Abstract
We evaluated the ability of emmetropic and myopic observers to detect and discriminate blur across the retina under monocular or binocular viewing conditions. We recruited 39 young (23–30 years) healthy adults (n = 19 myopes) with best-corrected visual acuity 0.0 LogMAR (20/20) or better in each eye and no binocular or accommodative dysfunction. Monocular and binocular blur discrimination thresholds were measured as a function of pedestal blur using naturalistic stimuli with an adaptive 4AFC procedure. Stimuli were presented in a 46° diameter window at 40 cm. Gaussian blur pedestals were confined to an annulus at either 0°, 4°, 8°, or 12° eccentricity, with a blur increment applied to only one quadrant of the image. The adaptive procedure efficiently estimated a dipper shaped blur discrimination threshold function with two parameters: intrinsic blur and blur sensitivity. The amount of intrinsic blur increased for retinal eccentricities beyond 4° (p < 0.001) and was lower in binocular than monocular conditions (p < 0.001), but was similar across refractive groups (p = 0.47). Blur sensitivity decreased with retinal eccentricity (p < 0.001) and was highest for binocular viewing, but only for central vision (p < 0.05). Myopes showed worse blur sensitivity than emmetropes monocularly (p < 0.05) but not binocularly (p = 0.66). As expected, blur perception worsens in the visual periphery and binocular summation is most evident in central vision. Furthermore, myopes exhibit a monocular impairment in blur sensitivity that improves under binocular conditions. Implications for the development of myopia are discussed.
Keywords: blur perception, peripheral visual field, myopia, binocular vision
Introduction
Blur perception is an elemental feature of the human visual system. A blurred retinal image serves to drive the accommodation and vergence responses, allowing one to see clearly and resolve fine target details (Ciuffreda, 1991, 1998; Ciuffreda, Wang, & Vasudevan, 2007; Fisher & Ciuffreda, 1988; Horwood & Riddell, 2009; Lin et al., 2013; López-Gil et al., 2013; Schor, 1999; Yamaguchi et al., 2013). Retinal blur also may provide information about the range of depths in one's environment (Maiello, Chessa, Solari, & Bex, 2014; Mather, 1997; Mather & Smith, 2002; Vishwanath, 2012; Vishwanath & Blaser, 2010; Watt, Akeley, Ernst, & Banks, 2005) (but see Langer & Siciliano, 2015; Maiello, Chessa, Solari, & Bex, 2015). As such, blur perception is crucial to one's ability to navigate the world easily, and to accurately perform daily tasks such as reading and driving (Poulere, Moschandreas, Kontadakis, Pallikaris, & Plainis, 2013; Wood et al., 2014). Furthermore, it has been suggested that continuous retinal defocus may be a causative factor in refractive error development, although the evidence is conflicting (Cufflin, Mankowska, & Mallen, 2007; Flitcroft, 1998; Hess, Schmid, Dumoulin, Field, & Brinkworth, 2006; Norton, Siegwart, & Amedo, 2006; Rosenfield & Abraham-Cohen, 1999; Schmid, Iskander, Li, Edwards, & Lew, 2002; Smith III & Li-Fang, 1999; Smith III, Li-Fang, & Harwerth, 1994; Strang, Day, Gray, & Seidel, 2011; Taylor, Charman, O'Donnell, & Radhakrishnan, 2009; Vera-Diaz, Maiello, Kerber, Thorn, & Bex, 2015; Wallman & Winawer, 2004; Wildsoet & Wallman, 1995).
There are two major components in the perception of blur: blur detection and blur discrimination (Ciuffreda et al., 2007; Wang, Ciuffreda, & Irish, 2006). Blur detection refers to the amount of defocus necessary for an observer to first perceive or notice the presence of blur. Blur discrimination refers to the amount of defocus necessary for an observer to perceive an already blurry target as just noticeably blurrier. Both aspects of blur contribute to our perception of image quality (Wang & Ciuffreda, 2006). Overall, the perception of blur is a complex process that depends on the eye's optical quality (i.e., aberrations) as well as both retinal and higher level neurophysiology (Ciuffreda et al., 2007; Mather & Smith, 2002; Wang & Ciuffreda, 2004, 2005a, 2005b). Motion, visual attention, sharpness overconstancy, and target attributes such as luminance, contrast, texture, and size also contribute to the perception of image blur (Christman, 1990; Galvin, O'Shea, Squire, & Hailstone, 1999; Pääkkönen & Morgan, 1994; Wang & Ciuffreda, 2004, 2005b).
The perception of blur within central vision has been studied extensively (Campbell, 1957; Jacobs, Smith, & Chan, 1989; Oshima, 1958; Rosenfield & Abraham-Cohen, 1999; Walsh & Charman, 1988), but there is relatively limited information about how we perceive blur throughout the peripheral field of vision (Ronchi & Molesini, 1975; Wang & Ciuffreda, 2004, 2005a; Wang et al., 2006), even though peripheral blur strongly impacts peripheral visual function (Maiello, Harrison, Vera-Diaz, & Bex, 2015; Rosén, Lundström, & Unsbo, 2011). Altogether, the limited studies on peripheral blur perception suggest that blur detection and discrimination thresholds increase progressively with retinal eccentricity, and that blur detection may be less sensitive than blur discrimination to eccentric viewing. However, most of these studies do not measure blur thresholds directly, rather they infer blur sensitivity by evaluating the subject's depth-of-focus, either with ophthalmic lenses or by manually displacing the test target. Additionally, most of these studies have evaluated perception of blur only monocularly (Wang & Ciuffreda, 2004, 2005a; Wang et al., 2006).
To understand how blur is employed by the visual system it is necessary to further study blur perception throughout the peripheral field. Studying blur perception at different eccentricities may also shed light on emmetropization and its failure, as the interaction between peripheral and central vision may be significant to this process (Huang, Hung, & Smith III, 2012; Smith III, Campbell, & Irving, 2013; Smith III et al., 2007; Vera-Diaz, Kerber, Thorn, & Bex, 2013; Wallman & Winawer, 2004; Yamaguchi et al., 2013). Binocularity (the neuronal integration of information from the two eyes) needs to also be considered when investigating perception of blur. Previous studies have found that binocularity improves visual performance through probability summation under blur conditions (Banton & Levi, 1991; Heravian, Jenkins, & Douthwaite, 1990; Plainis, Petratou, Giannakopoulou, Atchison, & Tsilimbaris, 2011).
Binocularity improves retinal sensitivity to defocus blur, and defocus blur is a useful cue to increase the accuracy of binocularity (Hoffman & Banks, 2010; Mather, 1997), including binocular rivalry (Arnold, Grove, & Wallis, 2007). In addition, proof of interocular transfer of defocus information has been shown in guinea pig (McFadden et al., 2014) and human (Kompaniez, Sawides, Marcos, & Webster, 2013) models of refractive error development. There is also interocular transfer of accommodative responses (Flitcroft, Judge, & Morley, 1992), creating similarity in retinal image focus. Therefore, although emmetropization signals are found locally at the retinal level, binocular vision may play a significant role in retinal image focus and therefore in emmetropization.
Psychophysical studies on the perception of blur have repeatedly shown that blur discrimination follows a typical “dipper” shape (for a comprehensive review see Watson & Ahumada, 2011), meaning that performance in blur discrimination tasks is best with a nonzero amount of reference blur. These data are often described using a variance discrimination model (e.g., Mather & Smith, 2002), since blur perception can be conceptualized as variance discrimination of luminance gradients (Morgan, Chubb, & Solomon, 2008). The variance discrimination model of blur assumes that the visual system is attempting to estimate the local variance of the luminance profile of an image from a set of luminance samples. Each of these samples is, however, perturbed by some level of internal noise or intrinsic blur. This intrinsic blur arises from optical sources (the optical aberrations of the eye) and neural sources (neural noise within the visual pathways). At near-zero levels of reference blur, in order to discriminate blur, the system has to overcome this level of intrinsic blur. As the reference level of blur increases, blur discrimination is initially facilitated owing to the additivity of variance (hence the dipper shape). Finally, for levels of reference blur much greater than intrinsic blur, discrimination performance worsens in accordance with Weber's law. This model well describes the dipper data and is grounded in signal detection theories of sensory discrimination and decision making (Green & Swets, 1966).
How the visual system represents and computes blur is actually a matter of current scientific debate. Watson and Ahumada (2011) provide a thorough overview of the models that have been proposed in the literature to explain the discrimination of blurred edges and provide support for a model in which blur discrimination is accomplished by discriminating local image contrast energy after filtering by the contrast sensitivity function. Georgeson, May, Freeman, and Hesse (2007) propose instead a scale-space model that employs Gaussian derivative filters arranged in a structure that resembles the organization of simple and complex cells in visual cortex.
Here, we study blur perception throughout the visual field using the variance discrimination model of the blur dipper function that is agnostic to how the visual system computes and encodes blur. Using this model we ask how blur perception changes in the periphery and whether these chances are due to an increase in intrinsic noise (that combines all sources of visual blur, including optics and sampling) or because of different neural resources allotted to blur perception throughout the visual field (which would lead to changes in decision making—i.e., the Weber fraction of blur discrimination). We differentiate these accounts by estimating both intrinsic blur and blur sensitivity as a function of eccentricity, refractive status, and monocular or binocular viewing conditions.
The aim of the current study was to assess blur perception in the near peripheral field of vision, both monocularly and binocularly, in emmetropic and myopic subjects. To do so, we used naturalistic stimuli (Bordenave, Gousseau, & Roueff, 2006; Lee, Mumford, & Huang, 2001; Wallis & Bex, 2012) blurred at fixed eccentricities (up to 12°) and measured blur detection and discrimination threshold with an adaptive 4AFC paradigm (Vul, Bergsma, & MacLeod, 2010).
Methods
Subjects
A total of 39 young adult subjects (mean ± SD age: 24.8 ± 3.8 years; n = 19 myopes) were recruited from staff and students of the New England College of Optometry to participate in this study. Following a vision screening that comprised an ocular heath evaluation and an ocular history questionnaire, subjects who met all inclusion criteria were enrolled in the study. Criteria for subjects' inclusion were: (1) within 18 and 32 years of age, (2) best-corrected visual acuity (BCVA) 20/20 or better in each eye, (3) refractive error (spherical equivalent, SE) between +0.75 hyperopia and −14.00DS myopia with ≤1.50DC of astigmatism or ≤1.00D anisometropia, (4) contact lens wearer if myopic refractive correction was needed, (5) no current binocular vision or accommodative dysfunction, (6) not using drugs that may affect their vision, (7) no history of surgery or eye disease that may have resulted in visual consequences, and (8) adequate hearing, language skills, and mental ability to understand the consent process and the instructions given during the experiment.
Subjects' refractive error for each eye was determined by objective refraction with an open-field autorefractor (Grand Seiko WR5100K, Grand Seiko, Hiroshima, Japan) followed by binocular subjective refraction with binocular balancing and evaluated by the observer's best-corrected visual acuity. Axial length measurements were performed with a Haag–Streit Lenstar LS900 optical biometer (Haag-Streit AG, Koeniz, Switzerland; http://www.haag-streit.com/). Subjects were grouped based on their refractive error. Myopia (n = 19) was defined as a SE in each eye between −0.50DS and −11.00DS (mean: −5.88 ± 3.35DS). Emmetropia (n = 20) was defined as SE in each eye between −0.25DS and +0.50DS (mean: +0.15 ± 0.24DS).
This research followed the tenets of the Declaration of Helsinki; informed consent was obtained from all subjects after explanation of the nature and possible consequences of the study, and was approved by the New England College of Optometry's Institutional Review Board.
Stimuli and apparatus
The experiment was programmed with the Psychophysics Toolbox Version 3 (Brainard, 1997; Pelli, 1997) in Matlab. Stimuli were presented on a gamma-corrected ROG SWIFT PG278Q Asus monitor (AsusTek Computer Inc., Taipei, Taiwan) with a resolution of 2560 × 1440 pixels (display dot pitch: 0.233 mm) running at 120 Hz from an Nvidia GeForce GTX 780 graphics processing unit (Nvidia Corporation, Santa Clara, CA).
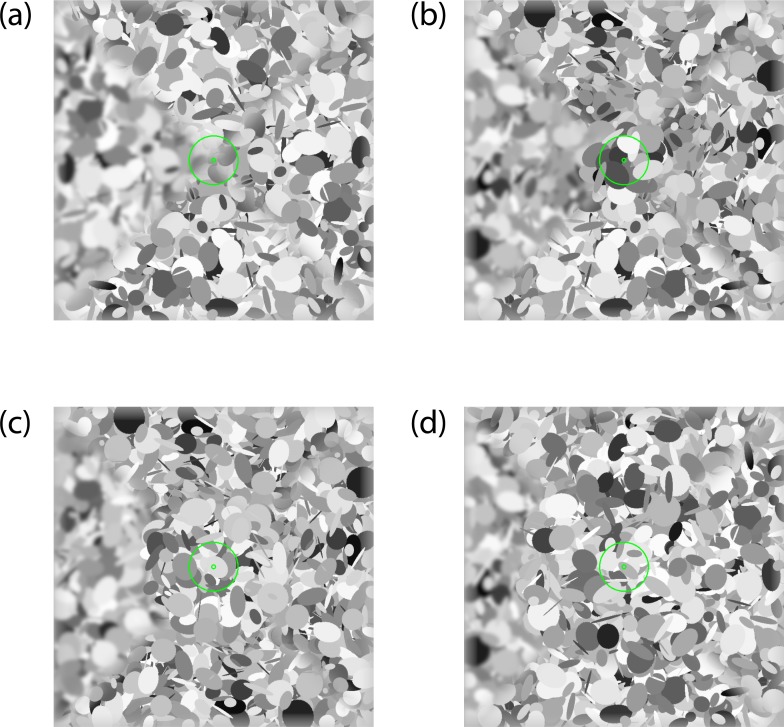
Subjects were seated 40 cm in front of the monitor with their heads stabilized in a chin and forehead rest. The monitor subtended 73 × 46°/visual angle. Stimuli were 46 × 46°/visual angle degree patches of “dead leaves” (Bordenave et al., 2006; Lee et al., 2001); examples can be seen in Figure 1. Stimuli were constructed from a set of 2,000 ellipses each assigned a center position, orientation, aspect ratio, and luminance drawn from pseudo-random uniform distributions. The length of each ellipse semi-axis was randomly selected to be between 0.1 and 10°/visual angle. Each image was divided in four sectors: upper, right, lower, and left. Blur was applied to each sector by Gaussian filtering in the frequency domain by an amount under the control of the adaptive algorithm described as follows. Three sectors were blurred by the same amount of “pedestal” blur, whereas the fourth was blurred by a greater amount of “pedestal + increment” blur. To measure blur discrimination throughout the selected visual field, a circular central portion of the stimulus up to 0°, 4°, 8°, or 12° of eccentricity was sharply rendered, with no blur (Figure 1a–d). Two green concentric circles of 0.3° and 3°, respectively, were presented in the middle of each stimulus image to serve as target for central fixation.
Figure 1.
Example of “dead leaves” stimuli employed in the study for each eccentricity condition (a–d). For each of these four example stimuli, the top, right, and bottom quadrants are blurred with 0.5 arcmin of “pedestal” blur, whereas the left quadrant is blurred by a greater amount of blur (0.5 + 6 arcmin of “pedestal” + “increment” blur). For the 0° condition (a), blur is present throughout the entire image from the center to the periphery. In the peripheral blur testing conditions (b–d), the image within the central 4°, 8°, or 12°, respectively, were sharply rendered, and blur was present only at an eccentricity beyond 4° (b), 8° (c), or 12° (d).
Design
It is known that the ability of human observers to discriminate differences in blur varies lawfully as a function of the amount of reference blur, and that this blur discrimination function is dipper shaped (Hamerly & Dvorak, 1981; Pääkkönen & Morgan, 1994; Watt & Morgan, 1983, 1984; for a review see Watson & Ahumada, 2011). Thus, blur perception is typically investigated by measuring blur discrimination thresholds at multiple levels of pedestal blur, and then fitting the threshold data with a dipper shaped function. Instead of measuring individual thresholds, in this study we employed an adaptive testing procedure (Vul et al., 2010) to estimate blur dipper functions at 0°, 4°, 8°, and 12° in the visual field both monocularly and binocularly.
We adopted a parameterization of the blur dipper function (Mather & Smith, 2002; Murray & Bex, 2010) with equation:
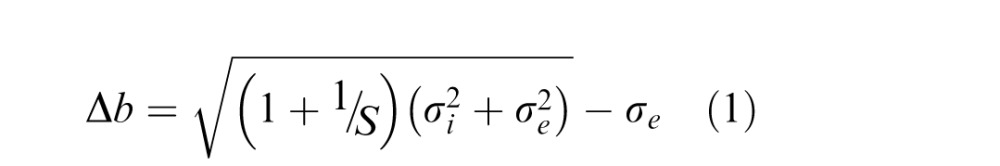 |
where blur increment threshold 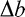 varies as a function of the external (pedestal) blur
varies as a function of the external (pedestal) blur 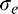 applied to the stimulus and is modulated by two parameters: blur sensitivity
applied to the stimulus and is modulated by two parameters: blur sensitivity 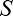 and intrinsic blur
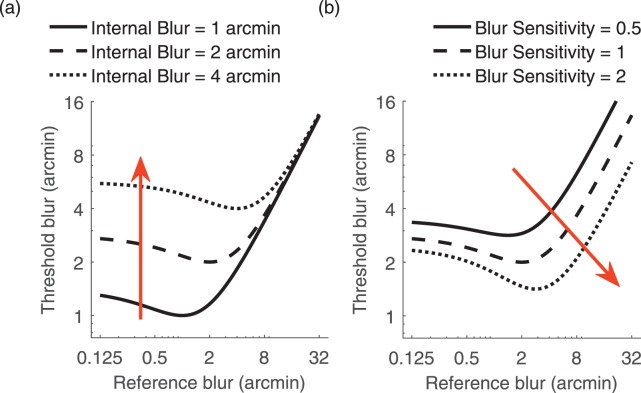
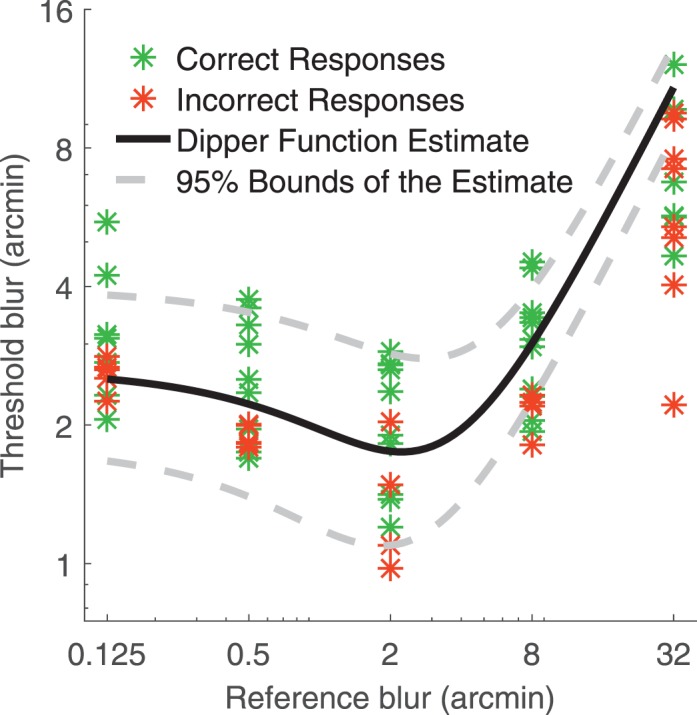
and intrinsic blur 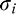 . The model's intrinsic blur parameter takes into account that a human observer's visual system has a baseline level of intrinsic blur arising from all optical and neurological sources. The blur sensitivity parameter takes into account the proportional increase of the blur threshold with increasing external blur: an observer with high sensitivity will have globally lower thresholds and will thus be overall better at discriminating blur. Figure 2 illustrates the effects of changing these two parameters on the shape of the dipper function. Increasing the internal blur parameter whilst holding constant the blur sensitivity parameter (Figure 2a) shifts the left side of the function upward but does not affect the rightward side of the function. Increasing blur sensitivity while holding internal blur constant (Figure 2b) shifts the entire curve downward and to the right.
. The model's intrinsic blur parameter takes into account that a human observer's visual system has a baseline level of intrinsic blur arising from all optical and neurological sources. The blur sensitivity parameter takes into account the proportional increase of the blur threshold with increasing external blur: an observer with high sensitivity will have globally lower thresholds and will thus be overall better at discriminating blur. Figure 2 illustrates the effects of changing these two parameters on the shape of the dipper function. Increasing the internal blur parameter whilst holding constant the blur sensitivity parameter (Figure 2a) shifts the left side of the function upward but does not affect the rightward side of the function. Increasing blur sensitivity while holding internal blur constant (Figure 2b) shifts the entire curve downward and to the right.
Figure 2.
Representation of the effect of modifying the various parameters of the blur dipper model. (a) Effect of holding the blur sensitivity parameter fixed at a value of 1 and increasing the internal blur parameter from 1 to 4 arcmin. (b) Effect of holding the internal blur parameter fixed at 2 arcmin and increasing the blur sensitivity parameter from 0.5 to 2.
We employed the functional adaptive sequential testing (FAST) (Vul et al., 2010) algorithm to obtain dipper function blur estimates. We validated the use of the FAST method by comparing estimates of the dipper function parameters estimated with FAST with estimates of the dipper function based on classical psychophysical methods with five interleaved staircases in the same observers; see Appendix 1 for details. An example of a dipper function estimated for one representative observer in one experimental condition is shown in Figure 3. Specifically, for each dipper function estimate we required the algorithm to select 15 trials at each of five pedestal blur levels [0.125, 0.5, 2, 8, 32] arcmin. At these reference blur levels, each trial the FAST algorithm selected test blur levels that maximized information gain, efficiently constraining the parameter estimates of the underlying dipper function.
Figure 3.
Example of a dipper function estimated for one representative observer in the binocular, 0° eccentricity condition. Asterisks represent individual trials: Green and red are correct and incorrect responses, respectively. Black curve is the estimated dipper function describing how the observer's threshold blur increment varies as a function of reference blur. Note that in this 4AFC task, 25% trials below threshold are guessed correctly (green asterisks). Dotted lines are the 95% bounds of the estimated curve.
Procedure
Subjects were tested both binocularly and monocularly (with their nondominant eye occluded) in two separate sessions. The session order was randomized across participants. Each session, subjects completed 15 trials at each of five pedestal blur levels for each of the four eccentricity conditions. Thus each subject completed 300 trials per sessions. When necessary, subjects were corrected with soft contact lenses.
Each trial, subjects were required to fixate the central fixation target. Stimuli were shown for 250 ms, which is too brief for subjects to initiate any stimulus driven changes in fixation. The 4AFC task was to identify, via button press on the keyboard placed in front of them, which of the four image sections (upper, left, lower or right) was most blurred. Visual feedback was provided by the fixation target, which was green following a correct response, or red following an incorrect response.
Data analyses
On some conditions and for certain subjects, the FAST algorithm failed to converge. Thus, we performed outlier removal by employing the following procedure. For each estimated parameter, we computed the size of the 95% confidence region of the estimate. For intrinsic blur (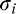 ) and Blur Sensitivity (
) and Blur Sensitivity (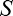 ) parameters separately, we then computed the upper 90th percentile of the size of the confidence regions. Finally, we excluded data from those dipper curves in which at least one of the parameter estimates confidence region was greater than this 90th percentile. This procedure thus excluded parameters estimated with high uncertainty (13% of dipper curves were excluded overall).
) parameters separately, we then computed the upper 90th percentile of the size of the confidence regions. Finally, we excluded data from those dipper curves in which at least one of the parameter estimates confidence region was greater than this 90th percentile. This procedure thus excluded parameters estimated with high uncertainty (13% of dipper curves were excluded overall).
Estimated intrinsic blur (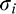 ) and blur sensitivity (
) and blur sensitivity (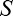 ) parameters were analyzed with a 2 (refractive status, between subjects factor) × 2 (viewing condition, within subjects factor) × 4 (eccentricity, within-subjects factor) mixed-design ANOVA. Blur sensitivity data did not respect the ANOVA assumption on the normality of the residuals. Thus blur sensitivity data was square-root transformed, and ANOVA was rerun on these transformed data that conformed to the ANOVA assumptions on the normality of the residuals. ANOVA results on the original and transformed data did not substantially differ; we report the ANOVA results on the transformed data for statistical rigor. Post-hoc comparisons were conducted via Bonferroni corrected t tests.
) parameters were analyzed with a 2 (refractive status, between subjects factor) × 2 (viewing condition, within subjects factor) × 4 (eccentricity, within-subjects factor) mixed-design ANOVA. Blur sensitivity data did not respect the ANOVA assumption on the normality of the residuals. Thus blur sensitivity data was square-root transformed, and ANOVA was rerun on these transformed data that conformed to the ANOVA assumptions on the normality of the residuals. ANOVA results on the original and transformed data did not substantially differ; we report the ANOVA results on the transformed data for statistical rigor. Post-hoc comparisons were conducted via Bonferroni corrected t tests.
Results
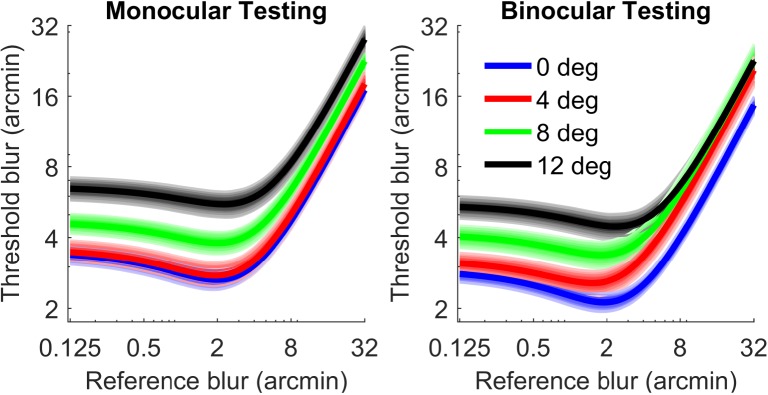
Figure 4 shows group data dipper functions estimated under monocular and binocular testing conditions at each tested eccentricity. In both monocular and binocular viewing, blur thresholds increase with eccentricity. Also blur discrimination thresholds are generally lower in the binocular testing condition.
Figure 4.
Blur dipper functions under monocular (left) and binocular (right) conditions. Curves are averaged across all subjects at each eccentricity (0° blue, 4° red, 8° green, and 12° black). Shaded regions are 95% bootstrapped confidence intervals of the mean.
Figure 5 shows the intrinsic blur parameter of the estimated dipper curves. Intrinsic blur is larger under monocular than binocular conditions, F(1, 42) = 12.75, p = 0.00091, and increases with eccentricity, F(3, 108) = 32.73, p = 10−14. There was no significant effect of refractive group, F(1, 38) = 0.54, p = 0.47, and no significant two or three way interactions (all ps > 0.6).
Figure 5.
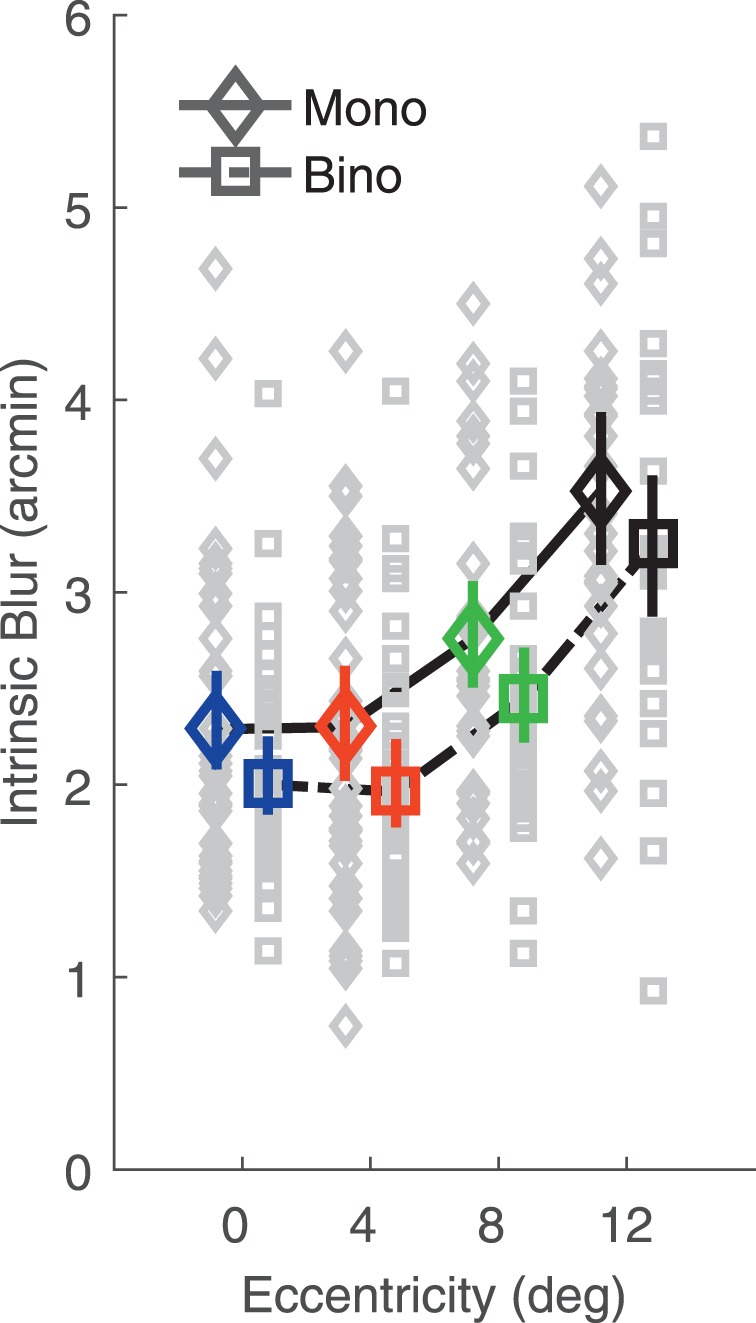
Intrinsic blur as a function of eccentricity in monocular and binocular viewing. Data are averaged across subjects for both monocular (diamonds) and binocular (squares) viewing conditions. Color-coding of eccentricity conditions is as in Figure 3. Error bars are 95% bootstrapped confidence intervals of the mean. Gray data points are individual subject data. Monocular and binocular data are shifted left and right for graphical purposes.
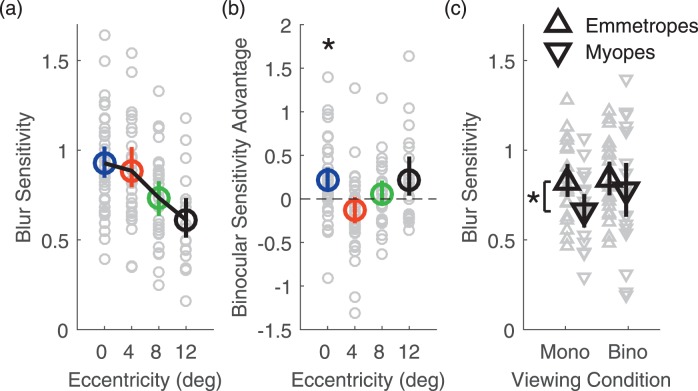
Figure 6a shows blur sensitivity under monocular and binocular viewing. Blur sensitivity decreases with eccentricity, F(3, 109) = 11.6, p = 10−6. Comparing monocular and binocular viewing, there was a significant main effect of viewing condition, F(1, 43) = 5.88, p = 0.020, and a significant two-way interaction between viewing condition and eccentricity, F(3, 75) = 4.91, p = 0.0036. Post-hoc test following the significant interaction between viewing condition and eccentricity revealed that blur sensitivity is higher under binocular viewing conditions at 0° eccentricity (p = 0.012). However, there was no significant difference in blur sensitivity between binocular and monocular viewing conditions at 4°, 8°, and 12° of eccentricity (all ps > 0.25). To summarize these results, in Figure 6b we plot binocular sensitivity advantage as a function of eccentricity. That is, we plot the difference between blur sensitivity measured binocularly and monocularly at each tested eccentricity. If binocular viewing boosts blur sensitivity, we would expect the binocular sensitivity advantage to be positive. We can see that on average, binocular sensitivity advantage was positive, but significantly so only with central vision.
Figure 6.
Blur sensitivity. (a) Blur sensitivity as a function of eccentricity averaged across subjects and viewing conditions. (b) Blur sensitivity advantage for binocular viewing compared with monocular viewing as a function of eccentricity averaged across all subjects. Color-coding of eccentricity conditions in (a) and (b) is as in Figure 4. (c) Monocular and binocular blur sensitivity in emmetropes (upwards pointing triangles) and myopes (downward pointing triangles). Data are averaged across eccentricities and across subjects in each refractive group. Error bars are 95% bootstrapped confidence intervals of the mean. Gray data points are individual subject data. *p < 0.05.
There was no significant main effect of refractive group on blur sensitivity, F(1, 38) = 3.49, p = 0.069, but a significant two-way interaction between viewing condition and refractive status, F(1, 39) = 4.11, p = 0.049. Bonferroni-corrected independent samples t tests revealed that myopes had significantly worse blur sensitivity than emmetropes monocularly (p = 0.037) but not binocularly (p = 0.66), as shown in Figure 5c. Lastly, there was no significant two-way interaction between eccentricity and refractive status, F(3, 109) = 0.28, p = 0.84, and no significant three-way interaction, F(3, 75) = 1.09, p = 0.36.
Discussion
We assessed monocular and binocular blur perception in the near peripheral vision in emmetropic and myopic subjects. It is known that the tolerance of blur increases in the far (Ronchi & Molesini, 1975) and near (Wang & Ciuffreda, 2004, 2005a) peripheral visual field. Blur perception is also known to follow a typical dipper shaped curve (Watson & Ahumada, 2011), with blur discrimination thresholds being lower than detection thresholds for small blur pedestals, and subsequently increasing with increasing levels of reference blur. This pattern is known to be maintained in the visual periphery, where blur discrimination thresholds with detectable levels of blur are lower than blur detection thresholds (Wang et al., 2006). We have replicated these findings by showing that blur perception worsens in the visual periphery and that performance can be described by a dipper-shaped function.
We have extended these findings to show that the increase in blur discrimination thresholds with increasing eccentricity can be attributed to two sources: (1) observers' increased intrinsic blur and (2) decreased blur sensitivity.
In the variance discrimination model we fit to our data, intrinsic blur represents the internal noise or the variance in luminance gradient due to the sensory system (Morgan et al., 2008). The intrinsic blur is thus due to the combined effects of aberrations in the eye's optics and to the noise in the neural transducer machinery. Intrinsic blur increased by 56% beyond 12° in the visual periphery. Part of this increase is likely due to the fact that the retinal surface is curved. Thus, when the eye is accommodating to place the flat surface of the monitor in focus at the fovea, the peripheral retina will experience defocus due to the curvature of the retina. Other aberrations, (astigmatism, coma, and higher order aberrations) also increase in the visual periphery and might contribute to increased intrinsic blur, although removing these aberrations via adaptive optics does not improve peripheral visual resolution (Lundström et al., 2007). Subsampling and consequent aliasing (Artal, Derrington, & Colombo, 1995; Thibos, Walsh, & Cheney, 1987; Williams, Artal, Navarro, McMahon, & Brainard, 1996) due to the decrease in sampling density occurring throughout the peripheral retina (Lindsay & Norman, 1972) are also likely responsible for the observed increase in Intrinsic Blur. Overall, Intrinsic Blur estimates with our “dead leaves” stimuli were greater than those reported for border blur discrimination (Watson & Ahumada, 2011) or for blur discrimination with fractal patterns (Mather, 1997), suggesting that blur perception with naturalistic stimuli may be mediated by receptive fields with larger space constants (Mather & Smith, 2002).
Blur sensitivity decreased by 34% beyond 12° in the visual periphery and was overall quite poor if compared with, for example, spatial frequency discrimination (Hirsch & Hylton, 1982) or even stereoscopic disparity (e.g., Badcock & Schor, 1985), a fair comparison given that blur is an important cue to depth (Langer & Siciliano, 2015; Maiello et al., 2014; Maiello, Chessa et al., 2015; Mather, 1997; Mather & Smith, 2002; Vishwanath, 2012; Vishwanath & Blaser, 2010; Watt et al., 2005). For discrimination of more fundamental image properties such as contrast, dipper functions are thought to arise from the derivative of a sigmoidal transduction response function (Solomon, 2009; Wilson, 1980). Given the observers' poor performance, it is unlikely that blur is represented in the human visual system by an analogous transducer function for image blur. The mechanisms employed for blur perception are a matter of debate. Watson and Ahumada (2011) criticize the variance discrimination model for not providing a mechanistic explanation of blur perception and propose an alternative model based on visible contrast energy detection in which the characteristic dipper shape of the blur discrimination function arises from the shape of the contrast sensitivity function (Campbell & Robson, 1968). Murray and Bex (2010), on the other hand, show that the contrast detection model cannot fit blur discrimination of Gaussian and Sinc-blurred images with the same contrast sensitivity function for the two different types of blur. Models based on luminance slope (such as the MIRAGE model, R. J. Watt & Morgan, 1985) rather than spatial frequency better predicted the data presented in Murray and Bex (2010).
Plainis et al. (2011) have shown that with visual blur induced by positive lenses, binocularity improved visual acuity and enhanced the P100 component of visual evoked potentials. We extend these findings by showing that binocularity also improves visual blur perception. Our results show that combining the information from the two eyes leads to a reduction in the system's internal blur, which is consistent with the notion that combining information from the two eyes reduces noise (Legge, 1984a, 1984b). We also observed a small but statistically significant advantage in blur sensitivity when viewing stimuli binocularly with central vision. This suggests there may be additional binocular processing of blur in the center of the visual field.
Evidence for differences in the perception of blur across refractive groups is conflicting. Rosenfield and Abraham-Cohen (1999) found that thresholds for blur detection are impaired in myopes, whereas Cufflin et al. (2007) found that early onset myopes adapt more strongly to optical blur, yet Schmid et al. (2002) found no statistically significant differences between myopic and emmetropic children in the ability to detect blur. In the current study we have found no differences in the amount of intrinsic blur between myopic and emmetropic subjects. We have found instead that myopes have, on average, worse blur sensitivity than emmetropes monocularly but not binocularly. This suggests that myopes may process blur less efficiently than emmetropes monocularly but not binocularly. We could speculate that monocular deficits in blur processing may be linked to refractive error, but functionally these deficits are masked by binocular summation. In future work it might be informative to assess blur sensitivity both binocularly and monocularly in each eye, not only the dominant eye, to verify the degree to which binocular summation occurs in different refractive groups. Future investigations should also include hyperopic subjects as well as progressing myopes to further study the relationship between blur sensitivity and refractive error development. However, it is thought that the mechanisms that lead to hyperopia are not the same as those that lead to myopia (Borchert et al., 2011; Llorente, Barbero, Cano, Dorronsoro, & Marcos, 2004). Thus hyperopia and myopia should be investigated separately as different processes within the failure of emmetropization. In the current study we assessed blur perception using rendered Gaussian blur. It is probable that blur perception would differ with different kinds of blur, and that the specific type of defocus blur arising from an observer's own optics may have a role in emmetropization. Future studies should thus assess the differences in blur perception with different kinds of blur, such as defocus blur through an observer's own optics (Akeley, Watt, Girshick, & Banks, 2004; Love et al., 2009; Sebastian, Burge, & Geisler, 2015) or sync blur, which contains phase reversals typical of the modulation transfer function of an optical system with a circular aperture such as the human pupil (Murray & Bex, 2010).
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health grant R01EY021553. Lenna Walker was supported by NEI T35 EY007149 to the New England College of Optometry; additional support was provided by internal funds from the New England College of Optometry.
Commercial relationships: Prevention and Treatment of Myopia Patent Application US 20160212404 A1 (GM, PJB, FAV).
Corresponding author: Guido Maiello.
Email: guido_maiello@yahoo.it.
Address: Department of Experimental Psychology, Justus-Liebig University, Gießen, Germany.
References
- Akeley, K., Watt, S. J., Girshick, A. R., & Banks, M. S.. (2004). A stereo display prototype with multiple focal distances. Paper presented at the ACM Transactions on Graphics (TOG). [Google Scholar]
- Arnold, D. H., Grove, P. M., & Wallis, T. S. A.. (2007). Staying focused: A functional account of perceptual suppression during binocular rivalry. Journal of Vision, 7 7: 7, 1–8, doi:10.1167/7.7.7. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Artal, P., Derrington, A. M., & Colombo, E.. (1995). Refraction, aliasing, and the absence of motion reversals in peripheral vision. Vision Research, 35 7, 939–947, doi:10.1016/0042-6989(94)00180-T. [DOI] [PubMed] [Google Scholar]
- Badcock, D. R., & Schor, C. M.. (1985). Depth-increment detection function for individual spatial channels. Journal of the Optical Society of America A, 2 7, 1211–1216, doi:10.1364/JOSAA.2.001211. [DOI] [PubMed] [Google Scholar]
- Banton, T., & Levi, D. M.. (1991). Binocular summation in vernier acuity. Journal of the Optical Society of America A, 8 4, 673–680. [DOI] [PubMed] [Google Scholar]
- Borchert, M. S., Varma, R., Cotter, S. A., Tarczy-Hornoch, K., McKean-Cowdin, R., Lin, J. H.,… Ibironke, J.. (2011). Risk factors for hyperopia and myopia in preschool children: The Multi-Ethnic Pediatric Eye Disease and Baltimore Pediatric Eye Disease Studies. Ophthalmology, 118 10, 1966–1973, doi:10.1016/j.ophtha.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Campbell, F. W. (1957). The depth of field of the human eye. Journal of Modern Optics, 4 4, 157–164. [Google Scholar]
- Campbell, F. W., & Robson, J. G.. (1968). Application of fourier analysis to the visibility of gratings. The Journal of Physiology, 197 3, 551–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman, S. (1990). Effects of luminance and blur on hemispheric asymmetries in temporal integration. Neuropsychologia, 28 4, 361–374. [DOI] [PubMed] [Google Scholar]
- Ciuffreda, K. J. (1991). Accommodation and its anomalies. Charman W.N. (Ed.), Vision and visual dysfunction: Visual optics and instrumentation (pp 231–279). London: MacMillan. [Google Scholar]
- Ciuffreda, K. J. (1998). Accommodation, pupil and presbyopia. Benjamin W.J. (Ed.), Borish's clinical refraction: Principles and practice (pp 77–20). Philadelphia: Saunders. [Google Scholar]
- Ciuffreda, K. J., Wang, B., & Vasudevan, B.. (2007). Conceptual model of human blur perception. Vision Research, 47 9, 1245–1252. [DOI] [PubMed] [Google Scholar]
- Cufflin, M. P., Mankowska, A., & Mallen, E. A.. (2007). Effect of blur adaptation on blur sensitivity and discrimination in emmetropes and myopes. Investigative Ophthalmology & Visual Science, 48 6, 2932–2939. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Fisher, S. K., & Ciuffreda, K. J.. (1988). Accommodation and apparent distance. Perception, 17 5, 609–621. [DOI] [PubMed] [Google Scholar]
- Flitcroft, D. (1998). A model of the contribution of oculomotor and optical factors to emmetropization and myopia. Vision Research, 38 19, 2869–2879. [DOI] [PubMed] [Google Scholar]
- Flitcroft, D., Judge, S., & Morley, J.. (1992). Binocular interactions in accommodation control: Effects of anisometropic stimuli. The Journal of Neuroscience, 12 1, 188–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin, S. J., O'Shea, R. P., Squire, A. M., & Hailstone, D. S.. (1999). Sharpness overconstancy: The roles of visibility and current context. Vision Research, 39 16, 2649–2657. [DOI] [PubMed] [Google Scholar]
- Georgeson, M. A., May, K. A., Freeman, T. C. A., & Hesse, G. S.. (2007). From filters to features: Scale–space analysis of edge and blur coding in human vision. Journal of Vision, 7 13: 7, 1–21, doi:10.1167/7.13.7. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Green, D. M., & Swets, J. A.. (1966). Signal detection theory and psychophysics. New York: Wiley. [Google Scholar]
- Hamerly, J. R., & Dvorak, C. A.. (1981). Detection and discrimination of blur in edges and lines. Journal of the Optical Society of America A, 71 4, 448–452. [Google Scholar]
- Heravian, J., Jenkins, T., & Douthwaite, W.. (1990). Binocular summation in visually evoked responses and visual acuity. Ophthalmic and Physiological Optics, 10 3, 257–261. [PubMed] [Google Scholar]
- Hess, R. F., Schmid, K. L., Dumoulin, S. O., Field, D. J., & Brinkworth, D. R.. (2006). What image properties regulate eye growth? Current Biology, 16 7, 687–691. [DOI] [PubMed] [Google Scholar]
- Hirsch, J., & Hylton, R.. (1982). Limits of spatial-frequency discrimination as evidence of neural interpolation. Journal of the Optical Society of America, 72 10, 1367–1374, doi:10.1364/JOSA.72.001367. [DOI] [PubMed] [Google Scholar]
- Hoffman, D. M., & Banks, M. S.. (2010). Focus information is used to interpret binocular images. Journal of Vision, 10 5: 13, 1–17, doi:10.1167/10.5.13. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood, A. M., & Riddell, P. M.. (2009). Receding and disparity cues aid relaxation of accommodation. Optometry and Vision Science, 86 11, 1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J., Hung, L.-F., & Smith E. L. III,. (2012). Recovery of peripheral refractive errors and ocular shape in rhesus monkeys (Macaca mulatta) with experimentally induced myopia. Vision Research, 73, 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, R., Smith, G., & Chan, C.. (1989). Effect of defocus on blur thresholds and on thresholds of perceived change in blur: Comparison of source and observer methods. Optometry & Vision Science, 66 8, 545–553. [DOI] [PubMed] [Google Scholar]
- Kompaniez, E., Sawides, L., Marcos, S., & Webster, M. A.. (2013). Adaptation to interocular differences in blur. Journal of Vision, 13 6: 19, 1–14, doi:10.1167/13.6.19. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer, M. S., & Siciliano, R. A.. (2015). Are blur and disparity complementary cues to depth? Vision Research, 107, 15–21. [DOI] [PubMed] [Google Scholar]
- Lee, A. B., Mumford, D., & Huang, J.. (2001). Occlusion models for natural images: A statistical study of a scale-invariant dead leaves model. International Journal of Computer Vision, 41 1-2, 35–59. [Google Scholar]
- Legge, G. E. (1984a). Binocular contrast summation—I. Detection and discrimination. Vision Research, 24 4, 373–383. [DOI] [PubMed] [Google Scholar]
- Legge, G. E. (1984b). Binocular contrast summation—II. Quadratic summation. Vision Research, 24 4, 385–394. [DOI] [PubMed] [Google Scholar]
- Lin, Z., Vasudevan, B., Liang, Y. B., Zhang, Y. C., Zhao, S. Q., Yang, X. D.,… Ciufreda, K. J.. (2013). Nearwork-induced transient myopia (NITM) in anisometropia. Ophthalmic and Physiological Optics, 33 3, 311–317. [DOI] [PubMed] [Google Scholar]
- Lindsay, P. H., & Norman, D. A.. (1972). Human information processing: An introduction to psychology. New York: Academic Press. [Google Scholar]
- Llorente, L., Barbero, S., Cano, D., Dorronsoro, C., & Marcos, S.. (2004). Myopic versus hyperopic eyes: Axial length, corneal shape and optical aberrations. Journal of Vision, 4 4: 5, 288–298, doi:10.1167/4.4.5. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- López-Gil, N., Martin, J., Liu, T., Bradley, A., Díaz-Muñoz, D., & Thibos, L. N.. (2013). Retinal image quality during accommodation. Ophthalmic and Physiological Optics, 33 4, 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, G. D., Hoffman, D. M., Hands, P. J., Gao, J., Kirby, A. K., & Banks, M. S.. (2009). High-speed switchable lens enables the development of a volumetric stereoscopic display. Optics Express, 17 18, 15716–15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström, L., Manzanera, S., Prieto, P. M., Ayala, D. B., Gorceix, N., Gustafsson, J.,… Artal, P.. (2007). Effect of optical correction and remaining aberrations on peripheral resolution acuity in the human eye. Optics Express, 15 20, 12654–12661, doi:10.1364/OE.15.012654. [DOI] [PubMed] [Google Scholar]
- Maiello, G., Chessa, M., Solari, F., & Bex, P. J.. (2014). Simulated disparity and peripheral blur interact during binocular fusion. Journal of Vision, 14 8: 13, 1–14, doi:10.1167/14.8.13. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiello, G., Chessa, M., Solari, F., & Bex, P. J.. (2015). The (in) effectiveness of simulated blur for depth perception in naturalistic images. PloS One, 10 10, e0140230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiello, G., Harrison, W., Vera-Diaz, F., & Bex, P.. (2015). Perceptual consequences of elongated eyes. Journal of Vision, 15 12: 111, doi:10.1167/15.12.111. [Abstract] [Google Scholar]
- Mather, G. (1997). The use of image blur as a depth cue. Perception, 26 9, 1147–1158. [DOI] [PubMed] [Google Scholar]
- Mather, G., & Smith, D. R.. (2002). Blur discrimination and its relation to blur-mediated depth perception. Perception, 31 10, 1211–1219. [DOI] [PubMed] [Google Scholar]
- McFadden, S. A.,, Tse, D. Y.,, Bowrey, H. E.,, Leotta, A. J.,, Lam, C. S.,, Wildsoet, C. F.,, To, C. H. (2014). Integration of defocus by dual power Fresnel lenses inhibits myopia in the mammalian EyeDefocus integration by dual power fresnel lenses. Investigative Ophthalmology & Visual Science, 55 2, 908–917. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, M., Chubb, C., & Solomon, J. A.. (2008). A ‘dipper' function for texture discrimination based on orientation variance. Journal of Vision, 8 11: 9, 1–8, doi:10.1167/8.11.9. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, S., & Bex, P. J.. (2010). Perceived blur in naturally contoured images depends on phase. Frontiers in Psychology, 1, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton, T. T., Siegwart, J. T., & Amedo, A. O.. (2006). Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Investigative Ophthalmology & Visual Science, 47 11, 4687–4699. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima, S. (1958). Studies on the depth-of-focus of the eye. Japanese Journal of Ophthalmology, 2, 63–72. [Google Scholar]
- Pääkkönen, A. K., & Morgan, M. J.. (1994). Effects of motion on blur discrimination. Journal of the Optical Society of America A, 11 3, 992–1002. [Google Scholar]
- Pelli, D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10 4, 437–442. [PubMed] [Google Scholar]
- Plainis, S., Petratou, D., Giannakopoulou, T., Atchison, D. A., & Tsilimbaris, M. K.. (2011). Binocular summation improves performance to defocus-induced blur. Investigative Ophthalmology & Visual Science, 52 5, 2784–2789. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Poulere, E., Moschandreas, J., Kontadakis, G. A., Pallikaris, I. G., & Plainis, S.. (2013). Effect of blur and subsequent adaptation on visual acuity using letter and Landolt C charts: Differences between emmetropes and myopes. Ophthalmic and Physiological Optics, 33 2, 130–137. [DOI] [PubMed] [Google Scholar]
- Ronchi, L., & Molesini, G.. (1975). Depth of focus in peripheral vision. Ophthalmic Research, 7 3, 152–157. [Google Scholar]
- Rosén, R., Lundström, L., & Unsbo, P.. (2011). Influence of optical defocus on peripheral vision. Investigative Ophthalmology & Visual Science, 52 1, 318–323. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Rosenfield, M., & Abraham-Cohen, J. A.. (1999). Blur sensitivity in myopes. Optometry & Vision Science, 76 5, 303–307. [DOI] [PubMed] [Google Scholar]
- Schmid, K. L., Iskander, D. R., Li, R. W., Edwards, M. H., & Lew, J. K.. (2002). Blur detection thresholds in childhood myopia: Single and dual target presentation. Vision Research, 42 2, 239–247. [DOI] [PubMed] [Google Scholar]
- Schor, C. (1999). The influence of interactions between accommodation and convergence on the lag of accommodation. Ophthalmic and Physiological Optics, 19 2, 134–150. [DOI] [PubMed] [Google Scholar]
- Sebastian, S., Burge, J., & Geisler, W. S.. (2015). Defocus blur discrimination in natural images with natural optics. Journal of Vision, 15 5: 16, 1–17, doi:10.1167/15.5.16. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. L., III, Campbell, M. C., & Irving, E.. (2013). Does peripheral retinal input explain the promising myopia control effects of corneal reshaping therapy (CRT or ortho-K) & multifocal soft contact lenses? Ophthalmic and Physiological Optics, 33 3, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. L., III, & Li-Fang, H.. (1999). The role of optical defocus in regulating refractive development in infant monkeys. Vision Research, 39 8, 1415–1435. [DOI] [PubMed] [Google Scholar]
- Smith E. L., III, Li-Fang, H., & Harwerth, R. S.. (1994). Effects of optically induced blur on the refractive status of young monkeys. Vision Research, 34 3, 293–301. [DOI] [PubMed] [Google Scholar]
- Smith E. L., III, Ramamirtham, R., Qiao-Grider, Y., Hung, L.-F., Huang, J., Kee, C.-s., … Paysse E.. (2007). Effects of foveal ablation on emmetropization and form-deprivation myopia. Investigative Ophthalmology & Visual Science, 48 9, 3914–3922. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, J. A. (2009). The history of dipper functions. Attention, Perception, & Psychophysics, 71 3, 435–443, doi:10.3758/app.71.3.435. [DOI] [PubMed] [Google Scholar]
- Strang, N. C., Day, M., Gray, L. S., & Seidel, D.. (2011). Accommodation steps, target spatial frequency and refractive error. Ophthalmic and Physiological Optics, 31 5, 444–455. [DOI] [PubMed] [Google Scholar]
- Taylor, J., Charman, W. N., O'Donnell, C., & Radhakrishnan, H.. (2009). Effect of target spatial frequency on accommodative response in myopes and emmetropes. Journal of Vision, 9 1: 16, 1–14, doi:10.1167/9.1.16. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Thibos, L. N., Walsh, D. J., & Cheney, F. E.. (1987). Vision beyond the resolution limit: Aliasing in the periphery. Vision Research, 27 12, 2193–2197, doi:10.1016/0042-6989(87)90134-9. [DOI] [PubMed] [Google Scholar]
- Vera-Diaz, F. A., Kerber, K. L., Thorn, F., & Bex, P. J.. (2013). Peripheral awareness and attention in myopia. Presented at the 14th International Myopia Conference, Asilomar, CA, August 20, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Diaz, F. A., Maiello, G., Kerber, K. L., Thorn, F., & Bex, P.. (2015). Myopes' ability to discriminate and detect blur. Investigative Ophthalmology & Visual Science, 56 7, 537 [Abstract] [Google Scholar]
- Vishwanath, D. (2012). The utility of defocus blur in binocular depth perception. i-Perception, 3 8, 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath, D., & Blaser, E.. (2010). Retinal blur and the perception of egocentric distance. Journal of Vision, 10 10: 26, 1–16, doi:10.1167/10.10.26. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Vul, E., Bergsma, J., & MacLeod, D. I.. (2010). Functional adaptive sequential testing. Seeing and Perceiving, 23 5, 483–515. [DOI] [PubMed] [Google Scholar]
- Wallis, T. S., & Bex, P. J.. (2012). Image correlates of crowding in natural scenes. Journal of Vision, 12 7: 6, 1–19, doi:10.1167/12.7.6. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman, J., & Winawer, J.. (2004). Homeostasis of eye growth and the question of myopia. Neuron, 43 4, 447–468. [DOI] [PubMed] [Google Scholar]
- Walsh, G., & Charman, W.. (1988). Visual sensitivity to temporal change in focus and its relevance to the accommodation response. Vision Research, 28 11, 1207–1221. [DOI] [PubMed] [Google Scholar]
- Wang, B., & Ciuffreda, K. J.. (2004). Depth-of-focus of the human eye in the near retinal periphery. Vision Research, 44 11, 1115–1125. [DOI] [PubMed] [Google Scholar]
- Wang, B., & Ciuffreda, K. J.. (2005a). Blur discrimination of the human eye in the near retinal periphery. Optometry & Vision Science, 82 1, 52–58. [PubMed] [Google Scholar]
- Wang, B., & Ciuffreda, K. J.. (2005b). Foveal blur discrimination of the human eye. Ophthalmic and Physiological Optics, 25 1, 45–51. [DOI] [PubMed] [Google Scholar]
- Wang, B., & Ciuffreda, K. J.. (2006). Depth-of-focus of the human eye: theory and clinical implications. Survey of Ophthalmology, 51 1, 75–85. [DOI] [PubMed] [Google Scholar]
- Wang, B., Ciuffreda, K. J., & Irish, T.. (2006). Equiblur zones at the fovea and near retinal periphery. Vision Research, 46 21, 3690–3698. [DOI] [PubMed] [Google Scholar]
- Watson, A. B., & Ahumada, A. J.. (2011). Blur clarified: A review and synthesis of blur discrimination. Journal of Vision, 11 5: 10, 1–23, doi:10.1167/11.5.10. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Watt, R. J., & Morgan, M. J.. (1983). The recognition and representation of edge blur: evidence for spatial primitives in human vision. Vision Research, 23 12, 1465–1477. [DOI] [PubMed] [Google Scholar]
- Watt, R. J., & Morgan, M. J.. (1984). Spatial filters and the localization of luminance changes in human vision. Vision Research, 24 10, 1387–1397. [DOI] [PubMed] [Google Scholar]
- Watt, R. J., & Morgan, M. J.. (1985). A theory of the primitive spatial code in human vision. Vision Research, 25 11, 1661–1674, doi:10.1016/0042-6989(85)90138-5. [DOI] [PubMed] [Google Scholar]
- Watt, S. J., Akeley, K., Ernst, M. O., & Banks, M. S.. (2005). Focus cues affect perceived depth. Journal of Vision, 5 10: 7, 834–862, doi:10.1167/5.10.7. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildsoet, C., & Wallman, J.. (1995). Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Research, 35 9, 1175–1194. [DOI] [PubMed] [Google Scholar]
- Williams, D. R., Artal, P., Navarro, R., McMahon, M. J., & Brainard, D. H.. (1996). Off-axis optical quality and retinal sampling in the human eye. Vision Research, 36 8, 1103–1114, doi:10.1016/0042-6989(95)00182-4. [DOI] [PubMed] [Google Scholar]
- Wilson, H. R. (1980). A transducer function for threshold and suprathreshold human vision. Biological Cybernetics, 38 3, 171–178, doi:10.1007/bf00337406. [DOI] [PubMed] [Google Scholar]
- Wood, J. M., Collins, M. J., Chaparro, A., Marszalek, R., Carberry, T., Lacherez, P., & Chu, B. S.. (2014). Differential effects of refractive blur on day and nighttime driving performance. Investigative Ophthalmology & Visual Science, 55 4, 2284–2289. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Yamaguchi, T., Ohnuma, K., Konomi, K., Satake, Y., Shimazaki, J., & Negishi, K.. (2013). Peripheral optical quality and myopia progression in children. Graefe's Archive for Clinical and Experimental Ophthalmology, 251 10, 2451–2461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.