Abstract
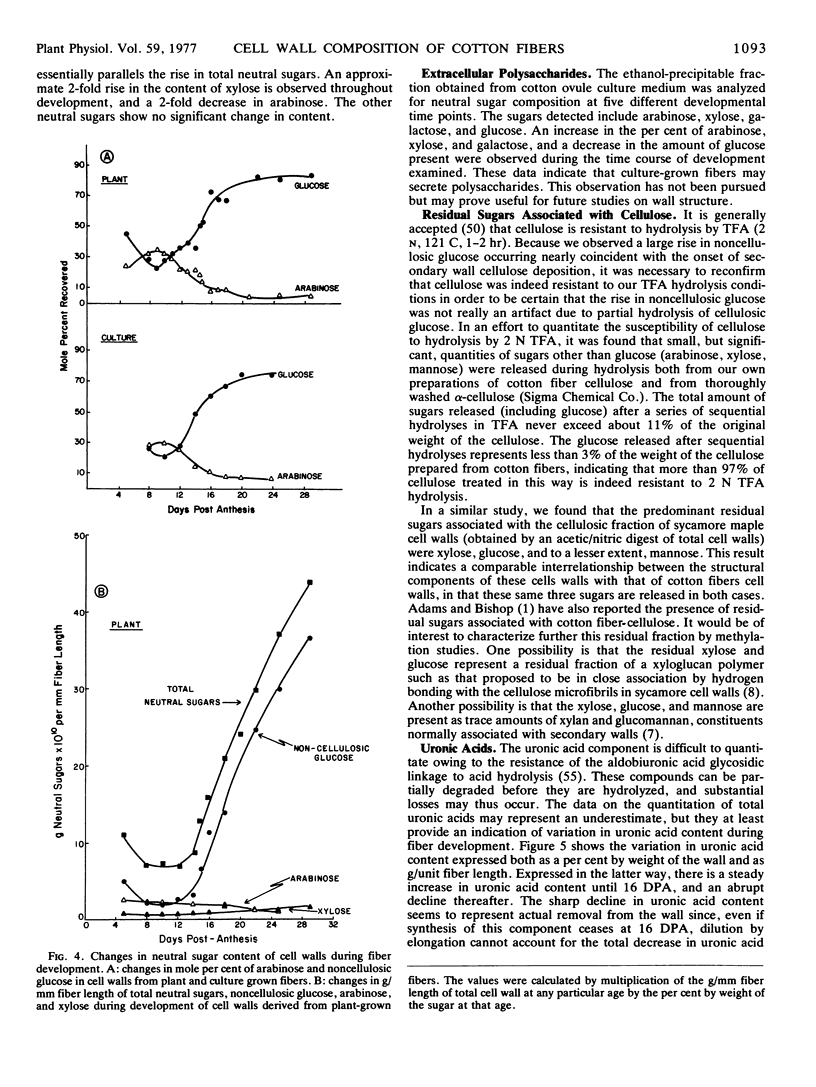
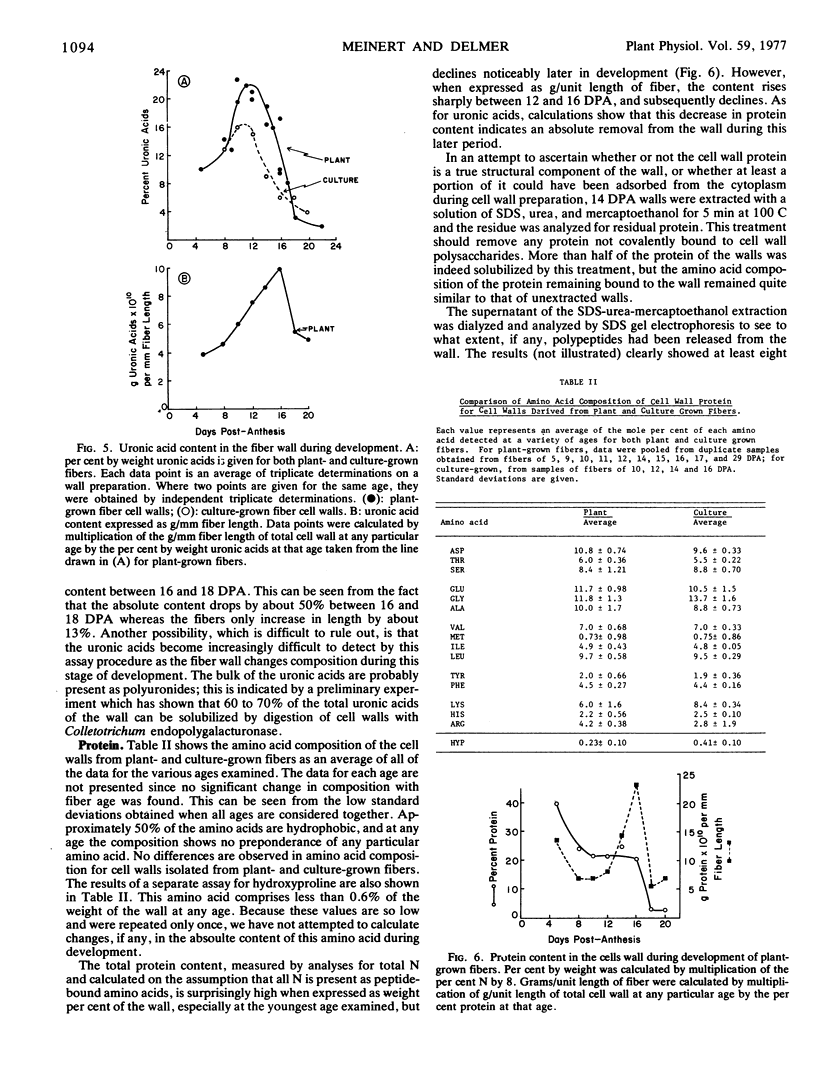
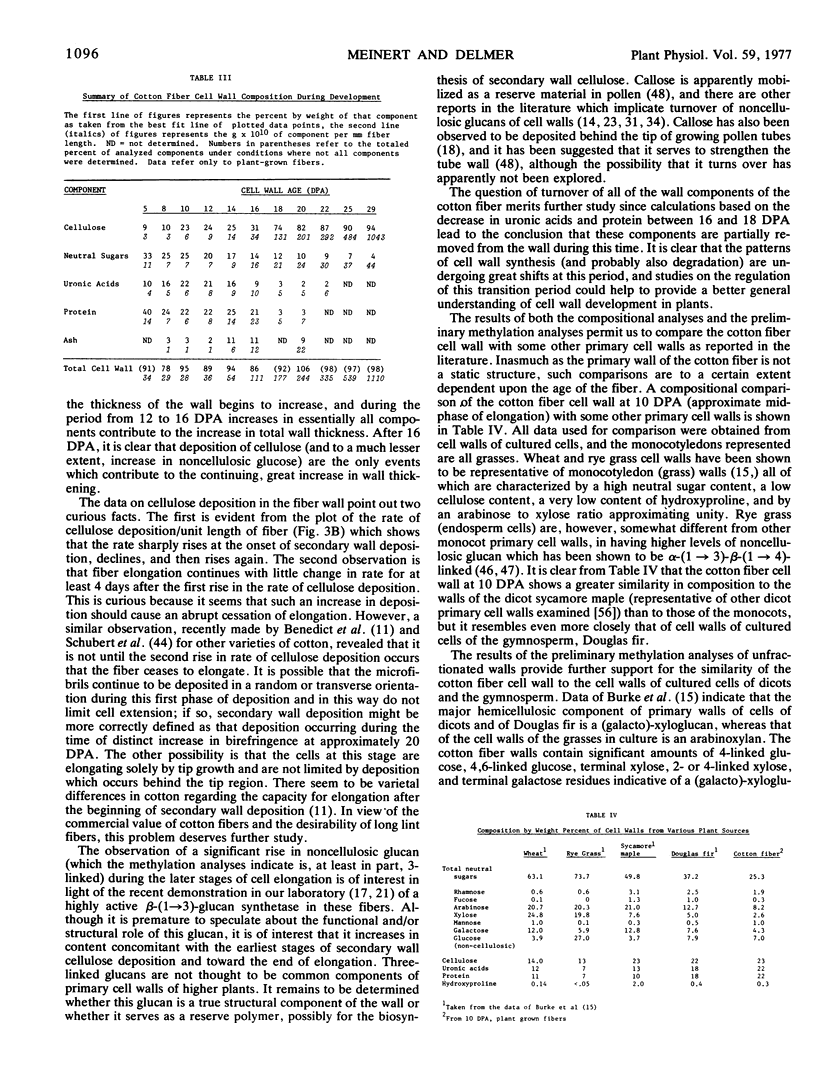
The composition of the cell wall of the cotton fiber (Gossypium hirsutum L. Acala SJ-1) has been studied from the early stages of elongation (5 days postanthesis) through the period of secondary wall formation, using cell walls derived both from fibers developing on the plant and from fibers obtained from excised, cultured ovules. The cell wall of the elongating cotton fiber was shown to be a dynamic structure. Expressed as a weight per cent of the total cell wall, cellulose, neutral sugars (rhamnose, fucose, arabinose, mannose, galactose, and noncellulosic glucose), uronic acids, and total protein undergo marked changes in content during the elongation period. As a way of analyzing absolute changes in the walls with time, data have also been expressed as grams component per millimeter of fiber length. Expressed in this way for plant-grown fibers, the data show that the thickness of the cell wall is relatively constant until about 12 days postanthesis; after this time it markedly increases until secondary wall cellulose deposition is completed. Between 12 and 16 days postanthesis increases in all components contribute to total wall increase per millimeter fiber length. The deposition of secondary wall cellulose begins at about 16 days postanthesis (at least 5 days prior to the cessation of elongation) and continues until about 32 days postanthesis. At the time of the onset of secondary wall cellulose deposition, a sharp decline in protein and uronic acid content occurs. The content of some of the individual neutral sugars changes during development, the most prominent change being a large increase in noncellulosic glucose which occurs just prior to the onset of secondary wall cellulose deposition. Methylation analyses indicate that this glucose, at least in part, is 3-linked. In contrast to the neutral sugars, no significant changes in cell wall amino acid composition are observed during fiber development.
Compositional analyses of cell walls derived from culture-grown fibers indicate that these walls are remarkably similar to those derived from fibers grown on the plant, both in terms of composition and in terms of relative changes in composition during development.
A comparison of our results on total cell wall composition and linkages of sugars as determined by a preliminary methylation analysis of unfractionated fiber walls indicates that the primary cell wall of cotton fibers is similar to that of primary cell walls of other dicotyledons and of gymnosperms as reported in the literature.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Neuberger A. The purification and properties of the lectin from potato tubers, a hydroxyproline-containing glycoprotein. Biochem J. 1973 Oct;135(2):307–314. doi: 10.1042/bj1350307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer W. D., Talmadge K. W., Keegstra K., Albersheim P. The Structure of Plant Cell Walls: II. The Hemicellulose of the Walls of Suspension-cultured Sycamore Cells. Plant Physiol. 1973 Jan;51(1):174–187. doi: 10.1104/pp.51.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Burke D., Kaufman P., McNeil M., Albersheim P. The Structure of Plant Cell Walls: VI. A Survey of the Walls of Suspension-cultured Monocots. Plant Physiol. 1974 Jul;54(1):109–115. doi: 10.1104/pp.54.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R., Karlsnes A. M. A possible role of hydroxyproline-containing proteins in the cessation of cell elongation. Plant Physiol. 1967 May;42(5):669–671. doi: 10.1104/pp.42.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Heiniger U., Kulow C. UDP-glucose: Glucan Synthetase in Developing Cotton Fibers: I. Kinetic and Physiological Properties. Plant Physiol. 1977 Apr;59(4):713–718. doi: 10.1104/pp.59.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Heiniger U., Delmer D. P. UDP-glucose: Glucan Synthetase in Developing Cotton Fibers: II. Structure of the Reaction Product. Plant Physiol. 1977 Apr;59(4):719–723. doi: 10.1104/pp.59.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K., Talmadge K. W., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: III. A Model of the Walls of Suspension-cultured Sycamore Cells Based on the Interconnections of the Macromolecular Components. Plant Physiol. 1973 Jan;51(1):188–197. doi: 10.1104/pp.51.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivilaan A., Bandurski R. S., Schulze A. A partial characterization of an autolytically solubilized cell wall glucan. Plant Physiol. 1971 Oct;48(4):389–393. doi: 10.1104/pp.48.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Turnover of cell wall polysaccharides in elongating pea stem segments. Plant Physiol. 1974 May;53(5):669–673. doi: 10.1104/pp.53.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine R. A., Young N. D., Gerber J. N., Sweeley C. C. Identification of 2-hydroxy fatty acids in complex mixtures of fatty acid methyl esters by Mass chromatography. Biomed Mass Spectrom. 1974 Feb;1(1):10–14. doi: 10.1002/bms.1200010105. [DOI] [PubMed] [Google Scholar]
- Lamport D. T., Miller D. H. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971 Oct;48(4):454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- Loescher W., Nevins D. J. Auxin-induced Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1972 Nov;50(5):556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins D. J., English P. D., Albersheim P. Changes in cell wall polysaccharides associated with growth. Plant Physiol. 1968 Jun;43(6):914–922. doi: 10.1104/pp.43.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'kelley J. C. The Use of C in Locating Growth Regions in the Cell Walls of Elongating Cotton Fibers. Plant Physiol. 1953 Apr;28(2):281–286. doi: 10.1104/pp.28.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- ROELOFSEN P. A. Orientation of cellulose fibrils in the cell wall of growing cotton hairs and its bearing on the physiology of cell wall growth. Biochim Biophys Acta. 1951 May;7(1):43–53. doi: 10.1016/0006-3002(51)90004-2. [DOI] [PubMed] [Google Scholar]
- Ray P. M. Radioautographic study of cell wall deposition in growing plant cells. J Cell Biol. 1967 Dec;35(3):659–674. doi: 10.1083/jcb.35.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Sugar composition of oat-coleoptile cell walls. Biochem J. 1963 Oct;89(1):144–150. doi: 10.1042/bj0890144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadava D., Walker F., Chrispeels M. J. Hydroxyproline-rich wall protein (extensin): biosynthesis and accumulation in growing pea epicotyls. Dev Biol. 1973 Jan;30(1):41–48. [PubMed] [Google Scholar]
- Sandford P. A., Conrad H. E. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. I. A reexamination using improved procedures for methylation analysis. Biochemistry. 1966 May;5(5):1508–1517. doi: 10.1021/bi00869a009. [DOI] [PubMed] [Google Scholar]
- Sokol F., Graves I. L., Ackermann W. W. Concerning the molecular weight, shape, and size of polyglucose isolated from HeLa cells. Biochim Biophys Acta. 1966 Jan 4;112(1):74–80. doi: 10.1016/s0926-6585(96)90010-2. [DOI] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Mense R. M. Characteristics of the process of enzyme release from secretory plant cells. Plant Physiol. 1972 Feb;49(2):187–189. doi: 10.1104/pp.49.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Westafer J. M., Brown R. M., Jr Electron microscopy of the cotton fibre: new observations on cell wall formation. Cytobios. 1976;15(58-59):111–138. [PubMed] [Google Scholar]
- Wilder B. M., Albersheim P. The Structure of Plant Cell Walls: IV. A Structural Comparison of the Wall Hemicellulose of Cell Suspension Cultures of Sycamore (Acer PseudoPlatAnus) and of Red Kidney Bean (Phaseolus Vulgaris). Plant Physiol. 1973 May;51(5):889–893. doi: 10.1104/pp.51.5.889. [DOI] [PMC free article] [PubMed] [Google Scholar]


