Abstract
The capacity to discriminate between safety and danger is fundamental for survival, but is disrupted in individuals with posttraumatic stress disorder (PTSD). Acute stressors cause a release of serotonin (5-HT) in the forebrain, which is one mechanism for enhanced fear and anxiety; these effects are mediated by the 5-HT2C receptor. Using a fear discrimination paradigm where a danger signal conditioned stimulus (CS+) coterminates with a mild footshock and a safety signal (CS-) indicates the absence of shock, we demonstrate that danger/safety discrimination and fear inhibition develops over the course of 4 daily conditioning sessions. Systemic administration of the 5-HT2C receptor antagonist SB 242084 (0.25 or 1.0 mg/kg) prior to conditioning reduced behavioral freezing during conditioning, improved learning and subsequent inhibition of fear by the safety signal. Discrimination was apparent in the first recall test, and discrimination during training was evident after 3 days of conditioning versus 5 days in the vehicle treated controls. These results suggest a novel therapeutic use for 5-HT2C receptor antagonists to improve learning under stressful circumstances. Potential anatomical loci for 5-HT2C receptor modulation of fear discrimination learning and cognitive performance enhancement are discussed.
Keywords: 5-HT2C, rat, anxiety, stress, fear, conditioned inhibition
1. Introduction
The ability to differentiate between danger and safety is necessary for survival. Exposure to traumatic stress can alter this fundamental process and individuals with post-traumatic stress disorder (PTSD) display an inability to utilize environmental safety signals (Jovanovic et al., 2009), overgeneralize fear (Rauch et al., 2006a), and fail to extinguish trauma-induced fear responses (Orr et al., 2000; Milad et al., 2009). A major effort in translational neuroscience has revealed much of the neural circuitry underlying fear learning and recall (LeDoux, 2000; Johansen et al., 2011; Beyeler et al., 2014) and we are beginning to understand how stressors modulate these systems (Baratta et al., 2007; Rodrigues et al., 2009; Martijena and Molina, 2012). Yet, little is known regarding the neural mechanisms underlying the discrimination learning that is critical to recognizing and utilizing environmental safety signals (Christianson et al., 2012).
In preclinical models of PTSD, exposure to uncontrollable traumatic stress leads to enhanced fear conditioning, expression, and interference with extinction (Rau et al., 2005; Baratta et al., 2007; 2008; 2015). Uncontrollable stress triggers a release of serotonin (5-HT) in the brain, specifically in regions known to modulate fear learning and recall including the medial prefrontal cortex (Kawahara et al., 1993; Bland et al., 2003), basolateral amygdala (Kawahara et al., 1993; Amat et al., 1998b) and hippocampus (Amat et al., 1998a). Acute increases in extracellular 5-HT are sufficient to induce anxiety-like states and enhance the expression of fear by action at 5-HT2C receptors (Martin et al., 2002; Campbell and Merchant, 2003; Burghardt et al., 2007; Greenwood et al., 2008). With regard to the consequences of uncontrollable stress, 5-HT2C receptor antagonists prevent the social anxiety (Christianson et al., 2010; Christianson et al., 2013), fear enhancement (Baratta et al., 2015) and instrumental learning deficits (Strong et al., 2009) that typically follow uncontrollable stress (for review see Christianson and Greenwood, 2014). Furthermore, selective activation of the 5HT2C receptor is sufficient to induce stress-like anxiety (Christianson et al., 2013) and fear expression (Campbell and Merchant, 2003; Greenwood et al., 2008).
The expression of fear and anxiety can be modulated by learned safety signals (Christianson et al., 2008; Pollak et al., 2008; Christianson et al., 2011; Christianson et al., 2013). A safety signal is a stimulus that is a good predictor of the non-occurrence of danger or aversive stimuli and is a specific type of a conditioned inhibitor (Christianson et al., 2013). Unlike conditioned exciters, which come to trigger the response that normally follows exposure to an unconditioned stimulus, i.e. freezing to a tone after pairing with footshock, conditioned inhibitors counteract the expression of conditioned responses even in the presence of conditioned exciters (Rescorla, 1969). Myers and Davis (2004) established conditioned inhibition of fear using a fear discrimination paradigm in which one conditioned stimulus (CS+) was repeatedly paired with a footshock, while another stimulus (CS-), the safety signal, was never paired with footshock. This approach leads to fear discrimination within one or two training sessions (Chen, Foilb & Christianson, under review); yet conditioned inhibition is only apparent after many training sessions (see Experiment 1). Thus, this paradigm allows for translational research into ways to improve or accelerate the acquisition of safety signals that might be useful in the treatment or prevention of PTSD.
Fear discrimination conditioning involves repeated sessions of unavoidable footshocks, which are sufficient to trigger acute increases in extracellular 5-HT (Shanks et al., 1991; Inoue, 1993; Kawahara et al., 1993; Kirby et al., 1997; Hajós-Korcsok et al., 2003). Given the fear-enhancing effects of 5-HT and the 5HT2C receptor, we hypothesized that fear discrimination and conditioned inhibition could be facilitated by 5HT2C receptor antagonist administrations prior to conditioning. Using a fear discrimination paradigm, in which discrete auditory or visual cues served as the conditioned stimuli, we established danger/safety discrimination. A recall test comprised of presentations of the CS+ cue, the CS+ and CS- cues in compound (CS+/− cue), and the CS- cue alone provided a means to assess fear recall, conditioned inhibition, and discrimination, respectively. In Experiment 1 we determined the number of training sessions necessary for CS+/CS− discrimination during training, and discrimination and conditioned inhibition measured in later recall tests. In Experiment 2 we tested the hypothesis that systemic 5HT2C receptor antagonist SB 242084 administration would improve fear discrimination learning, recall and conditioned inhibition.
2. MATERIALS & METHODS 2.1 Animals
A total of 48 adult (250–300g) male Sprague-Dawley rats from Charles River Laboratories (Wilmington, MA) were used. Rats were housed in groups of 2 and had free access to food and water at all times. Rats were given 7–10 days to acclimate to colony housing and were kept on a 12-hour light/dark cycle with lights on at 0700. All procedures were reviewed and approved the Boston College Institutional Animal Care and Use Committee.
2.2 Apparatus
Rats were conditioned in 10 x 11 x 6 in (L x W x H) cages made of black plastic with wire mesh lids and a floor of stainless bars attached to a shocking grid (Model H10-11R-TC-SF, Coulbourn Intruments, Whitehall, PA). Each cage was housed within a 15 x 12 x 27 in (L x W x H) light and sound-attenuated chamber. The chamber was illuminated from above by 2 infrared LEDs arrays (CMVision Model IR30) and behavior was recorded by overhead cameras (Model VX-5000, Microsoft, Redmond, VA) with the infrared blocking filters replaced with infrared passing filters. ANY-Maze software (version 4.99, Stoelting, Wood Dale, IL) was used for freezing detection using the manufacturer’s recommended settings as previously (Christianson et al., 2011). A white LED array (Model LPL620WTHD, Hampton Bay) and a speaker mounted at the top of the chamber were used for conditioned stimuli. A fan provided ventilation and masking noise of ~55dB.
2.3 CS+/CS- Conditioning and Discrimination Tests
As in Chen et al. (Chen, Foilb and Christianson, under review) and adapted from Myers and Davis (2004) to quantify fear using behavioral freezing, conditioning sessions consisted of 15 CS+ and 15 CS- trials. A flickering LED light (264.0 Lux, 20ms on/off) and a white noise pip (pip duration = 10ms, interval = 3 Hz, 75dB) were used as the stimuli. Assignment of light or pip as CS+ or CS- was counterbalanced in each experiment. Each conditioning trial began with a 5s, 1kHz tone (75dB), followed by a presentation of either the CS+ or CS- cue for 15 seconds. The cues were presented in a quasi-random order so that no cue was presented more than twice is succession. CS+ trials co-terminated with a 500ms, 1.2 mA shock (Model H13–15, Coulbourn Instruments); CS- cues were not accompanied with shock. Each training session consisted of 15 presentations of each cue, with a 70s inter-trial-interval, so that each training session was a total of 45 minutes. Training was conducted from 1200 to 1400 each day for 4 or 5 consecutive days.
In a pilot experiment we found that fear discrimination and conditioned inhibition recall manifest equally when tested in the familiar conditioning context or in a novel context (A. R. Foilb and J. P. Christianson, unpublished data). Therefore, recall tests were conducted in the conditioning apparatus. Discrimination recall tests were conducted at 0900 each day after conditioning. Rats were transferred to the conditioning apparatus and after 2 min of context exposure they were presented with the CS+, the CS+ in compound with the CS− (CS+/−) and finally the CS− alone.
2.4 Drugs
The highly selective 5HT2C receptor antagonist SB 242084 was purchased from Tocris and dissolved in 50% dimethyl sulfoxide (DMSO) in saline. The doses of 0.25 and 1 mg/kg were chosen to capture the range of effective doses (0.2mg/kg to 1mg/kg) found in several recent reports (Burghardt et al., 2007; Strong et al., 2009; Christianson et al., 2010; 2013). Importantly, these doses do not alter locomotor activity (Martin et al., 2002). Intraperitoneal (i.p.) injections were made at a volume of 1 ml/kg.
2.5 Experimental Procedures
2.5.1 Experiment 1
The purpose of Experiment 1 was to establish the time course of fear discrimination learning. To this end, 16 rats were given CS+/CS− conditioning on four consecutive days. Recall tests were given in the morning following the most recent conditioning session to gauge fear recall, CS+/CS− discrimination and conditioned inhibition.
2.5.2 Experiment 2
To determine the effect of 5HT2C receptor antagonist administration on the acquisition of conditioned fear discrimination 32 rats were assigned to one of thee treatment groups: vehicle (n = 10), 0.25 (n = 10) or 1.0 mg/kg (n = 12) SB 242084. Systemic SB242084 has been effective when given 45 min to 1 h before testing (Burghardt et al., 2007; Christianson et al., 2010), therefore injections were made in the vivarium 15 min before the 45 min conditioning sessions. Training and testing were performed as in Experiment 1.
2.6 Data Analysis
Time spent freezing to the relevant cues was converted to a percentage of time based on the length of each cue. For example, the total time spent freezing to the CS+ during training was divided by the number of cues (15) multiplied by the number of seconds per cue (15s) and then multiplied by 100 to provide a percentage. To examine discrimination and inhibition ratios were computed of freezing to the CS− relative to the CS+ (discrimination ratio) or the CS+/− compound to the CS+ (inhibition ratio). Group differences in percent freezing data were then evaluated by analyses of variance with drug treatment treated as a between-subjects factor, and cue, day or test treated as within-subjects factors. Main effects and interactions were deemed significant with p < 0.05 and the Tukey’s HSD post hoc multiple comparison procedure was used in GraphPad Prism 6.0 software to maintain experiment-wise error at α = 0.05.
3. Results
3.1 Experiment 1
3.1.1 Experiment 1: Conditioning
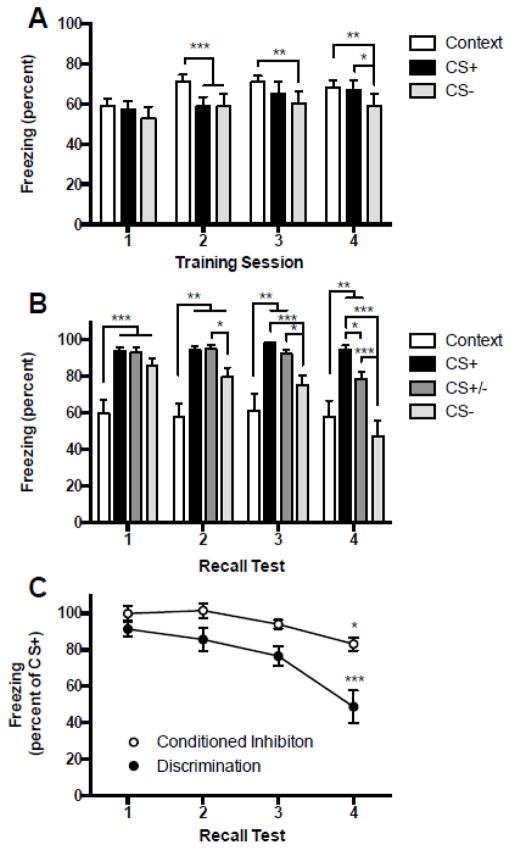
Mean percent time spent freezing to each type of cue presented during conditioning is depicted in Figure 1A. A 4 (day) by 3 (cue) ANOVA revealed a significant main effect of Day, F(3, 45) = 3.985, p = 0.013, but no significant effect for Cue, F(2, 30) = 3.268, p < 0.052 or Day by Cue interaction, F(6, 90) = 1.626, p = 0.149. Post hoc comparisons revealed a number of significant differences between group means. The main effect of Day reflected a significant increase in freezing levels regardless of cue over time: total freezing was significantly greater on Days 2, 3 and 4 than on Day 1 (ps < 0.03). Although the Day by Cue interaction did not reach significance, visual inspection of the data indicated cue effects on days 2, 3 and 4, which were explored with post hoc comparisons. There was significantly less freezing to the CS+ and CS− than the context on day 2 (ps < 0.001), less freezing to the CS- than the context on days 3 and 4 (ps < 0.01), and less freezing to the CS− than the CS+ on day 4 (p < 0.05).
Figure 1.
A: Mean (+ SEM) percent time spent freezing during the different cue conditions present during fear discrimination training. Fear discrimination conditioning evoked significant behavioral freezing. Freezing to the different cues was compared within each training day and significant differences are described in the text and indicated here by connecting lines. Bars, or sets of bars connected by overhead inverted “U” lines were found to have significantly different freezing levels in post hoc tests. Notably, freezing to the CS− was significantly less than freezing to the context on days 2, 3 and 4 and was significantly less than freezing to the CS+ on day 4. B: Mean (+ SEM) percent time spent freezing during different cue conditions in recall tests. Fear discrimination conditioning lead to significant freezing to the CS+ and discrimination to the CS− in recall tests. Freezing to the different cues was compared within each test day and significant differences are indicated by overhead brackets. On each day freezing to the CS+ and CS+/CS− compound was significantly greater than freezing to the context alone. Discrimination emerged on day two when freezing to the CS− alone was significantly less than to the CS+. As training continued the CS− appeared to become a conditioned fear inhibitor as freezing to the CS+, CS+/− compound and the CS− were all significantly different after 4 days of training. C: Mean (+/− SEM) discrimination (CS−) and conditioned inhibition (CS+/−) freezing expressed as the percentage of freezing relative to the CS+. These behaviors appear to follow a learning curve, with discrimination evident after less training. Asterisks indicate significantly more discrimination and inhibition in test 4 relative to all prior tests. *p < 0.05, **p < 0.01, ***p < 0.001.
3.1.2 Experiment 1: Recall
Mean percentages of time spent freezing during the different cue presentations of the recall tests are depicted in Figure 1B. A 4 (day) by 4 (cue) ANOVA revealed a significant main effect of Day, F(3, 45) = 6.521, p < 0.0001, Cue, F(3, 45) = 19.97, p < 0.0001 and a significant Cue by Day interaction, F(9, 135) = 3.907, p < 0.001. Pair-wise comparisons between cue conditions within each day identified a number of significant differences, which are indicated in Figure 2 and detailed here. On each day, freezing to the CS+ and CS+/− compound was significantly greater than freezing to the context (ps < 0.01) reflecting a robust conditioned fear to the CS+. Freezing during the CS− presentation was significantly less than freezing to the CS+ on days 2, 3 and 4 (ps < 0.05), and significantly less than the CS+/− combination on days 3 and 4 (ps < 0.05). Lastly, on day 4, freezing during the CS+/− compound was significantly less than freezing to the CS+ (p < 0.05) providing evidence that the CS− had become a conditioned inhibitor of fear. To convey the learning curve for discrimination and inhibition ratios (shown as percentages) of freezing to the CS− relative to the CS+ and to the CS+/− compound relative to the CS+ are provided in Figure 1C. A 2 (Discrimination versus Inhibition) by 4 (day) ANOVA revealed significant main effects of Discrimination, F(1, 15) = 22.68, p < 0.001, Day, F(3, 45) = 10.72, p < 0.0001 and a Discrimination by Day interaction, F(3, 45) = 3.30, p = 0.028. Discrimination was more robust than inhibition in tests 2, 3 and 4, ps < 0.05. Importantly, both discrimination and inhibition increased with training: on day 3 discrimination was significantly greater compared to day 1, and on day 4 was significantly greater than all other days, ps < 0.05; on day 4 inhibition was significantly greater than days 1 and 2, ps < 0.05.
Figure 2.
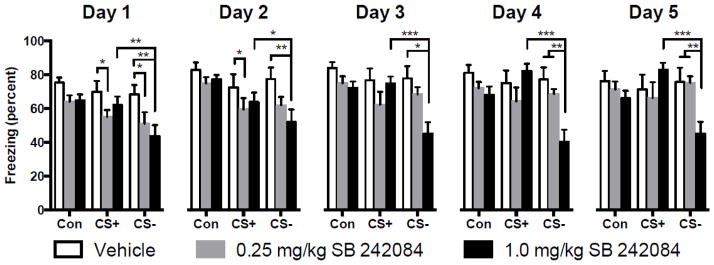
Mean (+ SEM) percent time spent freezing during different drug and cue conditions 15 min after injection during conditioning on days 1 through 5. Systemic administration of the 5-HT2C receptor antagonist SB 242084 (0.25 or 1.0 mg/kg) reduced freezing to some cues during conditioning and facilitated discrimination. Overhead brackets indicate significant hypothesis driven comparisons (see main text). With regard to discrimination, on the 1.0 mg/kg SB 242084 group spent less time freezing to the CS− than the CS+ indicating discrimination. *p < 0.05, **p < 0.01, ***p < 0.001
3.2 Experiment 2
3.2.1 Experiment 2: Conditioning
For clarity, mean percent freezing during the different cue conditions for vehicle and SB 242084 treated groups are depicted separately by day (Figure 2). Visual inspection of these data revealed a consistent reduction in freezing across cues and days by SB 242084 but the main effect of drug only approached significance on days 1 and 3. Data from each day were analyzed with a 3 (vehicle vs. drug) by 3 (cue) ANOVA. Significant main effects for Cue were found on Days 1, 2, 3, and 4 and significant Drug by Cue interactions on all Days. ANOVA results are summarized in Table 1. Our hypotheses predicted that SB 242084 would reduce freezing during conditioning and influence fear discrimination. Therefore, Tukey HSD post hoc tests were performed for between CS+ and CS− within each drug group on each conditioning day (drug effects on discrimination: i.e. CS+ vs CS− for 1.0 mg/kg on day 1), and between drug groups and vehicle for each cue (freezing effects: i.e. Vehicle vs 1.0 mg/kg for CS+ freezing on day 1). With regard to freezing effects, 1.0 mg/kg SB 242084 significantly reduced freezing to the CS− compared to vehicle all Days 1, 2, 3 & 4 (ps < 0.01) and 0.25 mg/kg SB 242084 reduced freezing to the CS+ on Day 1 (p < 0.05). With regard to discrimination effects, there was significant discrimination between CS+ and CS− within the 1.0 mg/kg SB 242084 group on all days (ps < 0.05). To summarize, pretreatment with SB 242084 reduced freezing to the CS− and facilitated discrimination emerged on conditioning Day 3, which did not occur even after 5 days in the vehicle group.
Table 1.
F statistics and significant p values in parenthesis for the main effects and interactions from the conditioning phase in Experiment 2.
| Day | Drug (df = 2, 29) | Cue (df = 2, 58) | Drug x Cue Interaction (df = 4, 58) |
|---|---|---|---|
| 1 | 3.176 (p = 0.057) | 16.03 (p < 0.014) | 2.824 (p = 0.032) |
| 2 | 2.122 | 15.73 (p < 0.001) | 2.697 (p = 0.039) |
| 3 | 3.121 (p = 0.059) | 8.119 (p < 0.001) | 6.227 (p < 0.001) |
| 4 | 2.690 | 6.434 (p = 0.003) | 8.334 (p < 0.001) |
| 5 | 0.977 | 2.073 | 6.777 (p < 0.001) |
3.2.2 Experiment 2: Recall
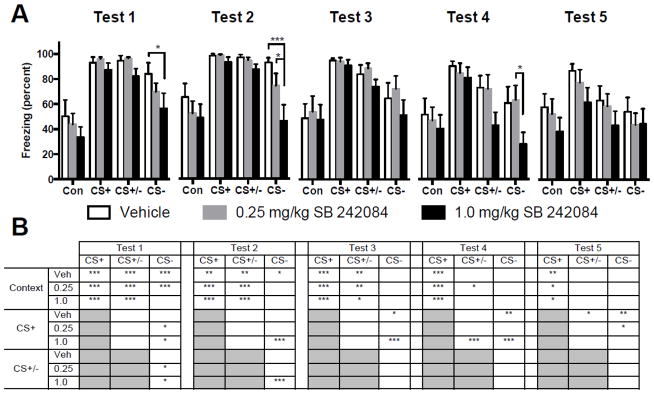
Mean time spent freezing to the context, CS+, CS+/− compound and CS− for the 5 daily recall tests are depicted in Figure 3. Data from each test were analyzed with separate 3 (vehicle vs. drug) by 4 (cue) ANOVAs. Significant main effects for Cue were found on each test day, and significant effects of Drug and Drug by Cue interaction on test day 2. ANOVA results are summarized in Table 2. The main effects of cue reflect a number of significant differences, all of which are indicated in Figure 3. In every test, freezing to the CS+ was significantly greater than to the context (ps < 0.001) regardless of drug treatment. The significant pair-wise comparisons between freezing to cues within each drug dose are summarized in Figure 3B. Regarding discrimination, as above, our hypotheses predicted effects of SB 242084 on discrimination and inhibition. Therefore, post hoc comparisons were conducted between drug groups (freezing effects) for each cue and between cues for each drug dose (discrimination and inhibition effects, as above). With regards to freezing, treatment with 1mg/kg SB 242084 before conditioning let to significantly less freezing to the CS− in Tests 1, 2 and 4 compared to vehicle, and compared to 0.25mg/kg in Test 2 (ps < 0.05). Regarding discrimination, both 0.25 and 1.0 mg/kg doses appeared to facilitate discrimination as evident in the difference between CS+ and CS− freezing in tests 1 and 2, (ps < 0.05). Conditioned inhibition was evident in the 1.0 mg/kg SB 242084 group on day 4 (CS+ versus CS+/−), p < 0.001 and in the vehicle group on day 5 (p < 0.05).
Figure 3.
A: Mean (+ SEM) percent time spent freezing during different cue conditions in recall tests 1 through 5. Systemic administration of the 5-HT2C receptor antagonist SB 242084 prior to fear discrimination conditioning facilitated CS+/CS− discrimination in recall tests 1 and 2, and facilitated conditioned inhibition (CS+ vs. CS+/−) on day 4. Brackets indicate significant differences between groups. B: Table summarizing significant comparisons between cues within each drug dose condition. Redundant pairwise comparisons are shaded in gray. In tests 1 through 5, regardless of drug conditions, freezing was significantly greater during presentation of the CS+ than to freezing in the context alone. However, in tests 1 and 2, the SB 242084 treated groups demonstrated discrimination with significantly less freezing during the CS− when compared to the CS+. In test 3, discrimination was evident in the vehicle treated groups with significantly less freezing to the CS− than to the CS+, and on day 5 conditioned inhibition was evident as less freezing during the CS+/CS− compound than to the CS+. *p < 0.05, **p < 0.01, ***p < 0.001
Table 2.
F statistics and significant p values in parenthesis for the main effects and interactions from the testing phase in Experiment 2.
| Test | Drug (df = 2, 28) | Cue (df = 3, 84) | Drug x Cue Interaction (df = 6, 84) |
|---|---|---|---|
| 1 | 2.380 | 38.97 (p < 0.001) | 0.630 |
| 2 | 4.181 (p = 0.026) | 25.36 (p < 0.001) | 2.136 (p = 0.058) |
| 3 | 0.674 | 25.36 (p < 0.001) | 0.426 |
| 4 | 1.782 | 23.19 (p < 0.001) | 1.814 |
| 5 | 0.954 | 14.54 (p < 0.001) | 0.587 |
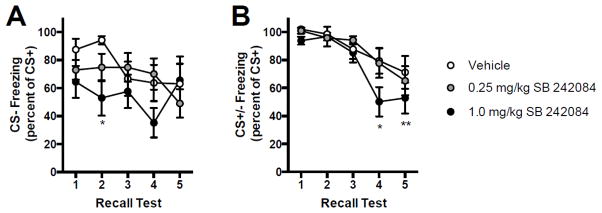
As in experiment 1, discrimination and inhibition ratios were computed for each group (Figure 4). A 3 (drug) by 5 (day) ANOVA revealed a main effect of day, F(4, 116) = 2.513, p = 0.045, for discrimination, but no other effects reached significance. Discrimination appeared to improve over days in the vehicle and 0.25 mg/kg SB 242084 groups but the 1.0 mg/kg SB 242084 exhibited more discrimination earlier with significantly less freezing to the CS− in test 2 compared to the vehicle group. Regarding inhibition, a 3 (drug) by 5 (day) ANOVA revealed a significant effect of day, F(4, 116) = 17.88, p > 0.001; no other effects reached significance. Again, 1.0 mg/kg SB 242084 appeared to accelerate inhibition with less freezing to the CS+/− compound than the CS+ in Test 4 compared to the 0.25mg/kg and vehicle groups, and compared to tests 1, 2, and 3 (ps < 0.05); All groups exhibited conditioned inhibition on day 5 as significantly lower CS+/− to CS+ ratio compared to tests 1 and 2 (ps < 0.01). To summarize, SB 242084 did not appear to influence recall of the context or the CS+ but these groups froze less to the CS− after only one day of conditioning while this did not emerge until day 3 in the vehicle treated rats. Accordingly, SB 242084 (1mg/kg) also accelerated the emergence of conditioned inhibition relative the to vehicle treated group.
Figure 4.
Discrimination (A) and Conditioned Inhibition (B) learning curves; data adapted from Figure 3A. A: Mean (+/− SEM) discrimination presented as percent freezing to the CS− relative to the CS+. *Significantly greater discrimination was evident in test 2 in the SB242084 group compared to vehicle. B: Mean (+/− SEM) conditioned inhibition presented as percent freezing to the CS+/− compound relative to the CS+. Conditioned inhibition appeared to strengthen over days. *Significantly improved conditioned inhibition in test 4, 1. 0 mg/kg SB 242084 versus vehicle and 0.25 mg/kg groups. Significantly improved conditioned inhibition in tests 4 (1.0 mg/kg) and 5 (all groups) compared to tests 1, 2 and 3. *p < 0.05, **p < 0.01.
4. Discussion
Here we report on the nature of conditioned fear discrimination, recall and the expression of conditioned inhibition in an animal model of safety learning. We demonstrate that although rats readily discriminate between a danger signal previously paired with shock (CS+) and a safety signal that was never paired with shock (CS−) during a test of recall, freezing behavior to CS+ and CS− during the training sessions is only distinguishable after several sessions. We hypothesized that pre-training administration of a 5HT2C receptor antagonist would improve fear discrimination and conditioned inhibition because it would counteract the anxiogenic effects of central 5-HT release during the training procedure, allowing for better learning. A number of effects of the 5HT2C receptor antagonist were consistent with this hypothesis including (i.) reduced freezing during conditioning, (ii.) earlier expression of fear discrimination during conditioning, (iii.) earlier recall of discrimination in the recall test (test 1 versus test 3) and (iv.) earlier expression of conditioned inhibition (test 4 versus test 5). Interestingly, the 5-HT2C receptor antagonist did not cause a general reduction of fear, it appeared to be specific to the CS− safety cue. These results have a number of implications for understanding the neural mechanisms underlying safety learning and this procedure may provide a valuable new approach for evaluating the next generation of therapeutics for PTSD and other fear-based psychiatric disorders.
The learning that occurs in CS+/CS− fear discrimination involves a transition of the associations formed to the CS− over the course of training. Initially, the CS− is presented within temporal and contextual proximity to the footshock; this arrangement results in conditioned excitation of fear. Over time, however, the conditioned excitation becomes very specific to the CS+, and the conditioned excitation of fear associated with the CS− diminishes to the point where the CS− predicts the non-occurrence of shock. This reversal of associations is evident as initial equal freezing to the CS+ and CS−, then differential freezing between CS+ and CS− and, finally, differential freezing to the CS+/− compound and the CS+. As noted, SB 242084 is known to reduce fear and anxiety expression in several paradigms and there is a general consensus that the 5-HT2C receptors are anxiogenic (for review see Martin et al., 2014) but these results are the first to indicate that blocking the 5-HT2C receptor could facilitate learning to distinguish between fear and safety cues.
Our understanding of 5-HT modulation of fear circuit function is informed by electrophysiological methods. While application of 5-HT to the amygdala yields a net inhibitory effect (Stutzmann et al., 1998; Rainnie, 1999), the 5-HT2C receptor is excitatory. Specifically, 5-HT2C receptors trigger depolarization in lateral amygdala neurons via a reduction of G-protein coupled inward rectifying potassium currents and an increase in a voltage insensitive cation current (Yamamoto et al., 2014). Thus, one mechanism of SB 242084 action could be to prevent excitatory modulation of the amygdala, which could favor the excitatory/inhibitory balance to favor learning about the CS−. Interestingly, in our preparation, SB 242084 did not interfere with fear learning to the CS+. Alternatively, SB 242084 may facilitate the reversal learning that occurs to the CS−; there is some empirical support for this possibility. Boulougouris and colleagues (2008) demonstrated that SB 242084 improved reversal learning in an instrumental task in which the test subject was required to change a behavioral response on a previously reinforced lever to an alternative lever (see Alsiö et al., 2015 for more detailed analysis; see also Baker et al., 2011 for a null effect). SB 242084 also improved a number of attention-related performance endpoints in the five choice serial reaction time task (Quarta et al., 2012; see also Fletcher et al., 2007). Therefore, in addition to the fear-reducing effects, SB 242084 may improve fear discrimination by augmenting attention and reversal learning processes. Further investigation of these possibilities could inform the future application or development of novel therapeutics.
The systemic effects of SB 242084 may help elucidate some of the anatomical loci of fear discrimination learning. The basolateral amygdala, a critical site of neuroplasticity mediating the acquisition and recall of conditioned fear in rodents (Maren and Quirk, 2004) and humans (Milad et al., 2006; Rauch et al., 2006b), is the locus of 5-HT action for stressor-induced anxiety (Christianson et al., 2010), stressor enhanced fear (Baratta et al., 2015) and selective serotonin reuptake inhibitor induced anxiety (Vicente and Zangrossi, 2012). Regarding reversal learning, the orbital frontal cortex, which is critical for task response switching as predicted values change (for reviews see Schoenbaum et al., 2011; McDannald et al., 2014) is also modulated by the 5-HT2C receptor, with SB 242084 improving reversal learning when applied by local microinjection (Boulougouris and Robbins, 2010). Together with the current results, we propose that stressor induced 5-HT release may bias learning and response toward fear by augmenting the output of the basolateral amygdala and promoting perseveration by interfering with response selection in the orbital frontal cortex. Indeed, these regions are affected by traumatic stress and implicated in the neural circuitry of PTSD (Hughes and Shin, 2011; Brown et al., 2014; Cisler et al., 2014; Huang et al., 2014) and deficits in fear extinction and generalization may be a consequence of experiential or genetic factors that alter the regulation of these systems by 5-HT (Hariri et al., 2002; Caspi et al., 2003; Canli et al., 2005; Telch et al., 2015). As others and we have suggested (Baratta et al., 2015; Christianson et al., 2010; Martin et al., 2014), 5-HT2C receptor antagonists appear to be very good targets for therapeutic development.
5. Conclusion
In the present study we provide a paradigm which allows us to study the acquisition and recall of fear discrimination and conditioned inhibition using behavioral freezing as a dependent measure. Fear discrimination and conditioned inhibition are fundamental capacities that are necessary for survival, yet are impaired in PTSD (Jovanovic et al., 2009). Adding to a growing, and generally coherent body of research, administration of the 5-HT2C receptor antagonist SB 242084 reduced fear during conditioning. Importantly, SB 242084 also augmented fear discrimination learning. This preclinical finding may offer a translational avenue similar to the “extinction enhancers” like D-cycloserine (Ressler et al., 2004) whereby administration of SB 242084 prior to a stressful task that requires precise distinction between danger and safety, such as in air traffic control or military surveillance, could enhance cognitive and behavioral performance.
Highlights.
Stress enhances fear learning by a 5-HT2C receptor dependent mechanism
Exposure to a danger versus safety discrimination conditioning protocol led to significant discrimination between shock-paired and unpaired conditioned stimuli, and significant inhibition of fear when the safe cue was provided in compound with danger cues
Pre-treatmend with the selective 5-HT2C receptor antagonist SB 242084 (0.25 and 1.0mg/kg) reduced the expression of fear during conditioning, accelerated discrimination and fear inhibition by the safety cue.
5-HT2C antagonists may selectively facilitate learning about safety cues, a desirable therapeutic effect for fear-related psychiatric diseases.
Acknowledgments
The authors wish to thank Mary C. Sarlitto for her assistance conducting Experiment 2. Funding in support of this project was provided by NIH grant MH093412 and the Brain and Behavior Research Foundation grant No. 19417 to JPC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alsiö J, Nilsson SR, Gastambide F, Wang RA, Dam SA, Mar AC, Tricklebank M, Robbins TW. The role of 5-HT2C receptors in touchscreen visual reversal learning in the rat: a cross-site study. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 1998a;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- 3.Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998b;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- 4.Baker PM, Thompson JL, Sweeney JA, Ragozzino ME. Differential effects of 5-HT(2A) and 5-HT(2C) receptor blockade on strategy-switching. Behav Brain Res. 2011;219:123–131. doi: 10.1016/j.bbr.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baratta MV, Kodandaramaiah SB, Monahan PE, Yao J, Weber MD, Lin PA, Gisabella B, Petrossian N, Amat J, Kim K, Yang A, Forest CR, Boyden ES, Goosens KA. Stress Enables Reinforcement-Elicited Serotonergic Consolidation of Fear Memory. Biol Psychiatry. 2015 Jul 2; doi: 10.1016/j.biopsych.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baratta MV, Lucero TR, Amat J, Watkins LR, Maier SF. Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learn Mem. 2008;15:84–87. doi: 10.1101/lm.800308. [DOI] [PubMed] [Google Scholar]
- 8.Beyeler A, Eckhardt CA, Tye KM. Deciphering memory function with optogenetics. Prog Mol Biol Transl Sci. 2014;122:341–390. doi: 10.1016/B978-0-12-420170-5.00012-X. [DOI] [PubMed] [Google Scholar]
- 9.Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–1596. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- 10.Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- 11.Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown VM, LaBar KS, Haswell CC, Gold AL, Mid-Atlantic MIRECCW, McCarthy G, Morey RA. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39:351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- 15.Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci U S A. 2005;102:12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 17.Christianson JP, Benison AM, Jennings J, Sandsmark EK, Amat J, Kaufman RD, Baratta MV, Paul ED, Campeau S, Watkins LR, Barth DS, Maier SF. The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. J Neurosci. 2008;28:13703–13711. doi: 10.1523/JNEUROSCI.4270-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christianson JP, Drugan RC, Flyer JG, Watkins LR, Maier SF. Anxiogenic effects of brief swim stress are sensitive to stress history. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:17–22. doi: 10.1016/j.pnpbp.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christianson JP, Fernando AB, Kazama AM, Jovanovic T, Ostroff LE, Sangha S. Inhibition of fear by learned safety signals: a mini-symposium review. J Neurosci. 2012;32:14118–14124. doi: 10.1523/JNEUROSCI.3340-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christianson JP, Greenwood BN. Stress-protective neural circuits: not all roads lead through the prefrontal cortex. Stress. 2014;17:1–12. doi: 10.3109/10253890.2013.794450. [DOI] [PubMed] [Google Scholar]
- 21.Christianson JP, Jennings JH, Ragole T, Flyer JG, Benison AM, Barth DS, Watkins LR, Maier SF. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol Psychiatry. 2011;70:458–464. doi: 10.1016/j.biopsych.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cisler JM, Steele JS, Lenow JK, Smitherman S, Everett B, Messias E, Kilts CD. Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: an exploratory fMRI study. J Psychiatr Res. 2014;48:47–55. doi: 10.1016/j.jpsychires.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- 25.Greenwood BN, Strong PV, Brooks L, Fleshner M. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise. Psychopharmacology (Berl) 2008;199:209–222. doi: 10.1007/s00213-008-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajós-Korcsok E, Robinson DD, Yu JH, Fitch CS, Walker E, Merchant KM. Rapid habituation of hippocampal serotonin and norepinephrine release and anxiety-related behaviors, but not plasma corticosterone levels, to repeated footshock stress in rats. Pharmacol Biochem Behav. 2003;74:609–616. doi: 10.1016/s0091-3057(02)01047-x. [DOI] [PubMed] [Google Scholar]
- 27.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 28.Huang MX, Yurgil KA, Robb A, Angeles A, Diwakar M, Risbrough VB, Nichols SL, McLay R, Theilmann RJ, Song T, Huang CW, Lee RR, Baker DG. Voxel-wise resting-state MEG source magnitude imaging study reveals neurocircuitry abnormality in active-duty service members and veterans with PTSD. Neuroimage Clin. 2014;5:408–419. doi: 10.1016/j.nicl.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes KC, Shin LM. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother. 2011;11:275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue T. Effects of conditioned fear stress on monoaminergic systems in the rat brain. Hokkaido Igaku Zasshi. 1993;68:377–390. [PubMed] [Google Scholar]
- 31.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawahara H, Yoshida M, Yokoo H, Nishi M, Tanaka M. Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett. 1993;162:81–84. doi: 10.1016/0304-3940(93)90565-3. [DOI] [PubMed] [Google Scholar]
- 34.Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- 35.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 36.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 37.Martijena ID, Molina VA. The influence of stress on fear memory processes. Braz J Med Biol Res. 2012;45:308–313. doi: 10.1590/S0100-879X2012007500045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin CB, Hamon M, Lanfumey L, Mongeau R. Controversies on the role of 5-HT(2C) receptors in the mechanisms of action of antidepressant drugs. Neurosci Biobehav Rev. 2014;42:208–223. doi: 10.1016/j.neubiorev.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Martin JR, Ballard TM, Higgins GA. Influence of the 5-HT2C receptor antagonist, SB-242084, in tests of anxiety. Pharmacol Biochem Behav. 2002;71:615–625. doi: 10.1016/s0091-3057(01)00713-4. [DOI] [PubMed] [Google Scholar]
- 40.McDannald MA, Jones JL, Takahashi YK, Schoenbaum G. Learning theory: a driving force in understanding orbitofrontal function. Neurobiol Learn Mem. 2014;108:22–27. doi: 10.1016/j.nlm.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Myers KM, Davis M. AX+, BX- discrimination learning in the fear-potentiated startle paradigm: possible relevance to inhibitory fear learning in extinction. Learn Mem. 2004;11:464–475. doi: 10.1101/lm.74704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 45.Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. An animal model of a behavioral intervention for depression. Neuron. 2008;60:149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quarta D, Naylor CG, Glennon JC, Stolerman IP. Serotonin antagonists in the five-choice serial reaction time task and their interactions with nicotine. Behav Pharmacol. 2012;23:143–152. doi: 10.1097/FBP.0b013e32834f9fb4. [DOI] [PubMed] [Google Scholar]
- 47.Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- 48.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Rauch SA, Morales KH, Zubritsky C, Knott K, Oslin D. Posttraumatic stress, depression, and health among older adults in primary care. Am J Geriatr Psychiatry. 2006a;14:316–324. doi: 10.1097/01.JGP.0000199382.96115.86. [DOI] [PubMed] [Google Scholar]
- 50.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006b;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Rescorla RA. Conditioned inhibition of fear resulting from negative CS-US contingencies. J Comp Physiol Psychol. 1969;67:504–509. doi: 10.1037/h0027313. [DOI] [PubMed] [Google Scholar]
- 52.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 54.Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value. Ann N Y Acad Sci. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shanks N, Zalcman S, Zacharko RM, Anisman H. Alterations of central norepinephrine, dopamine and serotonin in several strains of mice following acute stressor exposure. Pharmacol Biochem Behav. 1991;38:69–75. doi: 10.1016/0091-3057(91)90591-o. [DOI] [PubMed] [Google Scholar]
- 56.Strong PV, Greenwood BN, Fleshner M. The effects of the selective 5-HT(2C) receptor antagonist SB 242084 on learned helplessness in male Fischer 344 rats. Psychopharmacology (Berl) 2009;203:665–675. doi: 10.1007/s00213-008-1413-3. [DOI] [PubMed] [Google Scholar]
- 57.Stutzmann GE, McEwen BS, LeDoux JE. Serotonin modulation of sensory inputs to the lateral amygdala: dependency on corticosterone. J Neurosci. 1998;18:9529–9538. doi: 10.1523/JNEUROSCI.18-22-09529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Telch MJ, Beevers CG, Rosenfield D, Lee HJ, Reijntjes A, Ferrell RE, Hariri AR. 5-HTTLPR genotype potentiates the effects of war zone stressors on the emergence of PTSD, depressive and anxiety symptoms in soldiers deployed to iraq. World Psychiatry. 2015;14:198–206. doi: 10.1002/wps.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vicente MA, Zangrossi H. Serotonin-2C receptors in the basolateral nucleus of the amygdala mediate the anxiogenic effect of acute imipramine and fluoxetine administration. Int J Neuropsychopharmacol. 2012;15:389–400. doi: 10.1017/S1461145711000873. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto R, Hatano N, Sugai T, Kato N. Serotonin induces depolarization in lateral amygdala neurons by activation of TRPC-like current and inhibition of GIRK current depending on 5-HT(2C) receptor. Neuropharmacology. 2014;82:49–58. doi: 10.1016/j.neuropharm.2014.03.007. [DOI] [PubMed] [Google Scholar]