Abstract
CONTEXT
Persons with Alzheimer’s disease and other dementias experience behavioral symptoms that frequently result in nursing home (NH) placement. Managing behavioral symptoms in the NH increases staff time required to complete care, and adds to staff stress and turnover, with estimated cost increases of 30%. The Changing Talk to Reduce Resistivenes to Dementia Care (CHAT) study found that an intervention that improved staff communication by reducing elderspeak led to reduced behavioral symptoms of dementia or resistiveness to care (RTC).
OBJECTIVE
This analysis evaluates the cost-effectiveness of the CHAT intervention to reduce elderspeak communication by staff and RTC behaviors of NH residents with dementia.
DESIGN
Costs to provide the intervention were determined in eleven NHs that participated in the CHAT study during 2011–2013 using process-based costing. Each NH provided data on staff wages for the quarter before and for two quarters after the CHAT intervention. An incremental cost-effectiveness analysis was completed.
ANALYSIS
An average cost per participant was calculated based on the number and type of staff attending the CHAT training, plus materials and interventionist time. Regression estimates from the parent study then were applied to determine costs per unit reduction in staff elderspeak communication and resident RTC.
RESULTS
A one percentage point reduction in elderspeak costs $6.75 per staff member with average baseline elderspeak usage. Assuming that each staff cares for 2 residents with RTC, a one percentage point reduction in RTC costs $4.31 per resident using average baseline RTC.
CONCLUSIONS
Costs to reduce elderspeak and RTC depend on baseline levels of elderspeak and RTC, as well as the number of staff participating in CHAT training and numbers of residents with dementia-related behaviors. Overall, the 3-session CHAT training program is a cost-effective intervention for reducing RTC behaviors in dementia care.
Keywords: Intervention cost-effectiveness, Elderspeak, Resistiveness to care Behavioral symptoms, Cost-effectiveness, Nursing home care, Communication
INTRODUCTION
Extended lifespans have resulted in an aging population. An estimated one-third of persons living to 85 and beyond have Alzheimer’s disease or other dementias, and the current population of 5.3 million persons diagnosed with dementia in the United States is projected to reach 13.8 million by 2050 [1]. At least 50% of nursing home (NH) residents have dementia; as the number of family caregivers available to provide care for persons with dementia (PWD) at home declines [2], dependence on long term care facilities will increase. Cost-effective interventions that can minimize the costs of NH care and improve quality of care and quality of life for residents are of critical importance [1].
Behavioral symptoms such as aggression, withdrawal, vocal outbursts, and wandering develop in up to 90% of PWD at some point during the disease and precipitate NH placement [3]. Dementia behaviors challenge NH staff, disrupt care, and reflect unmet needs of residents [4, 5]. Decreased care quality and quality of life result from dementia behaviors or resistiveness to care (RTC) and result in increased use of psychotropic medication and restraints [6]. It is estimated that RTC increases the costs of dementia care by 25 to 35% [7]. As average national NH costs exceed $91,000 per resident annually, interventions to control costs are critical [1, 8].
As part of the culture change movement with a goal of making care less institutional and more homelike and person centered, a number of strategies to improve care for PWD are being tested [9]. Person centered care is especially important for PWD who struggle to maintain their sense of self and person-centered care can reduce dementia-related behaviors [10].
Communication is a key factor for maintaining personhood, a sense of self and connection to others. However, most direct care for NH residents is provided by nursing assistants who have minimal training in communication and who focus on tasks. Our research demonstrated a link between commonly-used elderspeak (infantilizing) communication and behavioral symptoms or RTC in NH residents with dementia [11, 12]. Elderspeak, which is similar to baby talk, is characterized by diminutives, “we” pronoun substitutions, and simplified vocabulary and grammar. Elderspeak provides a message of incompetence to older adults and is associated with a rise in RTC in PWD [12]. RTC extends the time needed to complete care and intensifies staff stress, contributing to burnout, job dissatisfaction, and turnover, thus increasing the costs of care. The Changing Talk (CHAT) study demonstrated that a 3-session staff training program reduced elderspeak communication and subsequently decreased behavioral symptoms or RTC in NH residents with dementia [13]. The current study is a cost-effectiveness analysis, conducted to evaluate the feasibility and value of disseminating CHAT across NH settings.
METHODS
This analysis evaluated the costs of providing CHAT to NH staff in relation to reductions in staff elderspeak communication and resident RTC. Our purpose was to obtain accurate cost estimates of the CHAT intervention and to compute a simple cost-effectiveness ratio — the added cost associated with CHAT divided by the reduction in elderspeak or RTC associated with CHAT. We used a traditional Cost-Effectiveness Analysis (CEA) approach to evaluate the costs relative to effectiveness of the CHAT intervention compared to no intervention [14]. The two effectiveness criteria considered in this study were the percentage of time staff used elderspeak communication and the percentage of time residents exhibited RTC. We calculated the incremental cost-effectiveness ratio (ICER) separately for changes in elderspeak and for changes in RTC.
Parent Study
The CHAT parent study that provided the data for this analysis was approved by the University institutional review board for the protection of human subjects. The study was designed as a cluster-randomized waitlist-controlled trial to test the effect of CHAT training on NH staff communication and resident RTC in 13 NHs [13]. The NHs assigned originally as controls crossed over and received the intervention; thus, all the NHs in the study received the intervention at some point in their participation, and we assessed elderspeak and RTC before and after the intervention for each NH. Staff-resident dyads (N = 42) were video recorded during morning care. Computer-assisted behavioral coding was used to evaluate post-CHAT intervention changes in the proportion of time staff used elderspeak in communication with residents and proportion of time residents showed RTC.
Resident participants in the parent study (N=27) were primarily older women (mean age = 88 years, range 72–104) of non-Hispanic white race and ethnicity and suffering from moderate to severe dementia. Staff in the parent study (N=29) also were primarily Caucasian women who ranged in age from 21 to 67 years and had varied experience in health care and as employees at their current NH.
For each dyad, changes in staff elderspeak and resident RTC were calculated for data collected before and after the intervention. The changes occurring before the intervention were calculated using data collected at time points before the intervention and the dyad’s first assessment. Post-intervention changes were calculated using data collected after the intervention and the mean of all pre-intervention assessments available for the dyad. Employing a linear mixed models approach, models were fit to the changes in elderspeak and RTC. The analyses were adjusted for important covariates.
The parent study’s results were consistent with earlier findings of reductions in staff elderspeak communication and resident time exhibiting RTC related to CHAT intervention [13]. Explanatory variables in the final model for change in elderspeak were the intervention (b = −12.20, p = .028) and baseline elderspeak (b = −0.65, p < .001). Explanatory variables in the final model for change in RTC were change in elderspeak (b = 0.43, p < .001) and baseline RTC (b = −0.58, p < .001). In addition, two covariates were included in the model for RTC: communication disability (b = 6.05, p = .03) and co-morbidity (b = 1.80, p = .002).
Determining Costs of the Intervention
The current analysis included 11 NHs that provided complete data for cost-effectiveness analysis to evaluate each facility’s costs for the CHAT intervention in relation to changes in elderspeak and RTC following the CHAT intervention. Process-based costing methods were used to determine the cost of the intervention. Data on costs was prospectively collected using established micro-costing methods [15]. Costs for providing the intervention (including set up and administration of the individual sessions) were computed and tracked across each NH. This included costs for interventionist teaching time, staff time, materials, and supplies. Costs for staff time to attend CHAT sessions were based on mean hourly wages.
A process-based costing flow diagram was developed to document the system being studied and provided a framework for cost data collection (See Figure 1). Costs for providing the CHAT intervention were collected for each facility and averaged overall for training and time. The costs included: facilitator time for preparation and presentation, staff time based on the time they attended and average wages for each job position (i.e. RN, LPN, CNA), time and materials for making handouts for the course, and administrative time for CEU preparation and coordination of the sessions.
FIGURE 1.
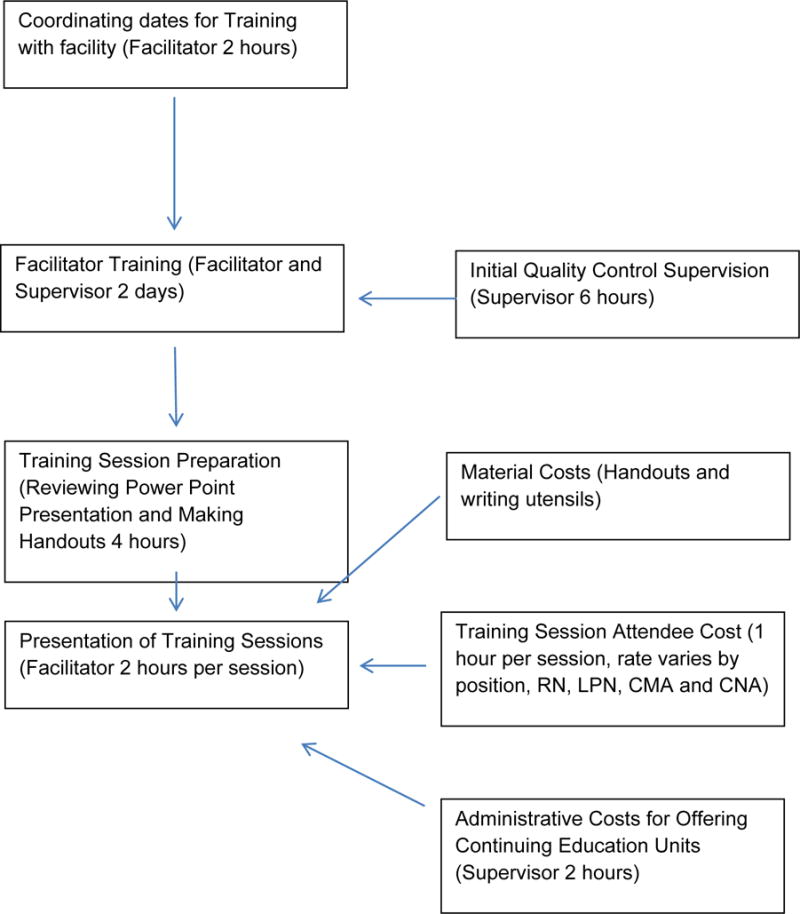
Process Diagram for CHAT Intervention
Data characterizing the participating NHs were extracted from Nursing Home Compare and NH cost reports. Medicaid case mix is a measure of resident acuity or care needs that is used to determine Medicaid reimbursement rates and has been used to control differences in care needs across NHs in prior research [16, 17]. In addition, each NH provided data on the average wages for staff job positions for the quarter when the CHAT training took place.
Calculation of Costs
For each training session, we identified the number of nursing staff (registered nurses [RNs], licensed practical nurses [LPNs], and certified nursing assistants [CNAs]) who attended CHAT training in each NH and their mean hourly wage rates. The total cost of staff participation in each one-hour session was calculated by summing hourly rates for all attendees in the session.
Next, for each NH the average cost per session per staff member was calculated by dividing the total cost of staff participation for each session by the number of attendees. Finally, the average costs per session per staff member were summed across the three training sessions to obtain the average cost per staff member for each facility and then averaged across facilities.
Because the study was conducted over a three-year period to include all the NHs, costs were adjusted for inflation using the Consumer Price Index (CPI) and discounted assuming a 3% discount rate. We also performed sensitivity analysis using a 0% and 5% discount rate. The sensitivity analysis accounts for different rates used to make future costs comparable to the present (i.e. discounting).
Incremental Cost-Effectiveness Ratio (ICER) Analysis
The ICER for elderspeak reduction was calculated by dividing the cost per staff member by the estimated change in staff elderspeak use due to CHAT that was obtained using model estimates for elderspeak. Although the CHAT intervention is targeted towards NH staff, we also were interested in evaluating the costs for a given reduction in resident RTC. To do this, we calculated costs for two scenarios: the first assuming a 2:1 resident to staff ratio and the second assuming a 10:1 resident to staff ratio. Thus, per resident costs were calculated by dividing cost for each staff by 2 for the first scenario and by 10 for the second scenario. We then calculated the ICER for resident RTC by dividing per resident cost by the estimated change in resident RTC due to CHAT that was obtained using model estimates for RTC. Because the cost-effectiveness of the CHAT intervention is likely to vary depending on baseline elderspeak and RTC, we also estimated the cost per unit reduction in RTC for several scenarios.
RESULTS
Table 1 provides descriptive characteristics about the participating NH facilities that were all Medicare Certified Skilled Nursing Facilities ranging in size from 43–163 beds. The NHs were typical for the Midwestern United States. Seven NHs were located in metro areas containing an urban core of 50,000 or more population; and four NHs were located in micro areas containing an urban core of 10,000 (but less than 50,000) population. Quality ratings (Centers for Medicare and Medicaid Services star ratings) ranged from 1 to 5. Average hourly wages ranged from $23.49 to $26.96 for RNs, from $16.81 to $19.41 for LPNs, and from $10.05 to $12.14 for CNAs. Average attendance at the CHAT training sessions ranged from 11 to 40 staff per session.
TABLE 1.
Participating Nursing Home Characteristics
| Facility | Resident beds | Special care units | Medicaid Case Mix (MCCM)* | Locale | CMS star rating | Average attendance at CHAT sessions | Average RN hourly wage | Average LPN hourly wage | Average CNA hourly wage |
|---|---|---|---|---|---|---|---|---|---|
| Nursing Home A | 60 | 1 | 0.92 | metro | 4 | 11 | $24.22 | $18.40 | $12.14 |
| Nursing Home B | 60 | 0 | 1.02 | micro | 4 | 32.3 | $24.13 | $18.89 | $11.60 |
| Nursing Home C | 163 | 6 | 1.07 | metro | 3 | 12 | $25.37 | $19.41 | $12.00 |
| Nursing Home D | 156 | 2 | 0.96 | metro | 3.7 | 39.7 | $26.51 | $19.27 | $11.86 |
| Nursing Home E | 90 | 0 | 1.21 | metro | 5 | 17.7 | $24.26 | $19.41 | $11.48 |
| Nursing Home F | 60 | 1 | 1.03 | micro | 5 | 27 | $24.00 | $17.20 | $11.02 |
| Nursing Home G | 43 | 0 | 1.05 | micro | 5 | 14 | $23.49 | $17.85 | $10.75 |
| Nursing Home H | 60 | 0 | 1 | micro | 5 | 9.7 | $24.81 | $17.65 | $10.05 |
| Nursing Home I | 90 | 1 | 1.03 | metro | 2.7 | 20.3 | $24.26 | $19.17 | $11.48 |
| Nursing Home J | 70 | 0 | 1.03 | metro | 1 | 30 | $26.86 | $19.41 | $11.43 |
| Nursing Home K | 60 | 1 | .99 | metro | 4 | 30 | $23.70 | $16.81 | $12.11 |
Note. MCCM= Medicaid Case Mix. Metro area contains a core urban area of 50,000 or more population; micro area contains an urban core of at least 10,000 (but less than 50,000) population. Each metro or micro area consists of one or more counties and includes the counties containing the core urban area, as well as any adjacent counties that have a high degree of social and economic integration (as measured by commuting to work) with the urban core.
Effectiveness in Relation to Outcomes
Using model results from the parent study with the average pre-intervention staff elderspeak (34.6% of the time) and resident RTC (35.7% of the time), we estimated that CHAT reduced the time staff engaged in elderspeak by 11.80 percentage points and the time residents exhibited RTC by 9.24 percentage points.
Calculation of Costs
Assuming a discount rate of 3%, the average cost of participation time in all three intervention sessions was $41.76 per staff member in 2011 U.S. dollars. The average materials cost (handouts) for the 3-session intervention was calculated in a similar fashion and amounted to $37.93 per staff member. Thus, the total cost of the intervention per staff member was $79.69, calculated as the sum of the participation time and material costs ($41.76 + $37.93).
Incremental Cost-effectiveness Ratio for Elderspeak
Panel A of Table 2 presents the cost per staff member and the ICER for reducing elderspeak calculated as described above. Assuming a discount rate of 3% and an average baseline elderspeak use of 34.6%, the ICER for elderspeak was $6.75 (= $79.69/11.80) in 2011 U.S. dollars. In other words, a one percentage point reduction in elderspeak cost $6.75 per staff member.
TABLE 2.
Cost-Effectiveness Ratios and Sensitivity Analysis
| Main estimates | Sensitivity analysis | ||
|---|---|---|---|
| 3% discount rate | 0% discount rate | 5% discount rate | |
| Panel A: Elderspeak | |||
|
| |||
| Cost per staff member | $79.69 | $82.47 | $77.95 |
| Change in elderspeak | −11.8 | −11.8 | −11.8 |
| Per staff ICER* | 6.75 | 6.98 | 6.61 |
|
| |||
| Panel B: Resistiveness to Care | |||
|
| |||
| Cost per resident, 2:1 patient-staff ratio | $39.84 | $41.24 | $38.97 |
| Cost per resident, 10:1 patient-staff ratio | $7.97 | $8.25 | $7.79 |
| Change in RTC | −9.24 | −9.24 | −9.24 |
| Per resident ICER* (2:1 patient-staff ratio) | 4.31 | 4.46 | 4.22 |
| Per resident ICER* (10:1 patient-staff ratio) | 0.86 | 0.89 | 0.84 |
Note. ICER = Incremental Cost-effectiveness Ratio. Results projected for staff with mean baseline elderspeak (34.6%) and resident with mean baseline RTC (35.7%).
Incremental Cost-effectiveness Ratio for Resistiveness to Care
Panel B of Table 2 presents the cost per resident and the corresponding ICERs for the two scenarios described above. Assuming that each staff member cares for, on average, two residents with an average RTC of 35.7%, the cost of the intervention per resident was calculated as $39.84 (=$79.69/2). The ICER per resident was then estimated as $4.31 (= $39.84/9.24). That is, a one percentage point reduction in RTC costs $4.31 per resident.
Assuming that each staff member cares for, on average, 10 residents with an average RTC of 35.7%, the cost of the intervention per resident was calculated as $7.97 (= $79.69/10). The ICER per resident was then estimated as $0.86 (= $7.97/9.24). That is, a one percentage point reduction in RTC costs $0.86 per resident. Table 2 also presents the results of the sensitivity analysis assuming a 0% discount rate and a 5% discount rate. Overall, the results are robust to these alternative discount rates.
Incremental Cost-effectiveness Ratio for Alternative Scenarios
Table 3 presents predicted results for varying levels of elderspeak and RTC prior to the intervention (25th percentile, median, and 75th percentile). Column 2 displays the predicted change in elderspeak due to the CHAT intervention by the level of baseline elderspeak (column 1), and column 4 displays the predicted change in RTC due to the CHAT intervention by the levels of baseline elderspeak and RTC. Columns 5 through 7 present the corresponding ICERs for three alternative staff-resident ratios. For example, at a baseline elderspeak of 20.1 (25th percentile) and a baseline RTC of 33.1 (median RTC) residents would experience a 3.9 percentage point reduction in RTC due to the CHAT intervention. Assuming a 2:1 staff-resident ratio at this NH, a one percentage point reduction in RTC would cost $10.30 per resident. In contrast, for a NH with a 2:1 staff-resident ratio and median baseline RTC but a higher baseline elderspeak of 51.4 (76th percentile), a one percentage point reduction in RTC only costs $3.14 per resident. We do not present the cost-effectiveness ratios for low baseline levels of both elderspeak and RTC since the intervention is not successful in reducing RTC in those situations.
TABLE 3.
Simulation of Cost-Effectiveness Ratios for Alternative Scenarios
| (1) | (2) | (3) | (4) | (5) | (6) | (7) |
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline elderspeak | Predicted change in elderspeak | Baseline RTC | Predicted change in RTC | ICER by patient-staff ratio | ||
| 2:1 | 5:1 | 10:1 | ||||
| 20.1 | −2.3 | 19.0 | 4.4 | N/A* | N/A* | N/A* |
| 33.1 | −3.9 | 10.30 | 4.12 | 2.06 | ||
| 47.8 | −12.5 | 3.20 | 1.28 | 0.64 | ||
|
| ||||||
| 30.0 | −8.8 | 19.0 | 1.6 | N/A* | N/A* | N/A* |
| 33.1 | −6.7 | 5.97 | 2.39 | 1.19 | ||
| 47.8 | −15.3 | 2.61 | 1.04 | 0.52 | ||
|
| ||||||
| 51.4 | −22.7 | 19.0 | −4.5 | 8.94 | 3.58 | 1.79 |
| 33.1 | −12.7 | 3.14 | 1.25 | 0.63 | ||
| 47.8 | −21.3 | 1.87 | 0.75 | 0.37 | ||
Note. Results projected for NHs with varying levels of baseline ES and RTC (25th percentile, median, and 75th percentile).
N/A = Not Applicable; ES= elderspeak; RTC = Resistiveness to Care
DISCUSSION
Overall, the CEA revealed relatively low costs for unit reductions in staff elderspeak communication and resident RTC behaviors. Thus, CHAT is a cost-effective, nonpharmacological approach to reduce RTC and its costs and may help to reduce the use of psychotropic medications used to control behavioral symptoms of dementia in NHs. These conclusions are limited because there are no established benchmarks for comparison of cost-effectiveness for improvements in elderspeak or RTC in NH settings. These findings should also be interpreted with caution due to limitations of this analysis, including the small sample of facilities located within one geographic area of the United States, the relatively small numbers of residents and staff in each NH who were included in the parent study evaluating staff communication and RTC outcomes, and existing differences in staff participation rates among facilities. Another limitation is that not all residents with RTC in the participating NHs consented to be in the study. Although beyond the scope of this study, probabilistic sensitivity analysis using nonparametric bootstrap methods that provide confidence intervals to confirm the certainty of the ICERs, would strengthen future cost-effectiveness analyses.
The cost-effectiveness of the intervention depended on a number of factors that varied in our sample. The number of staff who attended the intervention sessions varied by NH, and, obviously, for more attendees, the fixed cost per participant (i.e., paying the interventionist) was spread across participants. Attendance rates by staff at the CHAT session varied greatly (see Table 1). Two considerations for maximizing participation are: administrative support for staff to attend training and making it mandatory. In some NHs, staff from housekeeping and other non-nursing areas attended, which may add to the effect of the intervention by improving overall communication experiences for residents. These staff were not included in our cost computations. Considering the high turnover in many NHs, striving to reduce turnover rates could help to prolong the impact of the training.
The relative effect of the CHAT intervention on reducing elderspeak and RTC, and, subsequently, relevant NH expenses, depended on the baseline levels of elderspeak and RTC. Therefore the cost-effectiveness of the intervention can vary. Table 3 provides estimates of effects on elderspeak and RTC for different scenarios (by varying levels of baseline staff elderspeak use and resident RTC. As illustrated in the table, staff with higher baseline elderspeak (51.4% of the time) are predicted to have relatively greater reductions in elderspeak after training (22.7 percentage points), compared to staff with low initial elderspeak (20.1%) who are predicted an average decrease of 2.3 percentage points. Consequently, the intervention effects are accentuated if staff elderspeak use is high.
In addition, baseline levels of resident RTC made a difference in cost estimates. We worked with NHs with varied levels of resident RTC ranging from RTC occurring 0% to 92% of the time. As illustrated in Table 3, in staff-resident dyads with relatively low baseline elderspeak (20.1 %) and low RTC (19%), the predicted change in RTC was an increase of 4.4 percentage points (although this change is of limited clinical significance). Given the same amount of elderspeak, if baseline RTC is higher (47.8%), then our models predict a 12.5 percentage point decrease in RTC. The most benefit from the CHAT intervention is expected in NHs with high elderspeak use (51.4%) and high resident RTC (47.8%); reductions in RTC after training are anticipated to be 21.3 percentage points. Thus, the CHAT training may be the most beneficial in improving care in a cost-effective manner in special care units for residents with dementia
Staff-resident ratios are also important for the cost-effectiveness of the intervention. Since CHAT is targeted towards staff members who treat multiple residents with RTC, NHs with a higher resident-to-staff ratio will incur lower costs for a given reduction in RTC. Thus, we find the lowest cost-effectiveness ratios for scenarios with high baseline elderspeak and RTC and in NHs with a high resident-to-staff ratio. However, high resident-to-staff ratios may potentially influence the effects of the CHAT intervention on reducing staff elderspeak and resident RTC. Due to a small number of NHs in this study, this potential factor was not investigated. For the NHs in this study, staff-to-resident ratios ranged from 3.53 to 5.64 (M = 4.66).
We believe that some or all of the costs of the CHAT intervention will be offset by resulting savings in other areas that our study did not address. These include greater efficiency in completing routine care and reductions in staff turnover (due to increased job satisfaction). In addition, potential savings from reduced use of psychotropic medications were not measured in this study. Although we collected data from participating NHs regarding turnover rates for quarters before and after the intervention, the data were quite variable, had other potential explanations, and did not seem to relate to the CHAT intervention.
We also anticipate that potential cost savings from reductions in elderspeak and RTC represent only part of the benefits of CHAT. Reductions in elderspeak and RTC may improve the overall quality of care and quality of life for residents. However, evaluating other cost-related outcomes of interest, such as reduced time to complete care, may be difficult. For example, if CHAT results in less resident RTC behavior, staff may respond by offering more person-centered care, so that time savings may not be apparent.
Evaluating the cost-effectiveness of interventions is important for understanding intervention value in clinical settings. This study provides a first step in using ICER to evaluate the value of a nonpharmacological intervention in NH care. We conclude that CHAT costs per unit change in elderspeak and RTC are minimal. Findings from this analysis may be used as benchmarks for comparison of cost-effectiveness of other nonpharmacological interventions to overcome behavioral symptoms of dementia in NH care.
Acknowledgments
FUNDING
The research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR011455. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. ClinicalTrials.gov Identifier: NCT01324219. The full protocol is available from the author.
Footnotes
Disclosure: The authors have reported no conflicts of interest.
ClinicalTrials.gov Identifier NCT01324219
References
- 1.Alzheimer’s Association. 2015 Alzheimer’s Disease Facts and Figures. Alzheimer’s and Dementia. 2015;11(3):3–32+. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Redfoot D, Feinberg L, Houser A. Insight on the Issues. Vol. 85 AARP Public Policy Institute; Washington D.C.: Aug, 2013. The Aging of the Baby Boom and the Growing Care Gap: A Look at Future Declines in the Availability of Family Caregivers. 2013. [Google Scholar]
- 3.Kunik ME, et al. Consequences of agressive behavior in patients with dementia. Journal of Neuropsychiatry & Clinical Neuroscience. 2010;22:40–47. doi: 10.1176/jnp.2010.22.1.40. [DOI] [PubMed] [Google Scholar]
- 4.Souder E, O’Sullivan P. Disruptive behavors of older adults in an intituional setting: Staff time required to manage disruptions. Journal of Gerontological Nursing. 2003;29(8):31–36. doi: 10.3928/0098-9134-20030801-08. [DOI] [PubMed] [Google Scholar]
- 5.Zeller A, Hahn S, Needham I, Kok G, Dassen T, Halfens RJG. Aggressive Behavior of Nursing Home Residents Toward Caregivers: A Systematic Literature Review. Geriatric Nursing. 2009;30(3):174–187. doi: 10.1016/j.gerinurse.2008.09.002. 5// [DOI] [PubMed] [Google Scholar]
- 6.Gerdner L, Buckwalter K. Assessment and management of agitation in Alzheimer’s patients: A nursing challenge. Journal of Gerontological Nursing. 1994;20:11–20. doi: 10.3928/0098-9134-19940401-05. [DOI] [PubMed] [Google Scholar]
- 7.Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and pshychological symptoms of dementia (BPSD) in community dwelling AD patients. International Journal of Geriatric Psychiatry. 2002;17:403–408. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- 8.Genworth Financial. Genworth 2015 Cost of Care Survey. 2015 Available: https://www.genworth.com/dam/Americas/US/PDFs/Consumer/corporate/130568_040115_gnw.pdf, Accessed on: 5-29-15.
- 9.Buron B. Levels of Personhood: A Model for Dementia Care. Geriatric nursing (New York, NY) 2008;29(5):324–332. doi: 10.1016/j.gerinurse.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Kitwood T, Bredin K. Towards a theory of dementia care: Personhood and wellbeing. Ageing and Society. 1992;12:269–287. doi: 10.1017/s0144686x0000502x. [DOI] [PubMed] [Google Scholar]
- 11.Williams K. Improving outcomes of nursing home interactions. Research in Nursing and Health. 2006;29:121–133. doi: 10.1002/nur.20117. [DOI] [PubMed] [Google Scholar]
- 12.Williams K, Herman R, Gajewski B, Wilson K. Elderspeak communication: Impact on dementia care. American Journal of Alzheimer’s Disease and Other Dementias. 2009;24:11–20. doi: 10.1177/1533317508318472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams K, Perkhounkova Y, Herman R, Bossen A. A Communication Intervention to Reduce Resistiveness in Dementia Care: A Cluster Randomized Controlled Trial. The Gerontologist. 2016;00(00):1–12. doi: 10.1093/geront/gnw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond MF, Schulpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4th. Oxford, UK: Oxford University Press; 2015. [Google Scholar]
- 15.Lee RH, Bott MJ, Forbes S, Redford L, Swagerty DL, Taunton RL. Process-based cosing. Journal of Nursing Care Quality. 2003;18(4):259–266. doi: 10.1097/00001786-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Harrington C, Swan J. Nursing home staffing, turnover, and case mix. Medical Care Research and Review. 2003;60(3):366–392. doi: 10.1177/1077558703254692. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman S, et al. Attitudes, stress, and satisfaction of staff who care for residents with dementia. The Gerontologist. 2005;45(Special Issue 1):96–105. doi: 10.1093/geront/45.suppl_1.96. [DOI] [PubMed] [Google Scholar]


