Abstract
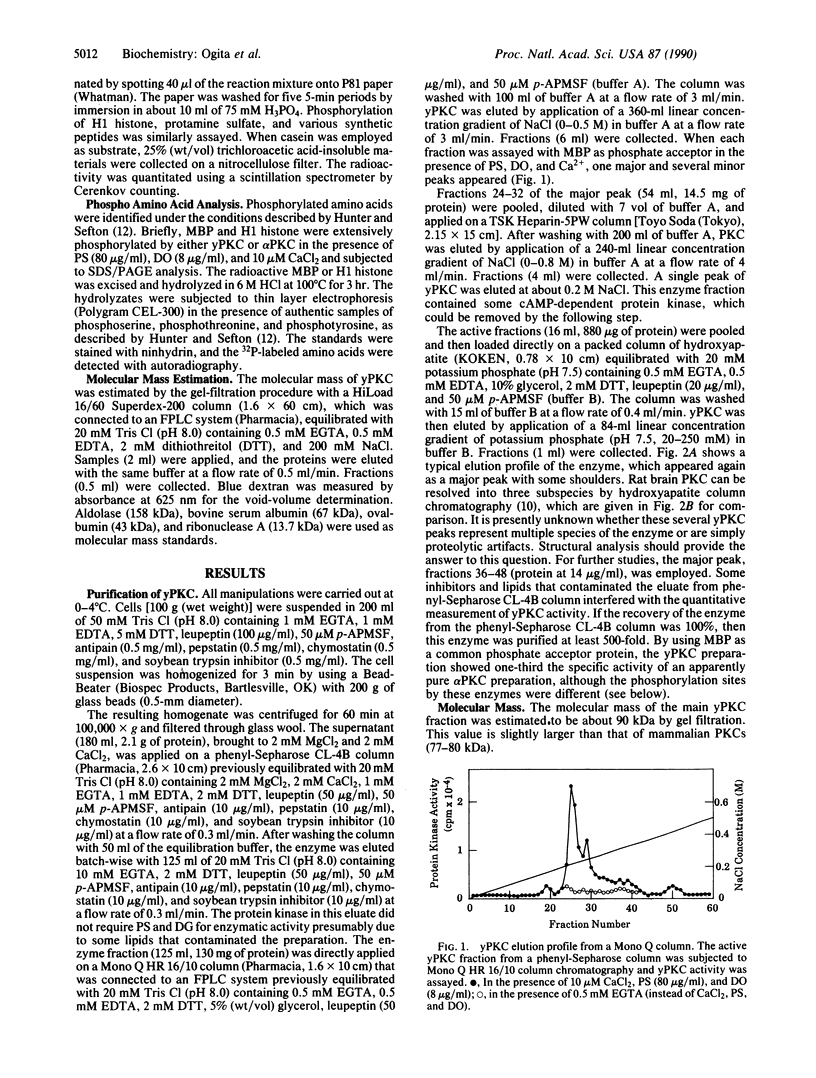
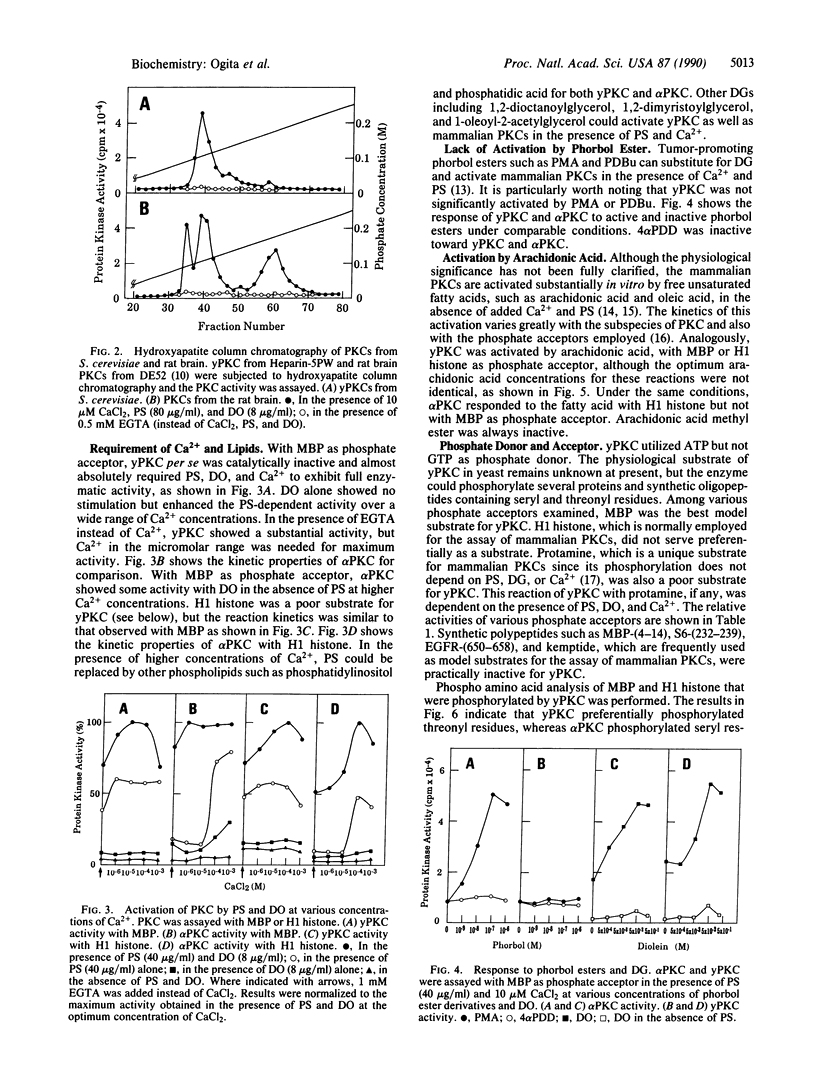
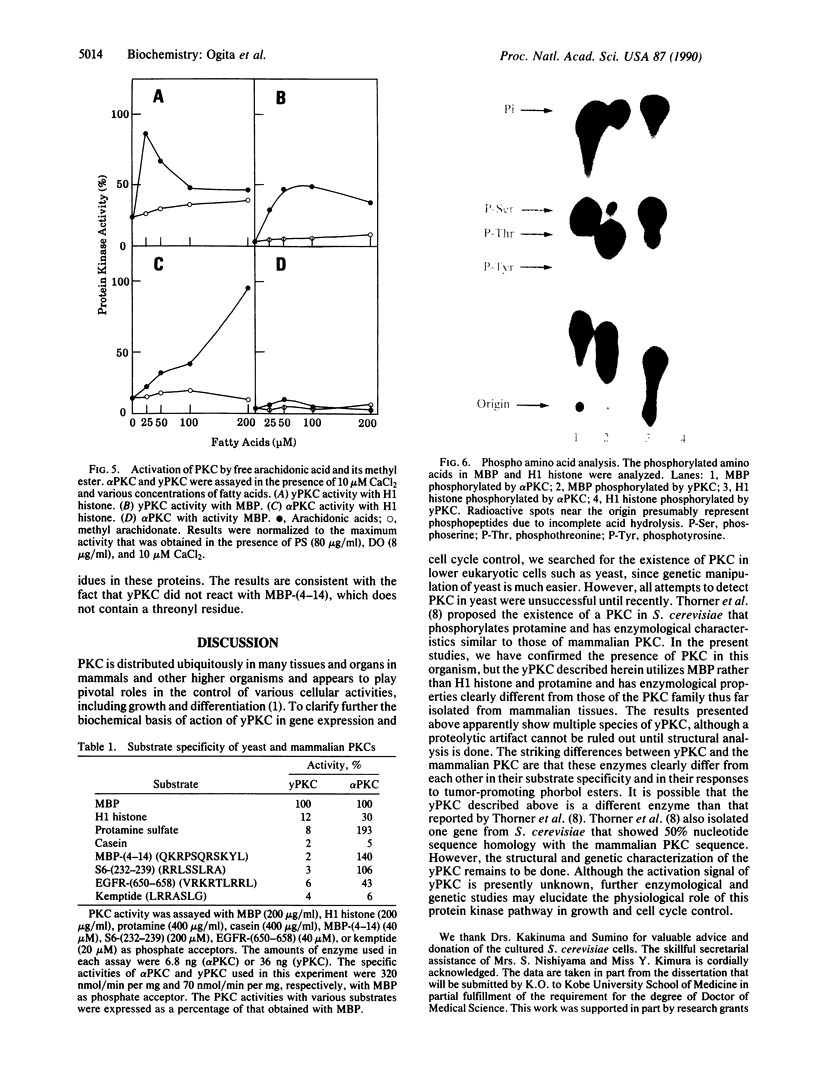
Protein kinase C (PKC) was detected in the yeast Saccharomyces cerevisiae with bovine myelin basic protein as the phosphate acceptor. The enzyme was purified at least 500-fold by a four-step column chromatographic procedure (phenyl-Sepharose CL-4B, Mono Q, Heparin-5PW, and hydroxyapatite). The molecular mass was approximately 90 kDa, as estimated by gel-filtration analysis. Yeast PKC was activated by the simultaneous addition of Ca2+, diacylglycerol, and phosphatidylserine. Free arachidonic acid alone could activate the enzyme to some extent. However, yeast PKC did not respond significantly to tumor-promoting phorbol esters. GTP did not serve as phosphate donor. The yeast enzyme showed substrate specificity distinctly different from that of mammalian PKCs. H1 histone and protamine were poor substrates. With myelin basic protein as a model substrate, yeast PKC phosphorylated threonyl residues preferentially, whereas rat brain PKCs phosphorylated seryl residues preferentially. Further studies should elucidate the role of yeast PKC in cellular regulation and cell cycle control.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chen K. H., Peng Z. G., Lavu S., Kung H. F. Molecular cloning and sequence analysis of two distinct types of Xenopus laevis protein kinase C. Second Messengers Phosphoproteins. 1988;12(5-6):251–260. [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez B., Pestaña A., Fernandez-Renart M. A phospholipid-stimulated protein kinase from Dictyostelium discoideum. Biochem J. 1989 Jun 1;260(2):557–561. doi: 10.1042/bj2600557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Ono Y., Ogita K., Fujii T., Asaoka Y., Sekiguchi K., Kosaka Y., Igarashi K., Nishizuka Y. Identification of the structures of multiple subspecies of protein kinase C expressed in rat brain. FEBS Lett. 1987 Jun 15;217(2):227–231. doi: 10.1016/0014-5793(87)80668-3. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Murakami K., Routtenberg A. Direct activation of purified protein kinase C by unsaturated fatty acids (oleate and arachidonate) in the absence of phospholipids and Ca2+. FEBS Lett. 1985 Nov 18;192(2):189–193. doi: 10.1016/0014-5793(85)80105-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Oliver D., Sommer K. R., Panyim S., Spiker S., Chalkley R. A modified procedure for fractionating histones. Biochem J. 1972 Sep;129(2):349–353. doi: 10.1042/bj1290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Rhee L., Yadegari R., Paro R., Ullrich A., Goeddel D. V. Structure and nucleotide sequence of a Drosophila melanogaster protein kinase C gene. EMBO J. 1987 Feb;6(2):433–441. doi: 10.1002/j.1460-2075.1987.tb04773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer E., Smith D., Mardon G., Quinn W., Zuker C. Isolation and characterization of two new drosophila protein kinase C genes, including one specifically expressed in photoreceptor cells. Cell. 1989 May 5;57(3):403–412. doi: 10.1016/0092-8674(89)90915-x. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Tsukuda M., Ogita K., Kikkawa U., Nishizuka Y. Three distinct forms of rat brain protein kinase C: differential response to unsaturated fatty acids. Biochem Biophys Res Commun. 1987 Jun 15;145(2):797–802. doi: 10.1016/0006-291x(87)91035-7. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Ricke L. A. Protein kinase C from sea urchin eggs. Comp Biochem Physiol B. 1989;92(2):251–254. doi: 10.1016/0305-0491(89)90274-5. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Yasuda I., Kishimoto A., Tanaka S., Tominaga M., Sakurai A., Nishizuka Y. A synthetic peptide substrate for selective assay of protein kinase C. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1220–1227. doi: 10.1016/0006-291x(90)90996-z. [DOI] [PubMed] [Google Scholar]




