological processes. A protein complex (GO: 0043234) that consisted of 66 genes was dominant in the main category of cellular components. We also observed that a high percentage of genes from functional groups for chloroplasts (GO: 0009507), with 29 genes, and plastids (GO: 0009536), with 29 genes, were enriched. In contrast, only four significantly different GO annotations belonging to the molecular function categories were enriched in LGTs vs. CK (Fig 2B). Nucleic acid binding transcription factor activity (GO: 0001071), with 27 genes, and sequence-specific DNA binding transcription factor activity (GO: 0003700), with 27 genes, were dominant in the molecular functions category.
Fig 2. Histogram of Gene Ontology classification.
The results are summarized in three main categories: biological process (BP), cellular component (CC), and molecular function (MF). The left Y-axis indicates the percentage of a specific category of genes in that main category. The right Y-axis indicates the number of genes in a category.
The 156 selected significant DEGs in the LGTs vs. DGIs comparison were categorized into 39 functional groups that were clustered in three main GO classification categories (biological processes, cellular components, and molecular functions) (Fig 2C, S11 Data). Biological processes made up the majority of the GO annotations (18 significantly enriched groups), followed by cellular components (11 significantly enriched groups) and molecular functions (10 significantly enriched groups). Among 18 different biological process categories, the oxidation-reduction process (GO: 0055114), with 30 genes, the lipid metabolic process (GO: 0006629), with 24 genes, and photosynthesis (GO: 0015979), with 22 genes, were the three most frequently identified GO terms for the genes that were part of the biological processes component. Within the cellular components, the major categories were chloroplasts (GO: 0009507) (16 genes) and plastids (GO: 0009536) (16 genes). Additionally, oxidoreductase activity (GO: 0016491) (33 genes) was dominant in the main category of molecular functions.
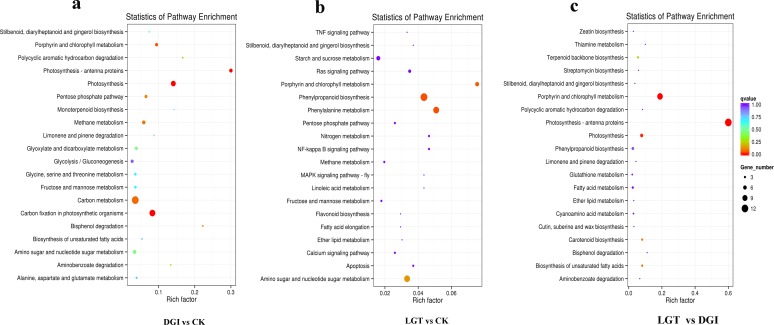
In addition to GO assignments, KEGG pathway mapping based on KO terms for assignments was also carried out with the total list of DEGs from the three comparisons. A total of 311 DEGs in the DGIs vs. CK comparison were assigned to 58 KEGG pathways. The top 20 enriched pathways were displayed in Fig 3A. The results indicated that the most frequently represented pathways were ‘photosynthesis-antenna proteins’ (PATH: ko00196), ‘photosynthesis’ (PATH: ko00195), ‘carbon fixation in photosynthetic organisms’ (PATH: ko00710), and ‘porphyrin and chlorophyll metabolism’ (PATH: ko00860), with 6, 9, 11 and 5 members, respectively.
Fig 3. Functional classification and pathway assignment of unigenes by KEGG.
The top 20 enriched pathways for LGTs vs. CK and LGTs vs. DGIs are displayed in Fig 3B and 3C, respectively. The pathways with the greatest representation in LGTs vs. CK were the phenylpropanoid biosynthesis pathway (PATH: ko00940), with 7 members, and phenylalanine metabolism (PATH: ko00360), with 6 members. The most frequently represented pathways in LGTs vs. DGIs were photosynthesis-antenna proteins (PATH: ko00196), porphyrin and chlorophyll metabolism (PATH: ko00860), and photosynthesis (PATH: ko00195), with 12, 10 and 5 members, respectively.
Validation of DGE data by qRT-PCR
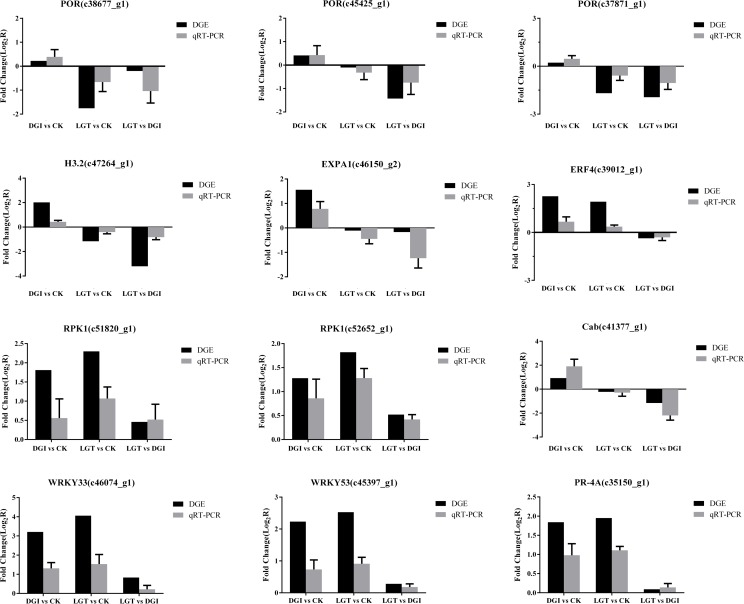
Validation of the DGE data for 12 unigenes with annotations was performed via qRT-PCR (Fig 4). The results showed that the qRT-PCR data for these genes were consistent with the DGE results. For example, both qRT-PCR and DGE analysis showed that the genes encoding expansin-A1 (EXPA1 c46150_g2), protochlorophyllide reductase (POR c45425_g1, 38677_g1, 37871_g1) and histone (H3.2 c47264_g1) were highly expressed in DGIs, while these genes were significantly suppressed in LGTs compared to CK. Additionally, the DGE results indicated that the genes encoding probable LRR receptor-like serine/threonine-protein kinase (RPK1 c51820_g1, c52652_g1), probable WRKY transcription factors (WRKY33 c46074_g1, WRKY53 c45397_g1) and ethylene-responsive transcription factor 4 (ERF4 c39012_g1) were significantly up-regulated in DGIs and LGTs compared to CK, which was consistent with the qRT-PCR expression patterns (Fig 4). Moreover, the expression levels of the genes encoding pathogenesis-related protein (PR-4A c35150_g1) and chlorophyll a/b binding protein (Cab c41377_g1) that were revealed by DGE analysis were also verified by the qRT-PCR analysis (Fig 4). Thus, the results of the qRT-PCR analysis were very similar to those obtained via RNA-seq analysis, which indicated that the changes in expression detected by RNA-seq were accurate.
Fig 4. Validation of the DGE analysis by quantitative real-time polymerase chain reaction (qRT-PCR).
Error bars represent the standard deviations of qRT-PCR signals (n = 3).
Expression of photosynthesis- and photosystem-related genes was up-regulated in DGIs
In the present study, we identified a substantial number of genes involved in different steps of photosynthesis and the photosystem that were up-regulated in DGIs (Table 3). In contrast, few expression differences for these genes were observed in the LGTs vs. CK comparison. In addition, an examination of GO terms suggested that the three main categories were devoted primarily to the control of photosynthesis-related processes and chloroplast components. For example, the GO terms photosynthesis (GO: 0015979), photosynthesis, light harvesting (GO: 0009765), photosystem I (GO: 0009522), photosystem II (GO: 0009523), chloroplast (GO: 0009507), photosynthetic membrane (GO: 0034357), chloroplast thylakoid lumen (GO: 0009543) and chloroplast thylakoid (GO: 0009534) were enriched in DGIs vs. LGTs and DGIs vs. CK. The KEGG pathway analysis also indicated that several pathways related to photosynthesis were enriched in DGIs vs. LGTs and DGIs vs. CK, including porphyrin and chlorophyll metabolism (PATH: ko00860), photosynthesis-antenna proteins (PATH: ko00196), photosynthesis (PATH: ko00195), carbon fixation in photosynthetic organisms (PATH: ko00710) and carotenoid biosynthesis (PATH: ko00906).
Table 3. Differentially expressed (log2Fold_change) of photosynthesis- and photosystem-related genes in DGI vs. CK, DGI vs. LGT and LGTs vs. CK.
| Gene ID | Swissprot Description | A | B | C |
|---|---|---|---|---|
| c44343_g2 | Chlorophyll a-b binding protein 36 | 1.96 | 1.37 | 0.54 |
| c44396_g1 | Chlorophyll a-b binding protein 13 | 1.85 | 1.08 | 0.78 |
| c48271_g1 | Chlorophyll a-b binding protein CP24 | 1.59 | 0.71 | 0.90 |
| c37314_g1 | Photosystem II repair protein PSB27-H1 | 1.48 | 0.71 | 0.79 |
| c44343_g1 | Chlorophyll a-b binding protein 37 | 1.42 | 1.37 | 0.07 |
| c35705_g1 | Photosystem II 5 kDa protein | 1.40 | 1.07 | 0.35 |
| c38029_g1 | Thylakoid lumenal 16.5 kDa protein | 1.16 | 1.07 | 0.11 |
| c41377_g2 | Chlorophyll a-b binding protein 4 | 1.12 | 1.73 | -0.58 |
| c42990_g1 | Chlorophyll a-b binding protein 8 | 1.11 | 1.13 | 0.02 |
| c41707_g1 | Photosystem II core complex proteins psbY | 1.10 | 0.93 | 0.19 |
| c38916_g1 | Photosystem I reaction center subunit II | 1.09 | 0.89 | 0.21 |
| c19662_g1 | Photosystem II reaction center W protein | 1.05 | 0.67 | 0.40 |
| c44710_g2 | Photosystem I reaction center subunit N | 1.05 | 1.11 | -0.04 |
| c41377_g1 | Chlorophyll a-b binding protein P4 | 0.92 | 1.16 | -0.22 |
| c42082_g1 | Chlorophyll a-b binding protein CP26 | 0.79 | 1.09 | -0.27 |
| c42990_g2 | Chlorophyll a-b binding protein 8 | 0.59 | 1.02 | -0.42 |
| c43018_g1 | Chlorophyll a-b binding protein CP29.2 | 0.94 | 1.18 | -0.22 |
| c43253_g1 | Chlorophyll a-b binding protein 6A | 0.58 | 1.02 | -0.42 |
| c48649_g1 | Chlorophyll a-b binding protein 21 | 0.72 | 1.04 | -0.31 |
| c48649_g2 | Chlorophyll a-b binding protein 16 | 0.77 | 1.10 | -0.31 |
| c8676_g1 | Photosystem I reaction center subunit VI | 0.89 | 1.06 | -0.15 |
“A” represents the log2Fold_change (DGI vs. CK), “B” represents the log2Fold_change (DGI vs. LGT) and “C” represents the log2Fold_change (LGT vs. CK).
Additionally, we also detected the chlorophyll contents in these tissues. The results suggested that the level of chlorophyll in DGIs was significantly higher than that observed in LGTs (S9A Fig). Meanwhile, the chlorophyll fluorescence parameters (Fm, Fv/Fm and Y(NO)) of the DGIs closely resembled those of CK, while these parameters were significantly impaired in LGTs (S9B Fig). Our previous study also suggested that DGIs contain perfect chloroplasts but LGTs do not [13]. Taken together, these results were consistent with the DGE analysis, which indicated that DGIs have a relatively normal photosynthetic system and photosynthesis in comparison to LGTs.
LGTs suffered more serious damage than DGIs
Apoptosis is a process of programmed cell death that occurs in multicellular organisms. Apoptosis is the outcome of severe injuries caused by changes in the environment of affected cells [47], as well as changes involved in defense, development and the stress response in plants [48]. The KEGG pathway analysis indicated that several pathways related to apoptosis, including apoptosis (PATH: ko04210), the NF-kappa B signaling pathway (PATH: ko04064) and the ras signaling pathway (PATH: ko04014), were enriched in LGTs vs. DGIs and LGTs vs. CK. In addition, the TNF signaling pathway (PHAT: ko04668), which refers to a group of cytokines that can cause apoptosis [49, 50], was also up-regulated in LGTs vs. CK. These results suggested that LGTs are more seriously damaged by virus attack than DGIs.
Paraffin sectioning showed that the histology within DGIs was very similar to that of CK, whereas the histology in the LGTs surrounding the DGIs was distinct (S10 Fig). For example, similar to CK, DGIs contained relatively large numbers of cell layers, including one or two layers of elongated palisade cells and large intercellular spaces between the elongated mesophyll cells. In contrast, LGTs had relatively few cell layers and contained abnormal palisade cells, which often failed to completely elongate and contained few plastids. The contrast between palisade and spongy mesophyll cells in LGTs was inconspicuous. In addition, the spongy mesophyll cells in LGTs were irregular and replaced by large swollen vacuoles. A previous study showed that aquaporins were enriched in zones of rapid cell division and expansion [51]. DGE analysis showed that the genes encoding aquaporins were significantly up-regulated in DGIs compared to LGTs (S2 Table), which may be correlated with the elongated palisade cells observed in DGIs but not in LGTs.
Additionally, we measured two additional physiological parameters, including MDA content (or TBARS content) and electrolyte leakage. LGTs contained significantly higher levels of MDA and electrolyte leakage in comparison to DGIs and CK (S11A and S11B Fig), which suggested that the stability and integrity of the plasma membrane was significantly reduced in LGTs in comparison to DGIs. This finding coincided with the results obtained from the paraffin section experiment. Meanwhile, the total H2O2 content and the transcriptional level of respiratory burst oxidase homolog D gene (RbohD) were significantly up-regulated in LGTs compared to DGIs (S11C and S11D Fig). Additionally, the expression levels of genes related to antioxidant enzymes were significantly up-regulated in DGIs compared to LGTs (displayed in S2 Table). These results indicated that LGTs suffered more serious oxidative damage than DGIs and had depressed antioxidant abilities in comparison to DGIs. DEG analysis showed that many genes that were strongly up-regulated in LGTs compared to DGIs and CK, including the CMV movement protein, CMV RNA-directed RNA polymerase 2a and CMV replication protein 1a (shown in S2 Table). This finding indicated that a large amount of virus in LGTs may cause more pressure in comparison to DGIs.
Biosynthesis and metabolism of IAA, CTK and expansins might be involved in the formation of DGIs and LGTs
The paraffin section results showed that the cells of LGTs were smaller than the cells of CK or DGIs, and the cell arrangement in LGTs was also disordered (S10 Fig). IAA and CTK had profound effects on plant growth and development, such as aspects of cell division, cell elongation and cell differentiation [52]. An analysis of DEGs indicated the genes encoding auxin binding protein 1 (ABP19A) were up-regulated in DGIs but down-regulated in LGTs in comparison with CK (S2 Table). We also detected endogenous IAA in these tissues. The results showed that the endogenous level of IAA was significantly higher in DGIs than in CK. In contrast, the IAA content of LGTs was slightly lower than that of CK (S12A Fig). Additionally, the KEGG pathway related to zeatin (a type of plant cytokinin) biosynthesis was significantly enriched in DGIs vs. LGTs and CK vs. LGTs. The DGIs exhibited much larger amounts of CTK than CK and LGTs (S12B Fig). Therefore, we hypothesized that IAA and CTK biological processes might be involved in the morphological development of DGIs and LGTs. However, further investigations are needed to clarify the roles of IAA and CTK in these tissues.
Expansins and expansin-like cell wall-loosening proteins can increase the extensibility of the cell wall [53]. According to the DEGs analysis, the genes encoding expansin A1/A4 were up-regulated in DGIs in comparison to CK and LGTs, and were also slightly down-regulated in LGTs vs. CK (S2 Table). The qRT-PCR analysis revealed that DGIs had the highest expression level for the gene encoding expansin-A1 (EXPA1), followed by CK (Fig 4). The cells in LGTs were smaller and were not completely elongated in comparison to the cells in DGIs and CK. Thus, we propose that expansins may have significant effects on the development of these tissues. To correlate the expression of expansin with its function in the formation of DGIs and LGTs, the responses of EXLA4-defective mutants (EXLA4 RNAi), overexpression lines (35S:EXLA4) and wild-type plants (WT) after CMV infection were examined. At approximately 20-dpi, we found that the formation of DGIs in the EXLA4 RNAi plants was repressed or reduced in comparison to WT. However, EXLA4-overexpressing plants developed more vivid and typical DGIs than WT plants. In addition, EXLA4 RNAi plants were more likely to develop LGTs (S13 Fig). The results obtained from the tobacco EXLA4 mutants indicated that the expression level of EXLA4 could affect the formation of DGIs and LGTs on CMV-infected N. tabacum plants.
Discussion
In this study, we used an RNA-seq and DGE approach to investigate the gene expression changes associated with the characteristic disease tissues induced in N. tabacum by CMV. We identified sets of DEGs that may be related to the formation or development of DGIs and LGTs. GO enrichment analysis and KEGG pathway analysis suggested that several pathways related to photosynthesis and chlorophyll metabolism are enriched in DGIs compared to LGTs. However, several pathways related to apoptosis are significantly up-regulated in LGTs compared to DGIs. The results obtained using tobacco expansin mutants indicated that NtEXPA4 has effects on DGI and LGT formation. Although the two tissues both arise through interactions between CMV and N. tabacum, they involved different metabolic and biological pathways.
In 1926, Goldstein reported that the chlorophyll contents of LGTs in Tobacco mosaic virus (TMV)-infected N. tabacum were significantly reduced in comparison to DGIs and healthy tissues [12]. Our previous study indicated that DGIs, which were very similar to healthy tissues, contained perfect chloroplasts and possessed a developed photosynthetic capacity, in contrast to LGTs [13]. In the present study, DGE analysis indicated that many genes in pathways related to photosynthesis were up-regulated in DGIs vs. CK and DGIs vs. LGTs but were down-regulated in LGTs vs. CK. Chloroplasts, which are important organelles in plants, are key components of photosynthesis. Photosynthesis is the basic energetic driver of plant biomass production [54]. Similar to healthy tissues, DGIs had a normal photosynthetic system and photosynthesis in comparison to LGTs. The interaction system of N. tabacum and CMV was compatible. On the one hand, DGIs allow the infected plant to survive longer. On the other hand, DGIs may be a nutrient resource for virus reproduction in the surrounding LGTs. Thus, we hypothesized that DGIs represent a win-win outcome in the battle between host plants and pathogens.
An analysis of DEGs showed that the genes encoding CMV RNA-directed RNA polymerase 2a, CMV replication protein 1a and CMV movement protein were significantly up-regulated in LGTs vs. DGIs and LGTs vs. CK. This finding was somewhat consistent with the previous suggestion that DGIs were free of viral RNAs and proteins [7, 9, 13, 16]. Previous studies reported that genes related to photosynthesis and pigment metabolism were suppressed by virus infection [55]. Therefore, a high viral concentration may also be correlated with damaged photosynthesis and pigment metabolism in LGTs.
The histology analysis performed in the present study was consistent with the previous view that DGIs more closely resemble healthy tissues and contain one or two layers of elongated palisade cells, as well as large intercellular spaces between elongated mesophyll cells [12]. In contrast, in LGTs, the palisade cells were abnormal and often failed to elongate completely, with few plastids, large swollen nuclei and few intracellular spaces [12]. Palisade cells of plants contain the largest number of chloroplasts per cell and are the primary site of photosynthesis in the leaves of plants that contain them. These cells convert the energy in light to the chemical energy of carbohydrates [56]. Palisade cells abnormalities can have unfortunate effects on the development and growth of cells in LGTs through damaged photosynthesis (S9 Fig).
DGIs are more similar to recovered tissues and are resistant to super-infection by the same virus [11, 13, 14]. Recovered tissue has been shown to undergo posttranscriptional degradation of viral and transgene RNAs [16]. Dicer-like, which is a type of double-stranded (ds) RNA-specific enzyme, plays a vital role in post-transcriptional gene silencing in plants [57]. Virus-associated molecular patterns are often generically recognized by dicerlike enzymes, which then produce virus-derived small interfering RNAs (vsiRNAs) that promote antiviral defenses through RNA silencing once they are loaded into argonaute proteins [58]. According to our DEGs analysis, the gene encoding endoribonuclease dicer homolog 2 (c52671_g1) was slightly up-regulated in DGIs in comparison to LGTs (S2 Table), which indicates that DGIs represent an inhospitable internal environment for virus replication. Therefore, high expression levels of endoribonuclease dicer homolog 2 may correlate with the relatively small amount of viruses in DGIs.
Previous studies reported that expansins are plant cell wall-remodelling proteins and are involved primarily in the pH-dependent extension of plant cell walls [59]. In the present study, DEGs analysis indicated that the expression levels of genes encoding expansins are up-regulated in DGIs in comparison to LGTs. Palisade cells failed to elongate completely in LGTs but not in DGIs. In addition, a preliminary study based on tobacco EXPA4 mutants showed that the formation of DGIs was repressed in EXLA4 RNAi plants. Taken together, our results suggested that tobacco EXPA4 may modulate DGI formation in CMV-infected N. tabacum. However, the underlying mechanisms by which expansin signaling affects DGI formation have yet to be determined. Further investigations are necessary to clarify these issues. Moreover, the other two phytohormones, IAA and CTK, also had profound effects on the division, elongation and differentiation of plant cells. Thus, further investigations are needed to clarify the roles of IAA and CTK in these tissues.
Overall, the combination of transcriptome sequencing and DGE analysis based on RNA-seq technology was shown to be a powerful method for identifying candidate genes involved in the response of N. tabacum to CMV. This study was also the first transcriptome study to compare DGIs and LGTs, and the obtained results will help researchers study the differences between DGIs and LGTs and the formation of these tissues in CMV-infected N. tabacum leaves. The knowledge obtained from this study will serve as a useful genetic resource for the study of DGIs and LGTs. Additionally, the identification of specific genes and the functional verification of candidate genes, including the genes that encode expansins, IAA, CTK and the dicer protein, are also needed to further analyze the formation mechanisms of disease tissues induced by mosaic viruses and to help us understand the mechanisms of plant resistance to virus infection.
Supporting information
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
The experiments were repeated three times with similar results.
(TIF)
(TIF)
(TIF)
(TIF)
Unigenes aligned to the KOG database were classified into 26 functional classes.
(TIF)
Unigenes with GO annotations were classified into three major functional categories (biological process, cellular components, and molecular functions).
(TIF)
Unigenes with KEGG annotations were distributed in 272 KEGG pathways.
(TIF)
(TIF)
The error bars represent the standard deviations of the mean values that were obtained from three biological replicates (n = 3).
(TIF)
Scale bar = 50um. The experiments were repeated three times with similar results.
(TIF)
Electrolyte leakage (a), malondialdehyde (MDA) contents (b), H2O2 content (c) and transcriptional level of respiratory burst oxidase homolog D gene (RbohD) (d) of DGI and LGT induced in N. tabacum by CMV. The error bars represent the standard deviations of the mean values that were obtained from three biological replicates (n = 3).
(TIF)
Accumulation of IAA (a) and CTK (b) in DGIs and LGTs induced in N. tabacum by CMV. The error bars represent the standard deviations of the mean values that were obtained from three biological replicates (n = 3).
(TIF)
Mock, N. tabacum were mock inoculated with phosphate buffer; EXLA4-defective mutants (EXLA4 RNAi); overexpression lines (35S:EXLA4) and wild-type plants (WT).
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank technical supports from Novogene Bioinformatics Technology Co., Ltd., in Beijing, China for helpful use of related software.
Data Availability
All relevant data are within the paper and its Supporting Information files. Additionally, the Illumina sequencing data sets have been uploaded to the NCBI SRA under accession number SAMN04404353 (https://www.ncbi.nlm.nih.gov/biosample/SAMN04404353/).
Funding Statement
This work was supported by the National Nature Science Foundation of China (31270290, 31171835, 91417305).
References
- 1.Allard HA. The mosaic disease of tobacco. Science. 1912:875–6. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell J. The Physiology of Virus Diseases in Plants: V. The Movement of the Virus Agent in Tobacco and Tomato. Annals of Applied Biology. 1934;21(2):191–205. [Google Scholar]
- 3.Fulton RW. Superinfection by strains of tobacco mosaic virus. Phytopathology. 1951;41(7):579–92. [Google Scholar]
- 4.Allard HA. A Review of Investigations of the Mosaic Disease of Tobacco, Together with a Bibliography of the More Important Contributions. Bulletin of the Torrey Botanical Club. 1914;41(9):435–58. [Google Scholar]
- 5.Atkinson PH, Matthews REF. On the origin of dark green tissue in tobacco leaves infected with tobacco mosaic virus. Virology. 1970;40(2):344–56. [DOI] [PubMed] [Google Scholar]
- 6.Šindelářová M, Šindelář L. TMV-RNA Biosynthesis in the Light-Green and Dark-Green Regions of Tobacco Leaves. Biologia Plantarum. 2004;48(3):419–23. [Google Scholar]
- 7.Burundukova OL, Sapotsky MV, Kochetov AV, Trifonova EA, Malinovsky VI. Dark and light green tissues of tobacco leaves systemically infected with tobacco mosaic virus. Biologia Plantarum. 2009;53(2):294–300. [Google Scholar]
- 8.Matthews R. Plant VirologyAcademic Press. San Diego. 1991:254–63.
- 9.Moore C, MacDiarmid R. Dark Greens Islands: the Phenomenon In: Loebenstein G, Carr J, editors. Natural Resistance Mechanisms of Plants to Viruses: Springer; Netherlands; 2006. p. 187–209. [Google Scholar]
- 10.Loebenstein G, Cohen J, Shabtai S, Coutts RHA, Wood KR. Distribution of cucumber mosaic virus in systemically infected tobacco leaves. Virology. 1977;81(1):117–25. [DOI] [PubMed] [Google Scholar]
- 11.Pang S-Z, Jan F-J, Tricoli D, Russell P, Carney K, Hu J, et al. Resistance to squash mosaic comovirus in transgenic squash plants expressing its coat protein genes. Molecular Breeding. 2000;6(1):87–93. [Google Scholar]
- 12.Goldstein B. A Cytological Study of the Leaves and Growing Points of Healthy and Mosaic Diseased Tobacco Plants. Bulletin of the Torrey Botanical Club. 1926;53(8):499–599. [Google Scholar]
- 13.Chen L-J, Liu J, Zhao F-F, Li J-Y, Wang S-X, Lin H-H, et al. Characterisation of the dark green islands of cucumber mosaic virus infected Nicotiana tabacum. Plant Cell Rep. 2015;34(7):1225–38. doi: 10.1007/s00299-015-1781-1 [DOI] [PubMed] [Google Scholar]
- 14.Moore CJ, Sutherland PW, Forster RLS, Gardner RC, MacDiarmid RM. Dark Green Islands in Plant Virus Infection are the Result of Posttranscriptional Gene Silencing. Molecular Plant-Microbe Interactions. 2001;14(8):939–46. doi: 10.1094/MPMI.2001.14.8.939 [DOI] [PubMed] [Google Scholar]
- 15.Honda Y, Matsui C. Electron microscopy of cucumber mosaic virus-infected tobacco leaves showing mosaic symptoms. Phytopathology. 1974;64(4):534–9. [Google Scholar]
- 16.Dougherty WG, Lindbo JA, Smith HA, Parks TD, Swaney S, Proebsting WM. RNA-mediated virus resistance in transgenic plants: exploitation of a cellular pathway possibly involved in RNA degradation. Mol Plant Microbe Interact. 1994;7(5):544–52. [PubMed] [Google Scholar]
- 17.Palukaitis P, Roossinck MJ, Dietzgen RG, Francki RIB. Cucumber MOSAIC Virus In: Karl Maramorosch FAM, Aaron JS, editors. Advances in Virus Research. Volume 41: Academic Press; 1992. p. 281–348. [DOI] [PubMed] [Google Scholar]
- 18.Zitter T, Murphy J. Cucumber mosaic. The Plant Health Instructor.
- 19.Wilhelm BT, Landry J-R. RNA-Seq—quantitative measurement of expression through massively parallel RNA-sequencing. Methods. 2009;48(3):249–57. doi: 10.1016/j.ymeth.2009.03.016 [DOI] [PubMed] [Google Scholar]
- 20.Zhang N, Zhang HJ, Zhao B, Sun QQ, Cao YY, Li R, et al. The RNA‐seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. Journal of pineal research. 2014;56(1):39–50. doi: 10.1111/jpi.12095 [DOI] [PubMed] [Google Scholar]
- 21.Cloonan N, Forrest ARR, Kolle G, Gardiner BBA, Faulkner GJ, Brown MK, et al. Stem cell transcriptome profiling via massive-scale mRNA sequencing. Nat Meth. 2008;5(7):613–9. [DOI] [PubMed] [Google Scholar]
- 22.Garber M, Grabherr MG, Guttman M, Trapnell C. Computational methods for transcriptome annotation and quantification using RNA-seq. Nat Meth. 2011;8(6):469–77. [DOI] [PubMed] [Google Scholar]
- 23.Xi D, Yang H, Jiang Y, Xu M, Shang J, Zhang Z, et al. Interference between Tobacco necrosis virus and Turnip crinkle virus in Nicotiana benthamiana. Journal of phytopathology. 2010;158(4):263–9. [Google Scholar]
- 24.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson Da, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotech. 2010;28(5):511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou L, Shi Y, Guo R, Liang M, Zhu X, Wang C. Digital Gene-Expression Profiling Analysis of the Cholesterol-Lowering Effects of Alfalfa Saponin Extract on Laying Hens. PLOS ONE. 2014;9(6):e98578 doi: 10.1371/journal.pone.0098578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei M, Niu J, Li C, Cao F, Quan S. Identification and expression analysis of genes related to calyx persistence in Korla fragrant pear. BMC genomics. 2016;17(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proceedings of the National Academy of Sciences. 2003;100(16):9440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storey JD. The positive false discovery rate: a Bayesian interpretation and the q-value. Annals of statistics. 2003:2013–35. [Google Scholar]
- 32.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Method Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14 doi: 10.1186/gb-2010-11-2-r14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y-N, Zhu X-Y, Wang W-P, Wang Y, Wang L, Xu X-X, et al. Reproductive switching analysis of Daphnia similoides between sexual female and parthenogenetic female by transcriptome comparison. Scientific Reports. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Research. 2008;36(suppl 1):D480–D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21(19):3787–93. doi: 10.1093/bioinformatics/bti430 [DOI] [PubMed] [Google Scholar]
- 36.Gal-On A, Kaplan I, Roossinck MJ, Palukaitis P. The kinetics of infection of zucchini squash by cucumber mosaic virus indicate a function for RNA 1 in virus movement. Virology. 1994;205(1):280–9. doi: 10.1006/viro.1994.1644 [DOI] [PubMed] [Google Scholar]
- 37.Chen L-J, Ren H, Deng X-G, Li Y-N, Cha W-Q, Lin H-H, et al. Effects of light intensity on the susceptibility of Nicotiana tabacum to cucumber mosaic virus. Journal of General Plant Pathology. 2015;81(5):399–408. [Google Scholar]
- 38.Ji H, Liu L, Li K, Xie Q, Wang Z, Zhao X, et al. PEG-mediated osmotic stress induces premature differentiation of the root apical meristem and outgrowth of lateral roots in wheat. Journal of experimental botany. 2014:eru255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kichtenthaler H, Wellburn A. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvent. Biochem Soc Trans. 1983;603:591–3. [Google Scholar]
- 40.Zhang L, Oh Y, Li H, Baldwin IT, Galis I. Alternative oxidase in resistance to biotic stresses: Nicotiana attenuata AOX contributes to resistance to a pathogen and a piercing-sucking insect but not Manduca sexta larvae. Plant physiology. 2012;160(3):1453–67. doi: 10.1104/pp.112.200865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teng N, Wang J, Chen T, Wu X, Wang Y, Lin J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytologist. 2006;172(1):92–103. doi: 10.1111/j.1469-8137.2006.01818.x [DOI] [PubMed] [Google Scholar]
- 42.Chen Q, Qi W-b, Reiter RJ, Wei W, Wang B-m. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. Journal of Plant Physiology. 2009;166(3):324–8. doi: 10.1016/j.jplph.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 43.Shang J, Xi D-h, Huang Q-r, Xu M-y, Yuan S, Wang S-d, et al. Effect of two satellite RNAs on Nicotiana glutinosa infected with Cucumber mosaic virus (CMV). Physiological and Molecular Plant Pathology. 2009;74(2):184–90. [Google Scholar]
- 44.Shang J, Xi D-H, Yuan S, Xu F, Xu M-Y, Qi H-L, et al. Difference of physiological characters in dark green islands and yellow leaf tissue of Cucumber mosaic virus (CMV)-infected Nicotiana tabacum leaves. Zeitschrift für Naturforschung C, A journal of biosciences. 2010;65(1):73. [DOI] [PubMed] [Google Scholar]
- 45.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotech. 2011;29(7):644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang LK, Yan HD, Zhao XX, Zhang XQ, Wang J, Frazier T, et al. Identifying differentially expressed genes under heat stress and developing molecular markers in orchardgrass (Dactylis glomerata L.) through transcriptome analysis. Molecular Ecology Resources. 2015;15(6):1497–509. doi: 10.1111/1755-0998.12418 [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Li J, Bostock RM, Gilchrist DG. Apoptosis: A Functional Paradigm for Programmed Plant Cell Death Induced by a Host-Selective Phytotoxin and Invoked during Development. The Plant Cell. 1996;8(3):375–91. doi: 10.1105/tpc.8.3.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reape TJ, McCabe PF. Apoptotic‐like programmed cell death in plants. New Phytologist. 2008;180(1):13–26. doi: 10.1111/j.1469-8137.2008.02549.x [DOI] [PubMed] [Google Scholar]
- 49.Liu Z-g. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005;15(1):24–7. doi: 10.1038/sj.cr.7290259 [DOI] [PubMed] [Google Scholar]
- 50.Rothe J, Gehr G, Loetscher H, Lesslauer W. Tumor necrosis factor receptors-structure and function. Immunologic research. 1992;11(2):81–90. [DOI] [PubMed] [Google Scholar]
- 51.Tyerman S, Niemietz C, Bramley H. Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant, Cell & Environment. 2002;25(2):173–94. [DOI] [PubMed] [Google Scholar]
- 52.Tran L-SP, Pal S. Phytohormones: a window to metabolism, signaling and biotechnological applications: Springer; 2014. [Google Scholar]
- 53.McQueen-Mason S, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall extension in plants. The Plant Cell. 1992;4(11):1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berry JA, Downton WJS. Environmental regulation of photosynthesis. Photosynthesis. 1982;2:263–343. [Google Scholar]
- 55.Hanssen IM, Peter van Esse H, Ballester A- R, Hogewoning SW, Parra NO, Paeleman A, et al. Differential Tomato Transcriptomic Responses Induced by Pepino Mosaic Virus Isolates with Differential Aggressiveness. Plant Physiology. 2011;156(1):301–18. doi: 10.1104/pp.111.173906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogelmann T, Martin G. The functional significance of palisade tissue: penetration of directional versus diffuse light. Plant, Cell & Environment. 1993;16(1):65–72. [Google Scholar]
- 57.Hannon GJ. RNA interference. Nature. 2002;418(6894):244–51. doi: 10.1038/418244a [DOI] [PubMed] [Google Scholar]
- 58.Pumplin N, Voinnet O. RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defence. Nature Reviews Microbiology. 2013;11(11):745–60. doi: 10.1038/nrmicro3120 [DOI] [PubMed] [Google Scholar]
- 59.Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407(6802):321–6. doi: 10.1038/35030000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
(XLS)
The experiments were repeated three times with similar results.
(TIF)
(TIF)
(TIF)
(TIF)
Unigenes aligned to the KOG database were classified into 26 functional classes.
(TIF)
Unigenes with GO annotations were classified into three major functional categories (biological process, cellular components, and molecular functions).
(TIF)
Unigenes with KEGG annotations were distributed in 272 KEGG pathways.
(TIF)
(TIF)
The error bars represent the standard deviations of the mean values that were obtained from three biological replicates (n = 3).
(TIF)
Scale bar = 50um. The experiments were repeated three times with similar results.
(TIF)
Electrolyte leakage (a), malondialdehyde (MDA) contents (b), H2O2 content (c) and transcriptional level of respiratory burst oxidase homolog D gene (RbohD) (d) of DGI and LGT induced in N. tabacum by CMV. The error bars represent the standard deviations of the mean values that were obtained from three biological replicates (n = 3).
(TIF)
Accumulation of IAA (a) and CTK (b) in DGIs and LGTs induced in N. tabacum by CMV. The error bars represent the standard deviations of the mean values that were obtained from three biological replicates (n = 3).
(TIF)
Mock, N. tabacum were mock inoculated with phosphate buffer; EXLA4-defective mutants (EXLA4 RNAi); overexpression lines (35S:EXLA4) and wild-type plants (WT).
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Additionally, the Illumina sequencing data sets have been uploaded to the NCBI SRA under accession number SAMN04404353 (https://www.ncbi.nlm.nih.gov/biosample/SAMN04404353/).