Abstract
The ventral hippocampus (VH) is involved in the both the acquisition and recall of conditioned fear. Here, we tested the role of VH in acquisition and recall of a conditioned fear discrimination. Intra-VH vehicle or muscimol injections were made 1 h prior to a CS+/CS− conditioning or prior to later recall. Vehicle treated rats exhibited discrimination with significantly greater freezing to the CS+ than to the CS− whereas muscimol treated rats did not freeze. Injections made before recall had no effect as both treatment groups displayed equal freezing in response to the CS+, and discrimination. While these results are consistent with several reports, the failure to influence fear discrimination upon recall appears to contrast with the hypothesized role of VH in recall of extinguished conditioned fear cues.
Keywords: rat, hippocampus, muscimol, fear, safety, ptsd
1. Introduction
Fear is important for survival and the neural mechanisms underlying fear learning, recall and inhibition is a rapidly progressing area of neurological research (1). Fear responses are typically specific to contexts or stimuli that were previously paired with aversive stimulation. Healthy individuals readily distinguish between danger-predicting and safety-predicting stimuli, while those suffering from posttraumatic stress disorder (PTSD) exhibit generalized fear and impaired danger versus safety discrimination (2). To study learned fear, a conditioned stimulus (CS) is first paired with footshock, and so becomes a conditioned exciter of fear responses. Preclinical research seeking to identify the neural mechanisms underling fear regulation typically employ either extinction or discrimination paradigms. Extinction occurs after repeated presentation of the CS without shock, which results in a decrease in the fear response. In a discrimination paradigm, training involves two stimuli, one that is consistently paired with a footshock (CS+), and another that is consistently unpaired (CS−). In a recall test, the CS+ evokes more fear than the unpaired cue.
A growing number of studies implicate the ventral hippocampus (VH) in the acquisition of conditioned fear (3–10) and the VH contributes to the modulation of fear in extinction and discrimination processes. The extinction of fear conditioned to a discrete cue depends upon the context in which extinction occurred because when the fear cue is presented in a novel setting, the fear response returns (11). The return of fear in a novel context depends upon excitatory input from the VH to the amygdala and prefrontal cortex (12). Thus, the VH contributes to both the initial acquisition of fear and the conditional expression of fear when context is a discriminate feature (13). Regarding discrete cues, hippocampal lesions interfere with the recall of a feature negative discrimination (14), but the VH was not isolated in this study. We tested the hypotheses that the VH may play a general role in conditioned fear discrimination by using a fear discrimination paradigm adapted from (15) wherein the fear expression is controlled by the conditioned cue instead of a context.
2. Materials and Methods
Sixteen adult male Sprague-Dawley rats were obtained from Charles River Labs (Wilmington, MA) weighing 250–300 g upon arrival. Rats were housed individually in plastic tub cages with free access to food and water and a short length of autoclaved manzanita wood for enrichment in the Boston College Animal Care Facility. The vivarium maintained a 12 h light/dark cycle. All rats were allowed to acclimate to the colony housing for 7 days prior to surgery. All experimental protocols were reviewed and approved by the Boston College Institutional Animal Care and Use Committee.
Under isoflurane anesthesia (3% in O2, Isothesia, Henry Schein, Dublin, OH) in a stereotaxic frame, as previously (3), stainless steel guide cannulae (22 g; Plastics One, Roanoke, VA) were implanted to target the VH as in (13) at −5.8 mm posterior to bregma, ±5.2 mm from midline, and −7.0 mm ventral to the surface of the skull. A stylet was placed in each cannula, which extended 1 mm below the tip of the guide. Immediately after surgery, each rat received loxicom (1mg/kg, Eloxiject, Henry Schein) and penicillin G procaine (15,000 Units, Combi-Pen-48, Henry Schein). Behavioral testing began 7–10 days after surgery.
Microinjections were made by gently restraining the rat in a cloth towel. Stylets were removed and replaced with a microinjector that extended 1 mm beyond cannula tip (33g; Plastics One). Each rat was injected bilaterally with 1 μL of either muscimol (Sigma, St. Louis, MO) in saline (1 μg per side) or saline alone at a rate of 1 μL/min. Injectors were left in place for an additional minute to allow for diffusion. The concentration was equal to that used by Hobin et al. (13). Injections were made 1 h prior to behavioral treatments as previously (16).
Fear conditioning occurred in chambers made of black plastic with wire mesh lids 10 x 11 x 6-in (L x W x H) with a stainless steel shock grid (Model H10-11R-TC-SF, Coulbourn Instruments, Whitehall, PA) enclosed within a 15 x 12 x 27-in (L x W x H) ventilated light and sound-attenuating chamber. Two infrared LED arrays (CMVision Model IR30) illuminated the chamber, and overhead cameras (Model VX-5000, Microsoft, Redmond, WA) with the manufacture’s infrared blocking filters replaced with infrared passing filters allowed for automated freezing detection with ANY-Maze software (version 4.99, Stoelting, Wood Dale, IL) as previously (17). Stimuli were delivered by a white LED array (Model LPL620WTHD, Hampton Bay) and speaker mounted at the top of the enclosure; a ventilation fan provided masking noise at ~55dB. The conditioning stimuli were a flickering white LED light (264.0 Lux, 20ms on/off) and a white noise pip (pip duration = 10ms, interval = 3 Hz, 75dB). Assignment of light or pip to CS+ or CS− was counterbalanced. No effect of the cue stimulus was evident as both the light and pip produced equivalent fear conditioning and discrimination behavior (data not shown).
To begin conditioning, rats were transferred from the vivarium, placed in the apparatus, and conditioning trials began immediately. Conditioning trials lasted 90 seconds beginning with a 70 s blackout (inter-trial-interval) after which a 5 s, 1 kHz tone (75dB) signaled the upcoming CS presentation. Then, either the CS+ cue or the CS− cue was administered for 15 seconds. CS+ trials concluded with a 500 ms, 1 mA shock (Model H13-15, Coulbourn Instruments). Training consisted of 15 trials of each cue presented in quasi-randomized order so that one trial type never occurred more than 2 times in series. Recall tests began by placing the rat in the conditioning apparatus. After 2 minutes rats received 6, 1 min presentations of each the CS+, the CS− or the context alone in a quasi-random order.
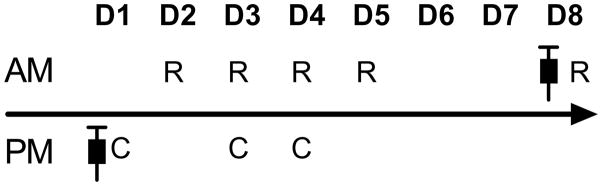
A schematic diagram of the procedures is provided in Figure 1. On day 1, rats were randomly assigned to either muscimol or vehicle conditions, injected and returned to the homecage. 1 h later all rats received CS+/CS− conditioning. Fear recall and discrimination were assessed on Days 2 and 3 in identical tests. To test the role of VH in fear discrimination recall, all rats received additional conditioning and testing until both the vehicle and muscimol treated rats exhibited equal fear and discrimination. This required two additional drug-free CS+/CS− training sessions, which began in the afternoon on Day 3 and again on Day 4. Recall tests were given on the morning of Day 4 and Day 5 at which point all rats exhibited equal freezing and discrimination, regardless of past drug treatment. Rats were then assigned to new muscimol and vehicle groups each consisting of 4 rats from the previous muscimol group and 4 rats from the previous vehicle group. To test the role of VH in fear discrimination recall, on day 8 rats received either muscimol or vehicle, according to their new groups and 1 h later given a final recall test.
Figure 1.
Conditioning sessions (C) were conducted in the afternoons of days (D) 1, 3 and 4 and recall (R) tests were conducted in the mornings on days 2, 3, 4, 5 and 8. Muscimol or vehicle infusions were made 1h before conditioning on day 1 or and before recall on day 8. Rats were assigned to new drug treatment groups based on recall on day 5. No treatment occurred on days 6 and 7.
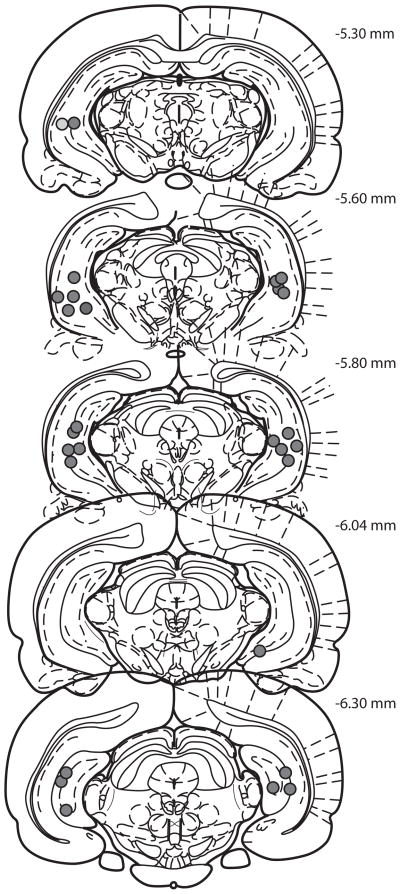
At the conclusion of the experiment rats were overdosed with tribromoethanol (Sigma), sacrificed and brains were flash frozen in 2-methylbutane on dry ice. Sections (40 μm) containing the ventral hippocampus were stained with cresyl violet and cannula placement was determined by comparison to the Rat Brain Atlas in Stereotaxic Coordinates (Paxinos and Watson, 2006). Only rats with cannula located in the ventral hippocampus were used in the statistical analysis (Figure 2).
Figure 2.
Reconstruction of ventral hippocampus cannula tip locations. Anterior to posterior coordinates relative to Bregma are shown in the upper right corner. Illustrations adapted from the atlas of Paxinos & Watson (2008, permission pending).
4. Results
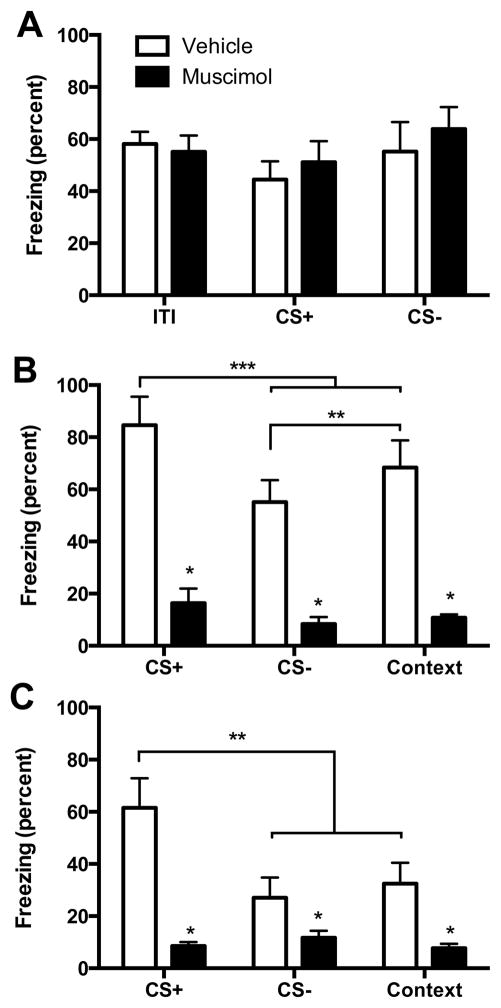
Behavioral data were analyzed using a two-way analysis of variance (ANOVA) with drug condition (muscimol or vehicle) as a between-subjects variable, and cue (CS+, CS−, ITI, or context) as a within-subjects variable. Freezing during each cue was averaged over the session for analysis. Significant main effects and interactions were subsequently explored using Tukey HSD post hoc tests to maintain an experiment-wise type I error rate α = 0.05. One rat with a misplaced cannula was excluded resulting in the following group sizes, muscimol: n = 8, vehicle: n = 7. Percent time spent freezing during conditioning was quantified during the 70 s inter-trial-interval and the 15 s CS+ and CS− presentations (Figure 3A). Although rats spent the majority of time freezing, there were no significant main effect of Cue F(2, 26) = 3.33, p = 0.051, Drug, F(1, 13) = 0.18, p = 0.679, or Cue by Drug interaction, F(2, 26) = 0.87, p = 0.430. In the recall on day 2, rats with prior muscimol exhibited reduced freezing to all cues (CS+, CS−, and context) compared to vehicle condition (Figure 3B). There was a main effect of Drug, F(1,13) = 37.95, p < 0.001, a main effect of Cue, F(2, 26) = 17.11, p < 0.001 and a significant Drug by Cue interaction, F(2, 26) = 5.61, p = 0.010. Freezing in the vehicle condition was significantly higher to all cues compared to the muscimol condition (ps < 0.05). In the vehicle condition, discrimination was evident as significantly different freezing to the CS+ compared to either CS− or context alone, and the CS− was significantly less than context (ps < 0.05). The recall test was repeated on day 3 (Figure 3C) with a main effect of Drug, F(1,13) = 15.34, p = 0.002, a main effect of Cue, F(2, 26) = 14.17, p < 0.001 and a Drug by Cue interaction, F(2, 26) = 17.35, p < 0.001. As on day 2, there was significantly greater freezing to all cues in the vehicle condition relative to pre-training muscimol and discrimination was evident in the vehicle condition as significantly greater freezing during the CS+ than during either the CS− or the context (ps < 0.05).
Figure 3.
(A) Mean (+ SEM) freezing during the inter-trial-interval (ITI), CS+ and CS− trials during conditioning. There was consistent freezing in all conditions with no effect of muscimol. (B) Mean (+ SEM) freezing during Recall on day 2. Rats with vehicle injections to the VH prior to conditioning were able to discriminate between the CS+ and the CS− and context alone (ps < 0.05) and there was significantly less freezing to the CS− than the context (p < 0.05). In contrast, VH muscimol injections reduced freezing to all cues (*ps < 0.01). (C) Mean + SEM freezing during Recall on day 3. Rats in the vehicle condition were able to discriminate between CS+ and CS− cues (ps < 0.05) and rats with prior muscimol displayed reduced freezing to all cues (*ps < 0.01).
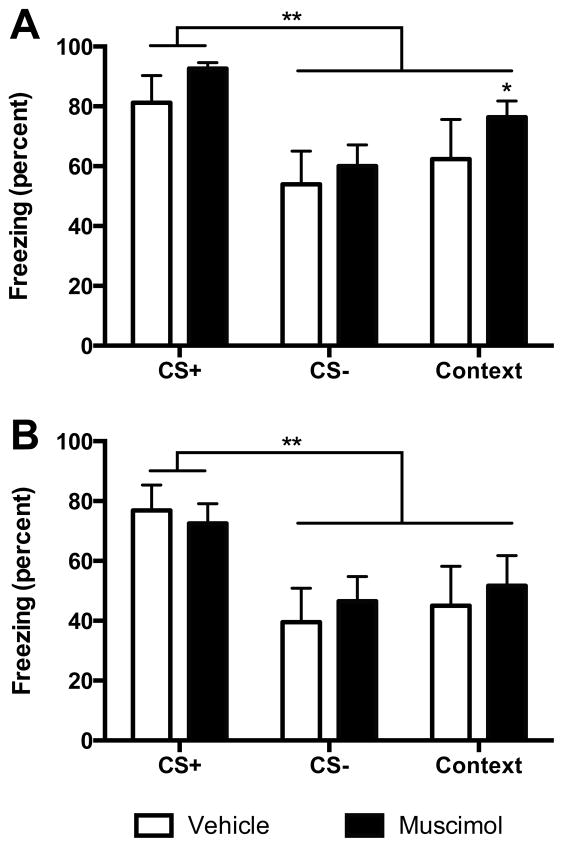
We next wished to determine if VH inactivation would interfere with fear recall and discrimination. As described above, rats received two additional sets of CS+/CS− conditioning and recall tests at which point fear discrimination was evident in all subjects. After assignment to new treatment groups (muscimol n=8, vehicle n=7), freezing was analyzed in the day 5 recall test (Figure 4A). There was a significant main effect of Cue, F (2, 26) = 20.35, p < 0.091 but no significant drug, F(1, 13) = 0.97, p = 0.340 or Drug by Cue interaction, F(2, 26) = 0.37, p = 0.693. Significantly greater freezing to occurred the CS+ than the CS− or context in each of the new treatment groups (ps < 0.01). Thus, prior to the final recall tests, the vehicle and muscimol treated rats exhibited equal fear recall and discrimination. On day 8, muscimol injected 1 h prior to the final recall test had no effect (Figure 4B). There was a significant main effect of Cue, F(2, 26) = 26.47, p < 0.001 but no effects of Drug, F(1, 13) = 0.06, p = 0.809, or Drug by Cue interaction, F(2, 26) = 0.96, p = 0.397. Discrimination was evident with significantly greater freezing to the CS+ compared to the CS−, and the CS+ compared to Context in both the muscimol and vehicle conditions (ps < 0.01).
Figure 4.
(A) Mean (+ SEM) freezing during Recall on Day 5 after reassignment to new muscimol and vehicle conditions. In both conditions, rats discriminated between the CS+ and the CS− and the context (ps < 0.05). There was significantly more freezing to the context compared to the CS− in the muscimol group (*p < 0.05). (B) Mean (+ SEM) freezing during Recall on day 8. Rats with vehicle injections or muscimol injections into the VH prior to the recall test were able to discriminate between the CS+ and the CS− and context (ps < 0.05).
5. Discussion
Our experiments add to the growing number of studies that investigated the VH in fear learning and recall. Pre-training infusion of muscimol into the VH impaired the acquisition of fear learning to all stimuli present during conditioning. In contrast, vehicle treated rats were able to discriminate between the cues with reduced freezing to the CS− than the CS+. The same pattern occurred in a second recall test at 48h post-training which suggests that lingering effects of muscimol did not influence recall. Fear discrimination was re-established in all rats which allowed us to test whether pre-testing injections of muscimol in the VH would impair the expression of fear. However, both the muscimol- and vehicle-treated rats displayed equal freezing to the CS+ and discrimination to the CS−.
Considering fear conditioned to discrete cues, our results corroborate a large number of studies implicating VH in fear acquisition (3–10), although see (8) for a discussion of exceptions. Concerning recall, however, pretest intra-VH muscimol sometimes has no effect (18) and other times causes a significant reduction in fear (19). An important procedural distinction between these studies may explain the conflicting results. Whereas Maren and Holt (18) used behavioral freezing, Sierra-Mercado et al (19) used a conditioned suppression of feeding paradigm (although freezing was also measured). Importantly, the current paradigm was more in keeping with the method of (18) which suggests that the VH may not always be critical the recall of fear.
With regard to discrimination, we expected VH inactivation to impair the recall of conditioned discrimination because of the noted role for VH in the return of fear phenomenon (12, 13) and feature-negative discriminations (14, 20). However, in the current experiments, there was no effect on discrimination, which leads to several possible interpretations. First, the repeated training used here could render the memory independent of the hippocampus. Although prior experiments involved repeated training (14), Wang et al (10) recently demonstrated that repeated conditioning trials can be mediated by either dorsal or ventral hippocampus, perhaps by engaging redundant or complementary consolidation processes. It is critical to note, however, that the use of repeated conditioning trials prior to the critical recall test may limit the generality of the null effect of VH inactivation of recall and discrimination. While this experiment does not preclude the possibility that a circuit including the VH would contribute in some way to conditioned discrimination acquisition, but it does call into question the necessity of the VH in the recall of the learned discrimination. This interpretation is consonant with a recent report that CS evoked VH potentials did not distinguish between auditory CS+ and CS− stimuli (21). The VH may be a common waypoint for all putative CSs to which the expression or association of fear is governed elsewhere, such as the amygdala and medial prefrontal cortex (21, 22).
To conclude, although the VH is critically important to the accurate discrimination between fear contexts in the return of fear phenomenon (12) the current results may indicate that this is a consequence of a more general role in which the VH distributes stimuli features to other nodes in a fear circuit but may not itself encode excitatory and inhibitory associations to discrete CSs. Additional research is warranted to determine what role, if any, the VH contributes to distinguishing between reinforced and unreinforced fear stimuli during conditioning.
Highlights.
We tested the role of the ventral hippocampus on cued fear learning and discrimination.
Inactivation of ventral hippocampus impaired cued fear learning.
Inactivation of ventral hippocampus did not influence fear expression.
Inactivation of ventral hippocampus did not influence fear discrimination.
Acknowledgments
This project was funded by National Institutes of Mental Health grant MH093412.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Exp Brain Res. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- 4.Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126:159–174. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]
- 5.Esclassan F, Coutureau E, Di Scala G, Marchand AR. Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus. 2009;19:33–44. doi: 10.1002/hipo.20473. [DOI] [PubMed] [Google Scholar]
- 6.Czerniawski J, Ree F, Chia C, Otto T. Dorsal versus ventral hippocampal contributions to trace and contextual conditioning: differential effects of regionally selective NMDA receptor antagonism on acquisition and expression. Hippocampus. 2012;22:1528–1539. doi: 10.1002/hipo.20992. [DOI] [PubMed] [Google Scholar]
- 7.Cox D, Czerniawski J, Ree F, Otto T. Time course of dorsal and ventral hippocampal involvement in the expression of trace fear conditioning. Neurobiol Learn Mem. 2013;106:316–323. doi: 10.1016/j.nlm.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Zhang WN, Bast T, Xu Y, Feldon J. Temporary inhibition of dorsal or ventral hippocampus by muscimol: distinct effects on measures of innate anxiety on the elevated plus maze, but similar disruption of contextual fear conditioning. Behav Brain Res. 2014;262:47–56. doi: 10.1016/j.bbr.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- 10.Wang SH, Finnie PS, Hardt O, Nader K. Dorsal hippocampus is necessary for novel learning but sufficient for subsequent similar learning. Hippocampus. 2012;22:2157–2170. doi: 10.1002/hipo.22036. [DOI] [PubMed] [Google Scholar]
- 11.Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J Exp Psychol Anim Behav Process. 1983;9:248–265. [PubMed] [Google Scholar]
- 12.Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- 14.Heldt SA, Coover GD, Falls WA. Posttraining but not pretraining lesions of the hippocampus interfere with feature-negative discrimination of fear-potentiated startle. Hippocampus. 2002;12:774–786. doi: 10.1002/hipo.10033. [DOI] [PubMed] [Google Scholar]
- 15.Myers KM, Davis M. AX+, BX− discrimination learning in the fear-potentiated startle paradigm: possible relevance to inhibitory fear learning in extinction. Learn Mem. 2004;11:464–475. doi: 10.1101/lm.74704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 17.Christianson JP, Jennings JH, Ragole T, Flyer JG, Benison AM, Barth DS, Watkins LR, Maier SF. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol Psychiatry. 2011;70:458–464. doi: 10.1016/j.biopsych.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- 19.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]