Introduction
Atrial fibrillation (AF) involves 1–2% of the adult general population, a rate that increases to 15% in those 80 years and above.1 Because of the aging of the general population, this epidemic of AF is expected to increase over the next decades,2-4 and to impose an increasing burden on the health care system due to the need for life-long care and pharmacologic treatment. Identification of the mechanisms underlying AF represents an unmet need and a first step towards developing more effective preventive measures.
Arterial hypertension (HT) is tightly associated with AF, as originally reported in the Framingham Heart Study,5 and thereafter confirmed by several studies.6-13 HT is a major predictor of AF and 50% to 90% of AF patients have HT.
Accumulating evidences point to a role for the renin-angiotensin-aldosterone system (RAAS)in the pathophysiology of cardiac inflammation, fibrosis, and hypertrophy.14-17 Aldosterone not only exerts well-known pressor effects, but also promotes inflammation, myocardial necrosis, cardiac collagen deposition, fibrosis, and left ventricular hypertrophy (LVH).18,19 Accordingly, there is renewed interest in aldosterone as one of the major culprits leading to chamber remodeling and ultimately creating the stage for AF in hypertensive patients.15,17,20-25 The evidence supporting a role for aldosterone in AF was, however, derived from observational studies performed in patients with heart diseases that are known to cause AF. This leaves open the question of whether aldosterone triggers AF per se, or only in the presence of structural heart disease.
In recent years, three reviews examined the relation between aldosterone and AF: one focused on the anti-arrhythmic potential of mineralocorticoid receptor (MR) antagonists in AF patients;26 another on the role of the MR in arrhythmias;27 and the last one on aldosterone-induced oxidative stress in atrial remodeling in AF.28 Thus, we thought it interesting to focus on the general role of hyperaldosteronism in AF starting with the epidemiologic data and moving on to discuss the molecular mechanisms whereby aldosteronism can induce AF in hypertension and the results of the clinical trials that either reduce aldosterone production and/or block its actions. To this aim the literature was searched using the PICO strategy (Table S1).29
The relationship between hyperaldosteronism and AF
The association of AF with hyperaldosteronism was not recognized for many years. About a decade ago, two case reports first described AF as the presenting sign of primary aldosteronism (PA), the most common, albeit often unrecognized, cause of secondary HT.30,31 In 2006, a 58 year-old man with PA was hospitalized four times for AF and hypokalemia; correction of the latter coincided with sinus rhythm restoration.32 In 2009, AF occurred despite optimal blood pressure (BP) and electrolytes control in another PA case.33 These cases led to the proposal that hypokalemia and/or aldosterone can cause paroxysmal AF. The relative importance of hyperaldosteronism and hypokalemia for AF remains unknown as disentangling the role of these two factors is challenging; hyperaldosteronism leads to hypokalemia whereas hemolysis at blood sampling can factitiously mask hypokalemia.
In 2005, a retrospective survey of cardiovascular complications in hypertensive patients with and without PA demonstrated a highly significant 12.1-fold increased risk of AF in patients with PA as compared to essential hypertensive patients.34 It is noteworthy that, along with age and known duration of hypertension, PA independently predicted AF on multivariate analysis, which strongly implicated hyperaldosteronism in the pathophysiology of AF in hypertensive patients. These results were confirmed in a larger prospective cohort study of systematically screened patients with HT, which found a somewhat lower rate of AF, likely because of the earlier diagnosis of PA; yet AF was significantly increased by 7-fold in the PA patients as compared to the essential hypertensive patients. Further, during long-term (median 36 months) follow-up, PA patients with greater increases in LV mass had a shorter AF-free survival.35
Given the lack of prospective studies, the on-going Prospective Appraisal of the Prevalence of Primary aldosteronism in HYpertensive patients (PAPPHY) Study was undertaken to assess prospectively the prevalence of PA and its subtypes, i.e. aldosterone producing adenoma (APA) and idiopathic hyperplasia (IHA), in consecutive hypertensive patients referred for evaluation of AF.36 The hypothesis that AF is a common clinical presentation of PA is very important from the practical standpoint; if verified, it would provide compelling evidence for a role for HT and hyperaldosteronism in the multitude of patients with AF. These patients may have underlying heart disease, but no other obvious cause for the arrhythmia. Currently, they only receive antihypertensive treatment and, if they have a CHA2DS2VASc (Congestive heart failure, Hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled), Vascular disease, Age 65–74, and Female Sex) score ≥ 2 or ≥ 3 in males and females, respectively, life-long anticoagulation along with drugs aimed at achieving rate control or restoring and maintaining sinus rhythm.1 All these measures impose a burden on the health care systems as well as on the patients' quality of life.
The demonstration that PA is a cause of AF in hypertensive patients might eventually change clinical practice in this field in that it may lead to systematic screening for PA. This is important since PA involves more than 11% of the hypertensive patients referred to specialized centers and surgical cure can be achieved in over 50% of PA patients.30 Moreover, cure of PA by surgery or treatment with MR blockade not only regresses LVH,35 but can even restore sinus rhythm as suggested in a long-term study.35
The vicious circle of aldosteronism and AF: does AF raise aldosterone?
Plasma aldosterone concentrations (PAC) were found to be elevated during AF and to fall with restoration of sinus rhythm in patients with persistent AF.37 Furthermore, PAC were described to be higher in patients with long-standing persistent AF than in patients with restored sinus rhythm.38 A decrease in PAC after successful DC cardio-version with maintenance of sinus rhythm was also reported in patients with normal LV function.39 One probable mechanism for the increase in aldosterone with AF is that AF decreases BP, which will activate the renin-angiotensin-aldosterone system. During AF the release of atrial natriuretic peptide (ANP),40 which potently inhibits aldosterone secretion,41 will serve to blunt the effects of AF on RAAS activation.
As mentioned above, the aldosterone-induced cardiac remodeling can create the substrate for AF, thus facilitating the persistence of the arrhythmia. Investigation of the role of aldosterone in AF seems, therefore, a ‘chicken-egg puzzle’, which needs well planned studies in different models to be solved. Moreover, we will examine below the possibility that AF alters sensitivity of the heart to the action of aldosterone.
Atrial mineralocorticoid receptor (MR) in AF
In 2010, the expression of the MR was reported to be higher in the right atrial appendages obtained from AF patients than from patients in sinus rhythm, thus implicating upregulation of this receptor in AF.42 It remained, however, unknown whether the onset of AF by itself was responsible for the enhanced MR expression, or if the latter facilitates the development of AF. Moreover, these data were generated using specimens from patients undergoing mitral and/or aortic valve replacement, in which the atrium could be remodeled, stretched, and/or affected by the underlying disease. Hence, it remained altogether unclear if the MR expression was increased just because of the concomitance of valvular diseases that are known to be associated with AF. Since the MR not only binds aldosterone, but also cortisol, that circulates at much higher (100 to 1000 fold) levels, it might be argued that some detrimental effects attributed to aldosterone were in fact driven by cortisol. However, the cortisol-inactivating enzyme 11βHSD2 has been reported to be more expressed in the atria of AF patients, which suggests that under physiological conditions MR activation in the atria of AF patients is mainly due to aldosterone, rather than to endogenous glucocorticoids.43 Given the difficulty of obtaining tissue, information on MR expression in the atria from hypertensive patients with or without AF is unavailable. Hence, the role of MR in the atria in mediating the onset and/or perpetuation of AF remains to be established.
This issue was addressed by an elegant experiment where rapid electrical field stimulation was used to induce depolarization of HL-1 atrial cardiomyocytes. This led to unambiguous increases in MR protein expression,44 via mechanisms involving intracellular Ca2+, as the MR increase was abrogated by chelating intracellular Ca2+ with BAPTA-AM and also by verapamil, a L-type Ca2+ channel blocker.43 Thus, it can be concluded that electrical remodeling by itself increases the expression of the MR via Ca2+-dependent pathways (Figure S1). 44
In turn, increased MR expression can amplify the effects of aldosterone, and possibly cortisol, on the cardiomyocytes. Of note, aldosterone increases T-type Ca2+ currents and induces sarcoplasmic reticulum Ca2+ overload in HL-1 cells, most likely by acting through the MR as these effects are blunted by the MR antagonist spironolactone.44 Since neither HL-1 nor human atrial cells can produce aldosterone,44 blood-borne aldosterone is the likely driver of these effects (Figure S1).
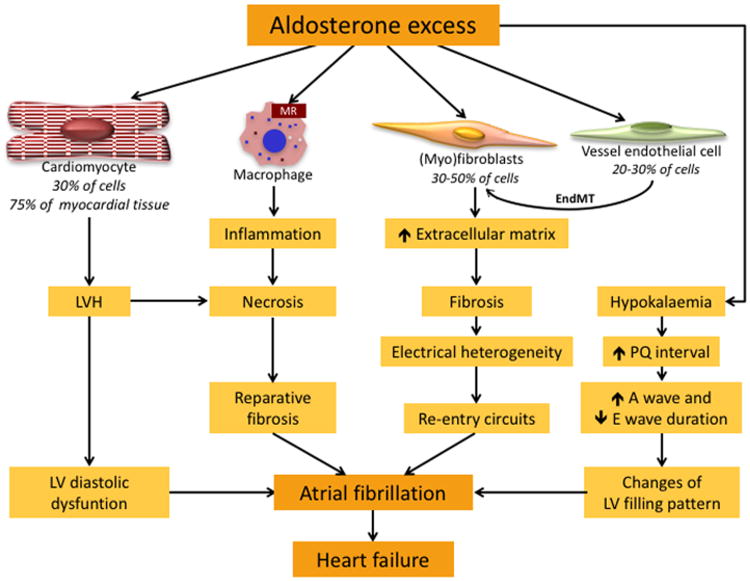
The following can, therefore, be a likely sequence of events: aldosterone enhances T-type Ca2+ currents and Ca2+ influx, thus increasing MR expression and reinforcing aldosterone's effects on the heart. Moreover, it favors re-entry mechanisms by inducing LVH and fibrosis, leading to stiffening of the LV, impaired LV filling, with ensuing atrial stretching and increasing left atrial size.45 Noteworthy, the hyperaldosteronism-associated hypokalemia prolongs the PQ interval and favors atrio-ventricular reentry mechanisms. By rendering the LV more dependent on the atrial kick for its filling, this can explain why PA patients can be more prone to pulmonary edema when they develop AF (Figure 1).21,46
Figure 1. Mechanisms by which aldosterone excess favors onset of atrial fibrillation.
Aldosterone affects all cell types that are the primary constituents of cardiac tissue: a) cardiomyocytes, which constitute 30% of myocardial cells, but 75% of myocardial tissue, b) fibroblasts (30-50% of myocardial cells), c) endothelial cells (20-30% of myocardial cells), and also activates macrophages. Aldosterone excess induces enlargement of cardiomyocytes and remodeling leading to left ventricular hypertrophy (LVH) that predisposes to diastolic dysfunction. Aldosterone excess also activates the transition of fibroblasts into myofibroblasts, which produce collagen and other extracellular matrix proteins, favoring fibrosis. Fibrotic tissue induces electrical heterogeneity of the myocardium that causes re-entry circuits, thereby leading to onset of atrial fibrillation. Endothelial cells exposed to aldosterone excess undergo transition into myofibroblasts (endothelial-to-mesenchymal transition, EMT), which contribute to the development of fibrosis.
The activation of the mineralocorticoid receptor (MR) on the monocytes/macrophages favors inflammation, necrosis and reparative fibrosis, and eventually atrial fibrillation.69 Aldosterone excess induces hypokalemia, which causes prolongation of PQ interval and changes in the duration of A and E waves, leading to abnormal LV filling that promotes AF, which in turn favors heart failure.
Aldosterone favors inducible atrial arrhythmias
In 2012, a well-designed experimental study provided a direct demonstration that aldosterone induces AF. Rats were infused with aldosterone for 8 weeks at a dose [0.5 ug/h] that does not affect ventricular function or atrial pressures, but lengthens the P-wave duration of and the total right atrial activation time. The rats developed AF after trans-esophageal atrial burst stimulation.47 In the same year, it was shown that aldosterone, even when infused for a shorter time (4 weeks) at a dose [0.5 ug/h] that did not increase BP, induced shortening of the left atria action potential and doubling of the mean time until spontaneous conversion into sinus rhythm.48 In both studies, the minimal changes in BP (systolic BP 134±10 vs 129±5 mmHg, aldosterone vs control) suggested that aldosterone per se can create a substrate for atrial arrhythmias without markedly affecting LV afterload.47
Besides its electrophysiological effects, aldosterone can promote arrhythmia by causing inflammation, vascular remodeling and possibly microcirculatory dysfunction.19,49 Aldosterone has been shown to increase the levels of pro-inflammatory genes (including cyclooxygenase-2, osteopontin, tumor necrosis factor α (TNFα), monocyte chemotactic protein-1 (MCP-1) and NADPH oxidase)50,51 with ensuing fibrosis, and to induce electrophysiological alterations that lead to early and delayed after-depolarization of the cardiomyocytes.52 The deposition of fibrotic tissue, by decreasing gap junctions coupling and creating muscle bundle discontinuities, alters the spatial location and propagation of depolarization waves, thereby reducing conduction velocity and promoting re-entry circuits.52,53 Because of the relatively low membrane potential of fibroblasts (-30 mV), fibroblast-cardiomyocyte coupling promotes delayed after-depolarization of the cardiomyocytes and ectopic firing.52 It also increases transient Ca2+ amplitude, leading to increased intracellular Ca2+ concentrations and spontaneous sarcoplasmic reticulum Ca2+ release, finally favoring AF maintenance 52 (Figure S1).
Electrophysiological changes were also documented with aldosterone in neonatal rat cardiomyocytes and atrial mouse cells. Aldosterone increased T-type channels in cardiomyocytes and L-type Ca2+ channels in atrial cells, and decreased the activity of the rapidly activating delayed rectifier potassium current IKr and transient outward K+ currents Ito1.54 Aldosterone also promoted the prolonged release of Ca2+ from the sarcoplasmic reticulum due to the opening of ryanodine receptors,55,56 finally leading to Ca2+ overload and thereby promoting AF (Supplemental Figure S1).
The ‘upstream’ therapy of AF and the randomized clinical trials (RCTs)
The term ‘upstream therapy’ refers to non anti-arrhythmic therapy that modifies the atrial substrate and can thereby prevent AF. Upstream therapy was encouraged in the treatment of AF in hypertensive patients, because theoretically it could prevent new onset and recurrent AF.14 The RCTs in arterial hypertension with agents that blunt aldosterone secretion or aldosterone effects 7,57,58 can therefore be regarded as ‘upstream’ therapy. Unfortunately, none of the RCTs carried out thus far was specifically designed to look at incident AF as a primary endpoint.
In the Losartan Intervention For End-point reduction (LIFE) study, which recruited hypertensive patients with LVH, the type 1 angiotensin receptor antagonist (ARB) losartan was found to be superior to atenolol in regressing LVH and in reducing new-onset AF.7 In the Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) Study, which recruited hypertensive patients at high cardiovascular risk, valsartan was more effective than amlodipine in preventing/regressing AF.57 Moreover, a meta-analysis of eleven studies with a total of 56,308 patients showed that ACE-inhibitors and ARBs reduced the overall relative risk of AF by 28%, thus confirming the beneficial effects of RAAS blockade in preventing AF 58 (reviewed in59). Needless to say, these results can be interpreted in two ways: either in terms of reducing AT1 receptor signaling and/or in terms of blunting aldosterone secretion.31
Studies with MR antagonists (MRAs) provided further evidence to sort out this issue. As already mentioned, the rationale for using these agents relies in the fact that PAC is increased in AF, and MR is up-regulated in the atria of AF patients, which led to consider MRAs as promising ‘upstream drugs’.
In 1999, the Randomized Aldactone Evaluation Study (RALES), the first large RCT evaluating the MRA spironolactone on top of standard care in New York Heart Association (NYHA) classes III–IV heart failure patients, was prematurely stopped on the steering committee's recommendation because the primary end-point, i.e. the decrease in mortality, was reached.60 Although data on incident AF were not collected, the authors speculated that a lower risk of sudden death in the spironolactone arm could be related to prevention of cardiac fibrosis with ensuing decreased susceptibility to arrhythmias. In 2003, the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) also found a decreased incidence of sudden death, thus supporting this hypothesis.
A decade later the multicenter Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) study randomized patients with an ejection fraction < 35%, but only mild symptoms (NYHA II) of heart failure; over 66% of the patients had hypertension. A post-hoc analysis of the study provided evidence that the MRA is more effective than placebo in preventing AF. During the treatment period (median 21 months), newly detected AF occurred in significantly fewer patients in the eplerenone group than in the placebo group (2.7% vs 4.5% hazard ratio [HR]: 0.58, 95 CI: 0.35-0.96).61
More recently, the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Study recruited patients with heart failure and preserved LV ejection fraction in North/South America and Eastern Europe. The patients were randomized to receiving spironolactone or placebo on top of other treatment. The study failed on its primary composite endpoint (that included time-to-cardiovascular death, aborted cardiac arrest, or hospitalization for heart failure). However, a post-hoc analysis showed marked regional heterogeneity in the recruited patients, with American patients demonstrating a significant reduction in the primary outcome with spironolactone, a result not seen in the patients recruited in Russia or Georgia.62 As in RALES and EPHESUS, data on incident AF have yet to be given and, unfortunately, to the best of our knowledge, no studies on these databases have been planned to look specifically at AF.
Of note, the randomized, placebo-controlled trial Atrial Fibrillation and Renin Angiotensin Aldosterone System study (ClinicalTrials.gov Identifier: NCT00141778) designed to investigate whether the antagonism of the RAAS reduces the risk of postoperative AF in patients undergoing surgery for coronary artery or valvular heart disease failed to show a beneficial effect of either ramipril or spironolactone over placebo.63 The duration of treatment before surgery (from 4 days to one week) could, however, have been too short to evidence such effect.
In a recent study involving patients with primary and secondary hyperaldosteronism, an association of excess aldosterone with changes of LV remodeling and function was documented.64 The PA patients had increased LV mass, high prevalence of LVH, inappropriate LV mass, subclinical LV systolic and diastolic dysfunction. The patients with secondary hyperaldosteronism (due to liver cirrhosis) also show increased LV mass, high prevalence of LVH and diastolic dysfunction, but not subclinical systolic dysfunction. The work overload caused by a hyper-dynamic circulatory state and the high renin with ensuing hyperaldosteronism could be driving these changes in patients with liver cirrhosis.64 Thus, in both PA and secondary hyperaldosteronism, aldosterone contributes to LVH and also cardiac fibrosis, causing LV diastolic dysfunction, increased atrial afterload, with ensuing atrial stretching, and wall stress, favoring AF onset.64,65
CYP11B2 Polymorphisms and AF in HT
Variations in the aldosterone synthase (CYP11B2) gene has been linked to cardiac remodeling,66-68 hypertension, albeit with inconsistent results as discussed in detail in the Supplemental data.
Conclusions
The success of current pharmacological strategies relying on blockade of ion channels that regulate conduction and/or atrial refractoriness for controlling AF is admittedly limited; AF recurs in most patients. The burden posed on the health care system and on patients' quality of life by AF requires swift actions to improve knowledge of the mechanisms underlying AF. This is particularly important given the expected rise in the prevalence of AF in the next decades. Improving our understanding of the mechanisms underlying AF is a key step towards developing more effective strategies for prevention of AF. Experimental and observational studies have provided compelling evidence for a direct role of aldosterone and the MR in promoting cardiac fibrosis and disrupting the conduction system, thus favoring the onset of AF. As Paul Dudley White, the Father of U.S Cardiology, used to say “Heart disease's death, before 80, is our fault, not God's or Nature's will”. This could not apply better to a condition like AF that can effectively be prevented by proper managing.
Supplementary Material
Acknowledgments
Sources of Funding. The work was supported by grant RF2011-02352318 from the Ministry of Health to TMS, and grants from the University of Padova to TMS and GPR and K24 HL103845 from the National Institutes of Health to GKA.
Footnotes
Conflict of Interest/Disclosure: None
References
- 1.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–521. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Naccarelli GV, Johnston SS, Dalal M, Lin J, Patel PP. Rates and implications for hospitalization of patients >/=65 years of age with atrial fibrillation/flutter. Am J Cardiol. 2012;109:543–549. doi: 10.1016/j.amjcard.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 6.Verdecchia P, Reboldi G, Gattobigio R, Bentivoglio M, Borgioni C, Angeli F, Carluccio E, Sardone MG, Porcellati C. Atrial fibrillation in hypertension: predictors and outcome. Hypertension. 2003;41:218–223. doi: 10.1161/01.hyp.0000052830.02773.e4. [DOI] [PubMed] [Google Scholar]
- 7.Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlof B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Devereux RB. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–719. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 8.Okin PM, Hille DA, Larstorp AC, Wachtell K, Kjeldsen SE, Dahlof B, Devereux RB. Effect of lower on-treatment systolic blood pressure on the risk of atrial fibrillation in hypertensive patients. Hypertension. 2015;66:368–373. doi: 10.1161/HYPERTENSIONAHA.115.05728. [DOI] [PubMed] [Google Scholar]
- 9.ONTARGET Investigators. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 10.Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119:2146–2152. doi: 10.1161/CIRCULATIONAHA.108.830042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundvold I, Skretteberg PT, Liestol K, Erikssen G, Kjeldsen SE, Arnesen H, Erikssen J, Bodegard J. Upper normal blood pressures predict incident atrial fibrillation in healthy middle-aged men: a 35-year follow-up study. Hypertension. 2012;59:198–204. doi: 10.1161/HYPERTENSIONAHA.111.179713. [DOI] [PubMed] [Google Scholar]
- 12.Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Lindholm LH, Nieminen MS, Edelman JM, Hille DA, Dahlof B. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA. 2006;296:1242–1248. doi: 10.1001/jama.296.10.1242. [DOI] [PubMed] [Google Scholar]
- 13.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P ESC Committee for Practice Guidelines (CPG) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki T, Yazaki Y. Role of tissue angiotensin II in myocardial remodelling induced by mechanical stress. J Hum Hypertens. 1999;13(1):S43–7. doi: 10.1038/sj.jhh.1000747. discussion S49-50. [DOI] [PubMed] [Google Scholar]
- 16.Dahlof B. Effect of angiotensin II blockade on cardiac hypertrophy and remodelling: a review. J Hum Hypertens. 1995;9(5):S37–44. [PubMed] [Google Scholar]
- 17.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res. 2015;116:1269–1276. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 18.Rossi GP, Sechi LA, Giacchetti G, Ronconi V, Strazzullo P, Funder JW. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol Metab. 2008;19:88–90. doi: 10.1016/j.tem.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Rossi GP, Bolognesi M, Rizzoni D, Seccia TM, Piva A, Porteri E, Tiberio GA, Giulini SM, Agabiti-Rosei E, Pessina AC. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension. 2008;51:1366–1371. doi: 10.1161/HYPERTENSIONAHA.108.111369. [DOI] [PubMed] [Google Scholar]
- 20.Rossi GP, Sacchetto A, Visentin P, Canali C, Graniero GR, Palatini P, Pessina AC. Changes in left ventricular anatomy and function in hypertension and primary aldosteronism. Hypertension. 1996;27:1039–1045. doi: 10.1161/01.hyp.27.5.1039. [DOI] [PubMed] [Google Scholar]
- 21.Rossi GP, Sacchetto A, Pavan E, Palatini P, Graniero GR, Canali C, Pessina AC. Remodeling of the left ventricle in primary aldosteronism due to Conn's adenoma. Circulation. 1997;95:1471–1478. doi: 10.1161/01.cir.95.6.1471. [DOI] [PubMed] [Google Scholar]
- 22.Carey RM. Aldosterone and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2010;17:194–198. doi: 10.1097/med.0b013e3283390fa4. [DOI] [PubMed] [Google Scholar]
- 23.Azibani F, Fazal L, Chatziantoniou C, Samuel JL, Delcayre C. Aldosterone mediates cardiac fibrosis in the setting of hypertension. Curr Hypertens Rep. 2013;15:395–400. doi: 10.1007/s11906-013-0354-3. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013;19:1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- 26.Dabrowski R, Szwed H. Antiarrhythmic potential of aldosterone antagonists in atrial fibrillation. Cardiol J. 2012;19:223–229. doi: 10.5603/cj.2012.0043. [DOI] [PubMed] [Google Scholar]
- 27.Gravez B, Tarjus A, Jaisser F. Mineralocorticoid receptor and cardiac arrhythmia. Clin Exp Pharmacol Physiol. 2013;40:910–915. doi: 10.1111/1440-1681.12156. [DOI] [PubMed] [Google Scholar]
- 28.Mayyas F, Alzoubi KH, Van Wagoner DR. Impact of aldosterone antagonists on the substrate for atrial fibrillation: aldosterone promotes oxidative stress and atrial structural/electrical remodeling. Int J Cardiol. 2013;168:5135–5142. doi: 10.1016/j.ijcard.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem. 2007;15:508–511. doi: 10.1590/s0104-11692007000300023. [DOI] [PubMed] [Google Scholar]
- 30.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 31.Rossi GP. A comprehensive review of the clinical aspects of primary aldosteronism. Nat Rev Endocrinol. 2011;7:485–495. doi: 10.1038/nrendo.2011.76. [DOI] [PubMed] [Google Scholar]
- 32.Al-Aloul B, Li JM, Benditt D, Tholakanahalli V. Atrial fibrillation associated with hypokalemia due to primary hyperaldosteronism (Conn's syndrome) Pacing Clin Electrophysiol. 2006;29:1303–1305. doi: 10.1111/j.1540-8159.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 33.Watson T, Karthikeyan VJ, Lip GY, Beevers DG. Atrial fibrillation in primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2009;10:190–194. doi: 10.1177/1470320309342734. [DOI] [PubMed] [Google Scholar]
- 34.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62:62–69. doi: 10.1161/HYPERTENSIONAHA.113.01316. [DOI] [PubMed] [Google Scholar]
- 36.Rossi GP, Seccia TM, Gallina V, et al. Prospective appraisal of the prevalence of primary aldosteronism in hypertensive patients presenting with atrial flutter or fibrillation (PAPPHY Study): rationale and study design. J Hum Hypertens. 2013;27:158–163. doi: 10.1038/jhh.2012.21. [DOI] [PubMed] [Google Scholar]
- 37.Goette A, Hoffmanns P, Enayati W, Meltendorf U, Geller JC, Klein HU. Effect of successful electrical cardioversion on serum aldosterone in patients with persistent atrial fibrillation. Am J Cardiol. 2001;88:906–9. A8. doi: 10.1016/s0002-9149(01)01905-1. [DOI] [PubMed] [Google Scholar]
- 38.Dixen U, Ravn L, Soeby-Rasmussen C, Paulsen AW, Parner J, Frandsen E, Jensen GB. Raised plasma aldosterone and natriuretic peptides in atrial fibrillation. Cardiology. 2007;108:35–39. doi: 10.1159/000095671. [DOI] [PubMed] [Google Scholar]
- 39.Wozakowska-Kaplon B, Bartkowiak R, Janiszewska G. A decrease in serum aldosterone level is associated with maintenance of sinus rhythm after successful cardioversion of atrial fibrillation. Pacing Clin Electrophysiol. 2010;33:561–565. doi: 10.1111/j.1540-8159.2009.02673.x. [DOI] [PubMed] [Google Scholar]
- 40.Rossi A, Enriquez-Sarano M, Burnett JC, Jr, Lerman A, Abel MD, Seward JB. Natriuretic peptide levels in atrial fibrillation: a prospective hormonal and Doppler-echocardiographic study. J Am Coll Cardiol. 2000;35:1256–1262. doi: 10.1016/s0735-1097(00)00515-5. [DOI] [PubMed] [Google Scholar]
- 41.Anderson JV, Struthers AD, Payne NN, Slater JD, Bloom SR. Atrial natriuretic peptide inhibits the aldosterone response to angiotensin II in man. Clin Sci (Lond) 1986;70:507–512. doi: 10.1042/cs0700507. [DOI] [PubMed] [Google Scholar]
- 42.Tsai CT, Chiang FT, Tseng CD, Hwang JJ, Kuo KT, Wu CK, Yu CC, Wang YC, Lai LP, Lin JL. Increased expression of mineralocorticoid receptor in human atrial fibrillation and a cellular model of atrial fibrillation. J Am Coll Cardiol. 2010;55:758–770. doi: 10.1016/j.jacc.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 43.Lavall D, Selzer C, Schuster P, Lenski M, Adam O, Schafers HJ, Bohm M, Laufs U. The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation. J Biol Chem. 2014;289:6656–6668. doi: 10.1074/jbc.M113.519256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai CF, Yang SF, Chu HJ, Ueng KC. Cross-talk between mineralocorticoid receptor/angiotensin II type 1 receptor and mitogen-activated protein kinase pathways underlies aldosterone-induced atrial fibrotic responses in HL-1 cardiomyocytes. Int J Cardiol. 2013;169:17–28. doi: 10.1016/j.ijcard.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 45.Gaddam K, Corros C, Pimenta E, Ahmed M, Denney T, Aban I, Inusah S, Gupta H, Lloyd SG, Oparil S, Husain A, Dell'Italia LJ, Calhoun DA. Rapid reversal of left ventricular hypertrophy and intracardiac volume overload in patients with resistant hypertension and hyperaldosteronism: a prospective clinical study. Hypertension. 2010;55:1137–1142. doi: 10.1161/HYPERTENSIONAHA.109.141531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi GP. Cardiac consequences of aldosterone excess in human hypertension. Am J Hypertens. 2006;19:10–12. doi: 10.1016/j.amjhyper.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Reil JC, Hohl M, Selejan S, Lipp P, Drautz F, Kazakow A, Munz BM, Muller P, Steendijk P, Reil GH, Allessie MA, Bohm M, Neuberger HR. Aldosterone promotes atrial fibrillation. Eur Heart J. 2012;33:2098–2108. doi: 10.1093/eurheartj/ehr266. [DOI] [PubMed] [Google Scholar]
- 48.Lammers C, Dartsch T, Brandt MC, Rottlander D, Halbach M, Peinkofer G, Ockenpoehler S, Weiergraeber M, Schneider T, Reuter H, Muller-Ehmsen J, Hescheler J, Hoppe UC, Zobel C. Spironolactone prevents aldosterone induced increased duration of atrial fibrillation in rat. Cell Physiol Biochem. 2012;29:833–840. doi: 10.1159/000178483. [DOI] [PubMed] [Google Scholar]
- 49.Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Muiesan P, Giulini SM, Agabiti-Rosei E. Vascular hypertrophy and remodeling in secondary hypertension. Hypertension. 1996;28:785–790. doi: 10.1161/01.hyp.28.5.785. [DOI] [PubMed] [Google Scholar]
- 50.Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802–10. doi: 10.1152/ajpheart.01096.2001. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am J Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka K, Ashizawa N, Kawano H, Sato O, Seto S, Nishihara E, Terazono H, Isomoto S, Shinohara K, Yano K. Aldosterone induces circadian gene expression of clock genes in H9c2 cardiomyoblasts. Heart Vessels. 2007;22:254–260. doi: 10.1007/s00380-006-0968-3. [DOI] [PubMed] [Google Scholar]
- 54.Benitah JP, Perrier E, Gomez AM, Vassort G. Effects of aldosterone on transient outward K+ current density in rat ventricular myocytes. J Physiol. 2001;537:151–160. doi: 10.1111/j.1469-7793.2001.0151k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ouvrard-Pascaud A, Sainte-Marie Y, Benitah JP, et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation. 2005;111:3025–3033. doi: 10.1161/CIRCULATIONAHA.104.503706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez AM, Rueda A, Sainte-Marie Y, et al. Mineralocorticoid modulation of cardiac ryanodine receptor activity is associated with downregulation of FK506-binding proteins. Circulation. 2009;119:2179–2187. doi: 10.1161/CIRCULATIONAHA.108.805804. [DOI] [PubMed] [Google Scholar]
- 57.Schmieder RE, Kjeldsen SE, Julius S, McInnes GT, Zanchetti A, Hua TA VALUE Trial Group. Reduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trial. J Hypertens. 2008;26:403–411. doi: 10.1097/HJH.0b013e3282f35c67. [DOI] [PubMed] [Google Scholar]
- 58.Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, Connolly SJ. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005;45:1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 59.Seccia TM, Caroccia B, Muiesan ML, Rossi GP. Atrial fibrillation and arterial hypertension: A common duet with dangerous consequences where the renin angiotensin-aldosterone system plays an important role. Int J Cardiol. 2016;206:71–76. doi: 10.1016/j.ijcard.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 61.Swedberg K, Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Shi H, Vincent J, Pitt B EMPHASIS-HF Study Investigators. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59:1598–1603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 62.Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 63.Pretorius M, Murray KT, Yu C, Byrne JG, Billings FT, 4th, Petracek MR, Greelish JP, Hoff SJ, Ball SK, Mishra V, Body SC, Brown NJ. Angiotensin-converting enzyme inhibition or mineralocorticoid receptor blockade do not affect prevalence of atrial fibrillation in patients undergoing cardiac surgery. Crit Care Med. 2012;40:2805–2812. doi: 10.1097/CCM.0b013e31825b8be2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cesari M, Letizia C, Angeli P, Sciomer S, Rosi S, Rossi GP. Cardiac Remodeling in Patients With Primary and Secondary Aldosteronism: A Tissue Doppler Study. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.004815. 10.1161/CIRCIMAGING.116.004815. [DOI] [PubMed] [Google Scholar]
- 65.Rosenberg MA, Manning WJ. Diastolic dysfunction and risk of atrial fibrillation: a mechanistic appraisal. Circulation. 2012;126:2353–2362. doi: 10.1161/CIRCULATIONAHA.112.113233. [DOI] [PubMed] [Google Scholar]
- 66.Delles C, Erdmann J, Jacobi J, Hilgers KF, Fleck E, Regitz-Zagrosek V, Schmieder RE. Aldosterone synthase (CYP11B2) -344 C/T polymorphism is associated with left ventricular structure in human arterial hypertension. J Am Coll Cardiol. 2001;37:878–884. doi: 10.1016/s0735-1097(00)01174-8. [DOI] [PubMed] [Google Scholar]
- 67.Isaji M, Mune T, Takada N, Yamamoto Y, Suwa T, Morita H, Takeda J, White PC. Correlation between left ventricular mass and urinary sodium excretion in specific genotypes of CYP11B2. J Hypertens. 2005;23:1149–1157. doi: 10.1097/01.hjh.0000170377.00591.7e. [DOI] [PubMed] [Google Scholar]
- 68.Stella P, Bigatti G, Tizzoni L, Barlassina C, Lanzani C, Bianchi G, Cusi D. Association between aldosterone synthase (CYP11B2) polymorphism and left ventricular mass in human essential hypertension. J Am Coll Cardiol. 2004;43:265–270. doi: 10.1016/j.jacc.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 69.Shen JZ, Morgan J, Tesch GH, Rickard AJ, Chrissobolis S, Drummond GR, Fuller PJ, Young MJ. Cardiac Tissue Injury and Remodeling Is Dependent Upon MR Regulation of Activation Pathways in Cardiac Tissue Macrophages. Endocrinology. 2016;157:3213–3223. doi: 10.1210/en.2016-1040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.