Abstract
Background
Wound heals it self spontaneously as physiological process. However, in some individuals, small wounds such as parenteral injections or body piercings may cause increased expression of collagen synthesis. The condition is known as keloid. Histopathology of keloid demonstrates extensive tissue proliferation that extends beyond the margin of primary wound. As a result, it develops uncontrolled or excessive fibrogenesis and tremendous source of collagen that still causes clinical problems until now. A wound, no matter how small the size is, will be followed by increased expression of collagen synthesis. Procollagen I and III is one of markers indicating the development of fibrosis. In fibrosis, there is hypoxia, which is characterized by stabilization of HIF-1α. Therefore, our study was aimed to obtain information about expression of collagen I and III in hypoxic keloid tissue.
Method
The study design was observational descriptive. Keloid specimens were obtained from biopsy and preputium skins as the control specimens were obtained from circumcision. There were 10 tissue specimens for each specimen group. The analysis performed were evaluation of mRNA expression on collagen I, collagen III and HIF-1α using RT-PCR, the evaluation of HIF-1α protein level using ELISA and the expression of collagen I and collagen III protein using immunohistochemistry. Statistically, data was analyzed by unpaired t-test.
Results
In keloid with excessive cell proliferation, we found that the expression of procollagen I mRNA increased 35 times and the expression of procollagen III mRNA increased 27.1 times compared to preputium control group (p<0.05). The expression of procollagen I protein in the dermal layer of keloid was 61% and in the preputium was 37% (p<0.05). The expression of collagen III protein in the dermal layer of keloid was 39% and in the preputium was 16% (p<0.05). There was a 5-fold increase on expression of HIF-1α mRNA in keloid tissue compared to those in preputium (p<0.05). The levels of HIF-1α protein in keloid tissue was 0.201 ng/mg protein and the level in preputium was 0.122 ng/mg protein (p<0.05). There was a strong positive and extremely significant correlation between the expression of HIF-1α protein and procollagen III (R=0.744; p<0.05, Pearson), but HIF-1α with procollagen I are weak correlation (R=0.360; p>0.05, Pearson)
Conclusion
Expression of collagen I and III have important role in hypoxic keloid tissue characterized by increased expressions. The expression of collagen I and III is associated with stable HIF-1α in keloid tissue.
Keywords: Hypoxia, HIF-1α, collagen, keloid
INTRODUCTION
The word keloid derives from Greek roots, i.e. kelis, which means wound blemish and eidos meaning a development of fibroma on the wound blemish.1 Wound heals it self as physiological process through fibrogenesis to replace damaged cells.2 Such wound healing is affected by secretion of various cytokines as a response to tissue damage including transforming growth factor-β1 (TGF-β1) that stimulates tissue recovery, activates fibroblast and collagen deposits through fibrogenesis.3 Moreover, there is vascular endothelial growth factor (VEGF) that stimulates degradation of extracellular matrix surrounding the damaged cells, increases cell proliferation and migration, enhances the development of tissue structure and granulation phase. The process of wound healing is also stimulated by increased activity of plasminogen activator inhibitor-1 (PAI-1) and reduced urokinase plasminogen activator (uPA).4
In some individuals, small wounds such as parenteral injections or body piercings5 may cause increased expression of collagen synthesis.6 The condition is known as keloid.1 Keloid tissues have typical characteristics, i.e. it appears as raised red nodule with shiny and smooth surface.7 Keloid is an active tissue that demonstrates signs of inflammation such as redness, itchy, and mild pain.5 The histopathological feature of keloid demonstrates extensive tissue proliferation that extends beyond the margin of primary wound. It appears in large size, particularly on the ears and face.5 Keloid can significantly reduce quality of life as it often disrupt the patients’ appearance since it makes the skin aesthetically unattractive.
Up to recent, there is no effective therapy and treatment for keloid. Moreover, the biochemical mechanism and pathogenesis of keloid remain vague.8 Keloid is often recurrent although it has been treated, either with pharmalogical agents or surgery.7 The probability of recurrent keloid following the surgery may reach 80–100%.9
Keloid is defined as a benign tumor. Several factors may induce the development of keloid is autosomal dominant inherited and it appears sporadically in each generation with a range of frequency about 38%–73%.6 Younger people during their growth and development stage have more risk for keloid than the elderly. Those with pigmented skin also have higher risk for keloid and it has been estimated that the incidence may reach 4–10%.2,7,10 Somatic mutation on p53 that inhibits apoptosis may also have roles.6 As a result, uncontrolled or excessive fibrogenesis occur. It indicates that there is inefficient fibrogenesis in keloid patients.2 Furthermore, the keloid may develop into cancer or malignant tumor.8
Increased collagen expression is also followed by increased needs in nutrition and oxygen.2 Energy is mainly obtained from glycolysis in cytoplasm, which continues into oxidative phosphorylation in mytochondria. There is increased activity of glycolysis enzymes and oxidative phosphorylation in fibroblasts of keloid tissue. Glucose intake, the amount of lactate and ATP formed in keloid tissue are also higher compared to the normal cell. There is also increased permeability of cell membrane that facilitates influx of substrate and oxygen into mytochondria for ATP synthesis.8
Increased collagen expression can be also caused by increased hydroxylation of proline residues in collagen molecules in order to form triple helix configuration. The hydroxylation process is catalyzed by an enzyme, prolyl hydroxylase (PHD), which needs oxygen for its activity. Oxygen is necessary for hydroxylation of proline and lysine into hydroxyproline and hydroxylysine, which subsequently form the triple helix configuration in collagen maturation, particularly for type I and type III collagen.3,10 Oxygen also has an essential role in inducing fibroblast differentiation into myofibroblast so that it can produce collagen deposits accordingly. Therefore, it can be said that the production of collagen is consistent with oxygen pressure.3
Since there is increased needs of oxygen in keloid, the cells are relatively in hypoxic state, which can activate hypoxia inducible factor-1α (HIF-1α) in fibroblast of keloid tissue.10 It means that individual with keloid experiences relative hypoxia.8
In addition to its role as a catalyst for collagen synthesis, the enzyme, prolyl hydroxilase, also has role in hypoxia sensing. It affects HIF-1α stability and activity through post-translational modification, which is also affected by oxygen level.
Under normoxic condition, HIF-1α undergoes hydroxylation by prolyl hydroxylase and subsequently undergoes ubiquitination by von Hippel Lindau (pVHL) protein for further degradation through proteasome. In hypoxia, HIF-1α does not undergoes degradation and becomes stable, which then translocates to nucleus and have further dimer with other HIF-1α, binds to p300 and CREB binding protein (CBP); afterward, it activates transcription of several target genes including the genes for collagen I and III.11 Therefore, a study should be conducted to analyze the expression of collagen I and III in hypoxic keloid tissue and to observe its probable role in tumor growth since keloid has characteristics that similar to tumor cells.
MATERIALS AND METHODS
Materials
Materials utilized in the study were as followed: for RT-PCR technique, the materials were primers for collagen I, collagen III, and 18S rRNA, KAPA SYBR FAST one step qRT-PCR universal (Kapa Bioseystems, KK4650), RNA mini Kit (Tissue) Geneaid, Water-Biotechnology Gradesterilized-nuclease proteases and pyrogen Free (BUF-1180), β-mercaptoeth; for histology technique, the materials were 10% formalin, a series of increasing alcohol concentration (70%, 80%, 95% and 100% alcohol), xylol and paraffin block; while for immunohistochemistry, the materials included anti-collagen I primary antibody (rabbit polyoclonal anti-collagen I/Novus Biologicals USA, NB600-408, 1:25), anti-collagen III primary antibody (rabbit polyoclonal anti-collagen III/Novus Biologicals USA, NBP1-67528, 1:25); novolink polymer labeled secondary antibody peroxidase in Detection Kit Immunohistochemistry (Leica Biosystem-Novocastra, Wetzlar, Germany/RE 7290-K); and the materials for ELISA technique were Human hypoxia inducible factor-1α (HIF-1α) elisa kit (Cusabio Biotech, Newark, New Jersey/CSB-E12112H), phosphate buffered saline (PBS) pH 7.4.
Methods
The study performed analytical descriptive observational study with cross-sectional design. Keloid tissues were obtained from biopsy or excision procedure and preputium tissues were obtained through circumcision as the control group. Keloid specimens were obtained from biopsy performed in 10 patients with keloid who visited several different hospitals. The patients with keloid participated in our study had given their written informed consent. Preputium tissues were obtained from 10 patients during mass circumcision. The study had been approved by the Medical and Health Research Ethic Committee, Faculty of Medicine, University of Indonesia.
The study was conducted in Faculty of Medicine, University of Indonesia. The evaluation of mRNA expression using RT-PCR and the measurement of collagen I, collagen III, and HIF-1α protein level using ELISA was performed at the Laboratory of Molecular Biology, Department of Biochemistry and Molecular Biology, Faculty of Medicine, University of Indonesia; while the evaluation of collagen I, and collagen III protein expression using immunohistochemistry was performed at Department of Histology, Faculty of Medicine, University of Indonesia.
1. RNA isolation of keloid and preputium tissue
RNA isolation from keloid and preputium tissue was performed using RNA mini Kit (Tissue) (Geneaid Biotech. Ltd.) Several steps were performed. In step 1, we performed cell lysis, i.e. 25 mg keloid and preputium fresh or frozen tissues were grinded using micropestle in 1.5 mL microtube. Afterward, 400 μL RB Buffer and 4 μL β-mercaptoethanol were added. The tissues were then homogenized using Potter-Elvehjehm tissue grinder and were spinned 10 times using syringe and subsequently incubated at room temperature for 3 minutes. The tissues were then passed through a strain using 2 mL filter collection tube. The result filtrate was subsequently centrifuged at 1000 g for 30 seconds.
Step 2, RNA binding: the result filtrate from step 1 was added with 400 μL 70% ethanol in ddH2O (RNase-free and DNase-free); afterward, it was passed thorugh a strain using 2 mL RB column-collection tube. The result filtrate was then centrifuged at 14,000–16,000 g for 1 minute, and then, it was restrained using 2 mL RB column-collection tube.
Step 3, washing: the result filtrate from step 2 was added with 400 μL Buffer W1 in RB column and was subsequently centrifuged at 14,000–16,000 g for 30 seconds. Then, it was restrained using 2 mL RB column-collection tube and 600 μL Wash Buffer and it was centrifuged at 14,000–16,000 g for 30 seconds. Afterward, it was passed through a strain again and transferred into 2 mL RB column-collection tube. It was then recentrifuged at 14,000–16,000 g for 2 minutes in dry matrix column.
Step 4, RNA purification: The result of RNA from dry column was then transferred into 1.5 mL microcentrifuge tube (RNase free). About 50 μL RNase-free Water was added to the matrix column and then we let it sit for 2 minutes. It was subsequently recentrifuged at 14,000–16,000 g for 1 minute. The mixture was added with 2 μL DNase I (2 KU/mL) and sit 10 minutes at room temperature resulting in purified RNA.
2. Measuring RNA Purification
Measurement of RNA purification was performed to identify the amount of total RNA used in RT-PCR in order to determine the purity and to estimate the amount of RNA resulting from the isolation. Spectrophotometry was used to measure the concentration. Results of total RNA were diluted 100 times by following procedure: 5 μL isolated RNA was added with 495 μL nuklease free water (NFW) and their absorbance was measured at 260 and 240 nm wavelengths. RNA purity was measured using nucleic acid purity index by quantifying its absorbance ratio on protein (A260/A280). Purity index of the isolates was considered good when the ratio of A260/A280 ≥ 1.7; therefore, it could be used as a template in RT-PCR. For RT PCR measurement, about 2 uL of 200 ng RNA template was used and it was diluted 10 times.
3. RT-PCR technique for detecting mRNA of HIF-1α, collagen I and collagen III
In our study, cDNA synthesis and PCR amplification were performed using an instrument, Real Time RT-PCR (MiniOpticon, BioRad) with KAPA SYBR FAST one step qRT-PCR universal (KAPA Bioseystems). SYBR Green in the reagent was binding dyes with increased fluorosence when bound to double-stranded DNA. Composition of the materials was as follows: 10 uL of KAPA SYBR “fast” qPCR master mix; 0.4 uL of KAPA RT Mix; 0.4 uL of Forward primer; 0.4 uL of Reverse primer; 6.8 uL of NFW and 2 uL of RNA template with total volume of 20 uL.
Primer genes for HIF-1α, collagen I and collagen III were designed using Primer 3 program. The sequence of HIF-1α, collagen I and collagen III was obtained from NCBI-Gene bank. Primers for collagen I: forward 5′-GGCGGCCAGGGCTCCGACCC′3, reverse 5′-AATTCCTCGTCTGGGGCACC-3′, product 192 bp. Primers for collagen III: forward 5′-TGGTGTTGGAGCCGCTGCCA-3′, reverse 5′-CTCAGCACTAGAATCTGTCC-3′, product 152 bp. Primers for HIF-1α: foward 5′-GGAAGCGCAAGTCTTCAAAG-3′, reverse 5′-TGGGTAGGAGATGGAGATGC-3′, product 187 bp. Primers for 18S: foward 5′-AGAAACGGCTACCACATCCA-3′, reverse 5′-CCCTCCAATGGATCCTCGTT-3′, product 258 bp.
The procedure of reaction with KAPA SYBR FAST one step qRT-PCR universal (KAPA Biosystems) was as follows: synthesis cDNA for 5 minutes at 42°C, inactivation of Reverse transcriptase for 2–5 minutes at 95°C, PCR cycle was performed as many as 40 cycles for 10 seconds at 95°C; 30 seconds at 56,1°C; for collagen I gene, it was at 59,8°C; for genes of collagen III and HIF-1α (through optimalization) at 72°C for 30 seconds; futhermore, Melt curve analysis was performed for 1 minute at 95°C; 1 minute at 55°C; 10 seconds at 55°C (80 cycles, with an increase of 0.5°C for each cycle).
In order to measure mRNA, the level of purity of total RNA isolates was quantified using spectrophotometer. The purity index was considered good when the ratio of total RNA absorbance (A260) to protein absorbance (A280) was more than 1.75. For real time polymerase chain reaction (RT-PCR) analysis, RNA samples were diluted until a concentration of 200 ng/uL was obtained. As a primer for control, a housekeeping gene, mRNA 18 S, was used.
HIF-1α, collagen I, collagen III and 18S human genes were obtained from tracking gene bank, which had been published by other researchers. Results of RT-PCR expression were detected and measured by quantifying fluorosence on SYBR green. The threshold was set to obtain the most optimal expression efficiency and it was matched so that all data were comparable. Ct (Cycle threshold) was the intersection between a curve and a threshold line. For the unmatched efficiency, Livak formula was used to measure the level of relative expression. Expression fluorosence curve of HIF-1α, collagen I, collagen III and 18S and the analysis melting curve showed that HIF-1α had a Tm of 80°C; while collagen I and collagen III had Tm of 87 °C and 18S had a Tm of 80 °C.
By using Real Time RT-PCR, we could determine the amount of cDNA copies resulting from amplification, which indicated the quantitative gene expressions of HIF-1α, Collagen I and Collagen III. 18S rRNA gene was used as the internal standard. Amplification of 18S rRNA gene was performed in equal condition including each gene for HIF-1α, Collagen I and Collagen III. As a negative control, nuclease free water was used as RNA replacer to exclude false positive results. Using real time RT-PCR, efficiency and Cycle Threshold (Ct) were obtained. Analysis of gene expression was performed by relative quantification and therefore, a relative level of mRNA was obtained by Livak method using the following formula:
Notes:
Target = gene X (HIF-1α/collagen I/collagen III)
Test = keloid tissue
Calibrator = preputium tissue
Ref = 18S gene
4. ELISA technique for detecting the level of HIF-1α protein
The level of HIF-1α protein was measured using ELISA Kit Cusabio. The specimens used were 30 mg homogenates of keloid and preputium tissues in 100 μL phosphate buffered Saline (PBS) pH 7.4. The steps were as follows: the method was optimalized by performing antigen titration through dilution of standard protein. The standard concentrations were 0; 0.0625; 0.125; 0.25; 0.5 (ng/mL) for quantifying protein and HIF-1α level. After the standard had been made, a microplate was prepared, which had been coated with primary antibody. About 100 μL of each specimen and standard were transferred to microplate well and subsequently were incubated for 2 hours at 37°C. Following the incubation, the supernatant was discarded and the well was rinsed 3 times with Wash Buffer. About 100 μL HRP-avidin was added and then it was incubated for 1 hour at 37°C. The supernatant was discarded and the well was rinsed 5 times with Wash Buffer. About 90 μL TMB-substrate was transferred into the well and it was incubated in a dark room for 15–30 minutes at 37°C. Furthermore, 50 μL stop solution was added and a color yielded, in which the absorbance could be read using ELISA reader at 450 nm wavelength.
5. Preparing Histological Slides/Sections12,13
Keloid fibroblast tissue obtained from biopsy was immersed in cold 0.9% NaCl; then, it was cut in 3–5 mm thickness. Furthermore, a fixation was performed by transferring it into 10% formalin solution. Next, dehydration was performed by immersing the specimen in increasing concentration of 70% alcohol incubated for 24 hours, 80% alcohol incubated for 24 hours, 95% alcohol incubated for 24 hours, 100% alcohol for 2 x 24 hours (in 12 hours, the 100% alcohol was removed). Afterward, clearing was performed by immersing the specimen in xylol 2 x 24 hours (in 12 hours, the xylol was removed). Then, the embedding was performed, i.e. by infiltrating the specimen with liquid paraffin. After the tissue specimen was ready, it was cut into sections with microtome in 4–5 μm thickness. The sections were then taken using a brush and were transferred to a water bath so that they were allowed to widen. The sections were carefully transferred to a warm water bath at 40–46°C. At this point, the sections were trimmed and transferred onto a slide that had been smeared with eiwit (egg white and glycerin), which served as an adhesive. The slide and tissue specimen on it were set in a special shelf and transferred into incubator at 40–60°C for 24 hours or until the slide was ready for staining. Histologic staining is done with Hematoxylin-Eosin.
6. Immunohistochemistry for detecting collagen I, collagen III and HIF-1α proteins
The available histological slides were ready for immunohistochemistry to detect cells expressing collagen I and collagen III proteins. The following steps were performed: deparaffinization by immersing the specimen in xylol for 5 minutes; dehydration by immersing the specimen in a serial of alcohol concentration of 100%, 95%, 90%, 80%, 75% for 5 minutes for each concentration and aquadest for 5 minutes. The next step was washing using PBS (pH 4) for 5 minutes. Then, a peroxidase blocking solution (0.3% H2O2 in methanol) was added for 15 minutes in order to inhibit peroxidase activity. The specimen was subsequently washed with PBS (2 x 5 minutes). A non-immune protein blocking solution was added for 15 minutes. The specimen was washed with PBS (2 × 5 minutes).
Afterward, it was incubated and collagen I and collagen III primary antibodies were added for each specimen with 1:25 dilution in PBS. The specimens were washed using PBS (2 x 5 minutes). Incubation was continued by adding secondary antibody (novolink polymer) to bind biotin for 1 hour. The specimens were washed again with PBS (2 x 5 minutes). Several drops of 3,3′-diaminobenzidine (DAB) solution were added to the specimen and it was rinsed with water quickly. The specimens were then counterstained by incubating them in hematoxylin solution for 15 minutes. The specimens were rinsed again with water quickly. Next, we performed dehydration using increasing concentration of alcohol, i.e. 70%, 96% and 100% for 5 minutes each and the specimens were incubated in xylol for 2 x 2 minutes, Entelan (Canada balsam) solution was added and the specimens were mounted with cover glass (coverslip), which was subsequently being sat in room temperature until dried. Furthermore, the slides were ready for observation under light microscope with 400× magnification. Later, quantification of cells expressing collagen I and III protein was performed in 5 power fields. The result was considered positive when there was brown stain in the cytoplasm and nucleus. The positive control specimen for evaluation of HIF-1α protein expression was breast cancer tissues; while for evaluation of collagen I and III protein expression, rat skin tissues were used.
The density of cells expressing protein, collagen I and III in keloid and preputium tissue was observed in dermal layer. Immunohistochemistry staining was performed using software Image J program; each cell that had been counted was marked (stained by the program) in order to prevent recounting. Each counting was performed by 2 different observers on the same slide and they were assisted with counting tool.
Cell quantification was performed in 5 high power field (HPF) for each slide of keloid or preputium tissue. High power field was determined as 40× magnification of dermal layer, which included: upper, lower, central, left, and right margins. Furthermore, the HPF was altered to 400× magnification to quantify the cells. The percentage of collagen I and III protein expressions was calculated and subsequently compared to the amount of total cells of the same power field and multiplied by 100.
7. Statistical Analysis
The data are presented in figures, tables and graphs. To analyze significant correlation, unpaired t-test was performed when the distribution in each group was normal; or Mann-Whitney test was performed when the distribution in one of the groups was abnormal. Difference was considered significan when the p < 0.05. To express the correlation between parameters, Pearson correlation test was performed.
RESULTS
1. Expression of Pro/collagen I
This study provide images of keloid tissue preparations with Hematoxylin-Eosin staining (figure 1). It aims to understand the structure and the cell and collagen (keloid and preputium). Extracellular collagen appeared large in keloid, and resemble the fibers in the preputium.
Figure 1.
Fibroblasts and collagen structure (arrows) are seen in keloid (A) and preputium (B) with hematoxylin-eosin staining (400 times magnification).
Immunohistochemical examination using positive and negative controls, was performed to confirm result by such techniques: according to the procedure, the target protein is detected, the location is clear and no contamination. Use of the tissues as a control following the instructions on the kit primary antibody used. Each antibody is specific primer, which detects specific proteins and expressed in large quantities in certain tissues. Antibodies to collagen proteins expressed in many tissues of the skin, so it should be used as a positive control. But normal human skin tissue is difficult to obtain (related to ethic issues), so in this study we were using the skin normal tissue of mice as a control. Principles of immunohistochemical examination using antigen-antibody and enzymatic reaction. Specific antibody-antigen complexes (antibody anti-collagen I or III) binds to a second antibody labeled with peroxidase to form a brown color.
The negative control was also used skin normal tissue of mice, but we don’t add primary antibody, and the enzimatc reaction showed blue. The negative control showed no expression for the protein collagen I or III. The both entities of differences between positive and negative controls have determined that the study, we conducted was valid (on the right track).
Collagen expressed in intracellular and extracellular by immunohistochemical staining (brown). Collagen extracellular looks irregular and endless. The quantitative calculations is too difficult. This study calculates the protein collagen I and III that are expressed in intracellular or cytoplasmic.
The expression of pro/collagen I is presented in figure 2. The mRNA expression of procollagen I in keloid tissue was found higher compared to those in preputium tissue (p<0.05; figure 2.A). Immunohistochemistry showed that collagen I protein was detected as intracellular and extracellular protein both in keloid and preputium cells (figure 2.C–F). To quantify the collagen expression, we calculated the percentage of total numbers of cells expressing intracellular collagen. The expression of collagen I protein in dermal layer of keloid tissue was significantly higher compared to those in preputium tissue (p<0.05; figure 2.B)
Figure 2.
Expressions of collagen I mRNA and protein. (A) Ratio of procollagen I mRNA expression, in keloid tissue it was significantly higher than those in preputium tissue (*p<0.05). Expression of collagen I protein in the cells of dermal layer was found both in nucleus and cytoplasm characterized by brown staining (arrow, 400 and 1000 times magnifications). (C) Expression of collagen I in dermal layer cells of preputium tissue; (D) Expression of collagen I in dermal layer cells of keloid tissue; (E) Expression of collagen I in positive control tissue; (F) negative control. (B) A graph shows percentage ratio of the number of cells expressing collagen I protein in dermal layer of keloid and preputium tissues using immunohistochemistry and it shows significant difference (*p<0.05).
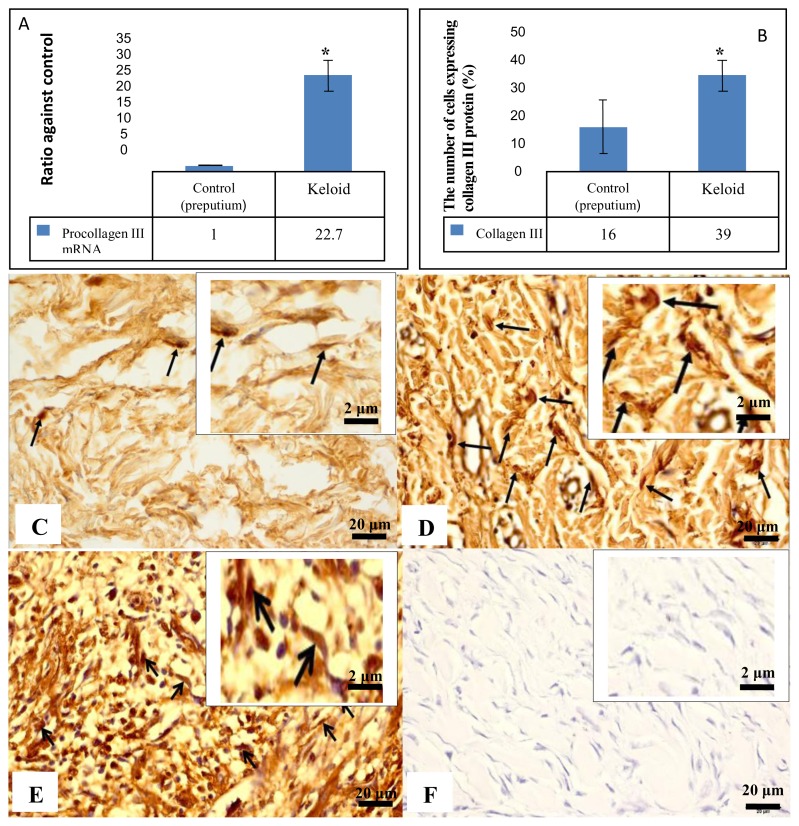
2. Expression of pro/collagen III
Expression of pro/collagen III is presented in figure 3. There was higher expression of procollagen III mRNA in keloid tissue compared to those in preputium (p<0.05; figure 3.A).
Figure 3.
Expression of collagen III mRNA and protein. (A) Ratio of procollagen III mRNA expression in keloid tissue was significantly higher than those in preputium tissue (*p <0.05). The expression of collagen III protein in the cells of dermal layer was found both in nucleus and cytoplasm characterized by brown staining (arrow, 400 and 1000 times magnifications). (C) Expression of collagen III in dermal layer cells of preputium tissue; (D) Expression of collagen III in dermal layer cells of keloid tissue; (E) Expression of collagen III in positive control tissue; (F) negative control. (B) A graph shows percentage ratio of the number of cells expressing collagen III protein in dermal layer of keloid and preputium tissues using immunohistochemistry and it shows significant difference (*p<0.05).
The immunohistochemistry showed that collagen III protein was detected intracellular and extracellular both for keloid and preputium cells. In our study, we performed quantification of collagen III protein expression, which was detected intracellular (figure 3.C–F). The percentage of the the number of cells expressing collagen III protein in keloid tissue was significantly higher than those in preputium tissue (p<0.05; figure 3.B).
3. Expressions of HIF-1α mRNA and protein
The results of analysis on HIF-1α mRNA expression and HIF-1α protein level are presented in figure 4. The results are showed that the mRNA and protein expression of HIV-1α in keloid was significantly higher than the preputium (unpaired t-test; p<0.05; figure 4.A and 4.B). With the increased expression of HIF-1α, It is proved keloid hypoxic.
Figure 4.
mRNA expression and the level of HIF-1α protein. (A) A ratio of HIF-1α mRNA expression in keloid and preputium tissue using RT-PCR shows significant difference (*p<0.05). Data had been previously normalized to the control (B) A ratio of HIF-1α protein level in keloid and preputium tissue using ELISA technique shows significant difference (*p<0.05).
4. Correlation between HIF-1α, procollagen I and III
The correlation between HIF-1α protein level and the cells expressing FGF protein, procollagen I and III mRNA can be seen in figure 5. HIF-1α protein and procollagen I mRNA had a weak correlation (figure 5A; Pearson; R=0,369; p>0,05). The correlation between the expression of HIF-1α protein and prokolagen III mRNA shows a strong positive and significant correlation (Figure 5B; Pearson; R=0.740; *p<0.05).
Figure 5.
Correlation between HIF-1α protein level with expressing procollagen I (A) and procollagen III (B).
DISCUSSION
Keloid is a benign tumor, but it as well look like a malignant tumor. Keloid is defined as excessive growth of scar. Keloid appears because the excessive increase in the synthesis of collagen by fibroblasts.8 The fibroblasts of keloid also showed a decrease in the activity of apoptosis and down-regulation of gene p53. In keloid also found decreased expression of connection, a protein that plays a role in gap junction on cell communication.3,9
In this study we used keloid as a model in the fibrosis case. Fibrosis is present in other organs as well. Keloid is purposed to study the growth properties of cells, especially in fibrosis or tumor case. In general, fibrosis apreass in hypoxia. In this study, we have proved that hypoxia occurs with an increase in HIF-1α as a marker of hypoxia. HIF-1α will act as a transcription factor and activates genes necessary for adaptation to fibrosis (including the expression of collagen I and III).14 Thus, this study is determine to know the expression of collagen I and III in hypoxia of keloid. In addition, there is open possibilities about the benefits of this research in the future both for diagnostic and therapeutic.
Fibrosis tissue is difficult to get in other organs, then we used keloid tissue which is easily got through surgery or excision. Distribution locations of keloid, we can find keloid on around the ears and face.5 In Indonesia in 2008 informed of the location of keloid found in the ears, chest and arms about 25%; while the stomach, legs and back about 5%. Keloids are also identified in some part other body. Keloid locations that are known at this time, in the chest (40%); shoulder and upper arm (20%); abdomen (13.33%); legs (6,67%); hand front (6,67%); buttocks (3.33%); breasts (3, 33%) and earlobes (3.3%).15
Locations of keloids is found at the extremity of about 62.5% in men and at the ear about 41.67% in women. The chest and arms are most locations the incidence of keloids reached 80% in patients aged over 30 years, while the age range of 10–30 years are the most locations in the ear that is equal to 40%. Keloids are also found in the retina of the eye.16
In this study was we can’t use a normal skin from keloid patient as control sample. Researchers have problem to get keloid sample voluntarily from keloid patient. Keloid patients already know it will appear again after surgery. The probability of postoperative keloid, it will recurrence about 80–100%.9 Generally, samples were obtained from patients with repeat caesarean section or reconstruct orthopedic patients appearing keloids. The other reason, if the keloids are in ears, it will be difficult to get around normal skin. If normal skin normal is taking around the keloid, it is likely to aggravate or extend the incidence area of keloids. It is difficult to get permission from the ethics committee.
From the previous literature, there is no information indicating that the penis or preputium organ has fibrosis or keloids. Yet there are also reports that patients with keloids, at the post-circumcision (removal of the preputium) found keloid in the penis organ. The problem in this research is there any genes or proteins that are suppressing the incidence of keloid in the skin tissue of the penis, which is different from skin tissue in another location. Therefore, in this study we have collected and selected the preputium as a control, which known as waste product of circumcised penis.
The excessive proliferation of fibroblasts in keloids, has proved mRNA expression of procollagen I and III at the transcriptional level are higher than preputium. Thus, at the translation level, protein expression of collagen I and III more entitied in keloid than preputium. There was a high expression of pro/collagen I and III starting from the transcription to the translational lavel at mechanisme of collagen biosynthesis (Figure 6).
Figure 6.
Biosynthesis of collagen. Biosynthesis of collagen stage include (i) the synthesis of procollagen in the reticulum endoplasmic (II) hydroxylation of proline and lysine (III) O-glycosylated hydroxylysine (IV) Three chain prokalogen are together and form a triple helix called monomers collagen (V and VI) the monomer collagen secreted from the cell (VII) termination propeptida (VIII) self-assemble into collagen fibrils and it is form crosslink covalent bonds between the monomer collagen (IX) aggregation Fibril.17
The Transcription stage is initiated by procollagen genes switched into RNA in the nucleus. Furthermore procollagen mRNA heading into the cytoplasm and translated to be a single polypeptide chain on the ribosome. Polypeptide chains that form attached to the membrane of the rough endoplasmic reticulum. That polypeptide go into the lumen of the cisterns. Furthermore, through the enzymatic reaction every three polypeptides forming procollagen. Procollagen through the maturation phase or post-translational modification becomes kolagen.17,18
Collagen maturation include hydroxylation reaction of the proline and lysine amino acids become hydroxyproline and hydroxylysine. The hydroxylation occurs after polypeptide chain of procollagen reaches a length of about 20–35 kD and It is still bound to the ribosome. The enzyme that catalyzes the reaction is prolil-4-hydroxylase and lysine-hydroxylase. During this hydroxylation process requires O2, cofactor ascorbic acid and 2-oxoglutarate.17,18
Then the chain of procollagen is transferred to the Golgi apparatus for improving the structure. The reaction of N-oligosaccharides to enhance procollagen structure adding additional glucose or mannose or galactose. At both the ends of the polypeptide (carboxyl and amino terminal), cysteine amino acids to make disulfide bonds, which helps the arrangement of procollagen into triple helical and called monomers of collagen. The Collagen monomers use transport vesicles to the Intracellular. The collagen monomers are secreted into the extracellular by exocytosis. In the extracellular, at both the ends of the terminal (carboxyl and amino terminal) monomer cut by activity of enzymes procollagen aminoproteinase and procollagen karboksiproteinase become an active collagen.19
Some collagen are together and draw up self-assembly into fibers Collagen. Fibers collagen is stabilized by hydrogen bonds (covalent) at amino acid hidroksiprolin among chain polypeptides. Collagen fibers aggregate and packaged in bundles fibril.20
In keloid tissue, collagen density was found higher in addition to increased expressions of pro/collagen I and III. It demonstrated that the accumulated collagen in the extracellular matrix of dermal layer was collagen I and III. In keloid proliferation, it was obvious that the fibroblasts expressed collagen I and III starting from the translation phase to protein synthesis. The increased expressions of collagen I and III in keloid tissue indicated that there are a lot of fibroblasts that activate procollagen I and III mRNA at the cellular endoplasmic reticulum into procollagen I and III protein. After the procollagen is modified by the activity of prolylhydroxilase, it is subsequently secreted as collagen I and III abundantly into the extracellular matrix.18,20
The cells role in secreting collagen I and collagen III protein are fibroblast, fibrocytes, dendrocytes, mast cells, macrophages or other cells in all tissues including the dermal layer.16 Fibroblasts are major types of cells composing the dermal layer of the skin; therefore, they have essential roles in synthesis of collagen I and III.16 Increased expression of collagen I and III in excessive cell proliferation is also strongly associated with the role of ascorbic acid, which stimulates the maturity of intracellular collagen. Since there are a lot of fibroblasts synthesizing procollagen I and III, proline hydroxylation occurs rapidly and they are promptly secreted into extracellular matrix and regulate the development of new procollagen synthesis by stimulating the production of procollagen mRNA.22,23
In other case, excessive fibroblast proliferation at fetal development in the uterine has caused increased type I collagen after birth.22 Ratio of type I : type III collagen in normal skin is 8:1.22,23 However, in excessive cell proliferation of keloid tissue, there is an increase percentage of collagen III although both collagen I and III seem to be increased. In our study, we demonstrated that the increased ratio of collagen I and III expression was 2: 1.
To provide evidences on the occurrence of hypoxia in keloid tissue, our study showed that the expression of HIF–1α was significantly higher, starting from the mRNA to protein levels compared to the preputium tissue as the control group. In keloid tissue, increased cell proliferation had caused the cells in relative hypoxia condition. Due to the absence of oxygen, there was an inhibition of prolylhydroxilase (PHD), an enzyme which has role in HIF–1α degradation. PHD activity requires co-factors, such as 2-oxoglutarate, oxygen and Fe2+. As a result, HIF–1α did not undergo hydroxylation for proline and lysine residues; and therefore, it was not recognized by the Von Hippel–Lindau protein for ubiquitination so that it was not degraded by proteasome.24 It caused increased HIF–1α level in keloid tissue, which usually low or nearly absent in physiological condition, although overall, there is adequate oxygen supply.
In hypoxia, there is an increase of ROS development due to oxidative stress that induces synthesis of HIF–1α mRNA through signal transduction pathway.25 ROS activates c–Jun N terminal–kinase (JNK), which subsequently activates c–Jun and activator protein–1 (AP–1); or increased ROS activates MAPK, which then activates c–fos and NF–κβ (nuclear factor kappa beta). After c–Jun, Ap–1, c–fos and NF–κB have been activated, they simultaneously induce target genes, including HIF–1α gene.24 Since increased ROS production in hypoxia can induce HIF–1α mRNA and relative hypoxia condition resulting in stable HIF–1α, then it can be understood that hypoxia due to excessive fibrosis may also have increased HIF–1α mRNA and protein. Moreover, in keloid, there is also increased needs of ATP due to fibrosis; therefore, the increased HIF–1α protein level is more or less required for increasing synthesis of glycolysis enzyme for the sake of producing energy.26
Statistical analysis of data showed a strong significant positive correlation between HIF–1α protein and expressions of procollagen III (Figure 5B; R=0.740; p<0.05). However, in our study, we could not explain how HIF–1α regulates the expression of collagen I and III. In a study conducted by Glikes et al, there are evidences that HIF–1α regulates the expression of procollagen in tumor metastasis.14 Another study demonstrates that HIF–1α stimulates fibroblasts in expressing prolylhydroxylase enzyme during the repair of hypoxic extracellular matrix.27
Stable HIF-1α will control expressions of various genes. In keloid excessive cell proliferation, we know that it requires a lot of energy or ATP through semi-anaerobic metabolism, i.e. anaerobic glycolysis metabolism, which is followed by oxidative phosphorylation.8 The role of HIF-1α is to regulate the transcription of glycolysis and oxidative phosphorylation enzymes, which have important roles in adaptation against oxygen depletion. The presence of energy and growth factors stimulates excessive fibroblast proliferation, which consequently also causes excessive procollagen synthesis. The abundant amount of procollagen encourages collagen maturity through the mechanism of proline hydroxylation into hydroxyproline. The excessive cell proliferation in keloid provides evidences that HIF-1α protein does regulate the development of hydroxyproline.26,28
Those statements explain that diminished HIF-1α activity can reduce tumor growth; while HIF-1α overexpression will increase the activity of HIF-1α transcription factors and encourage excessive cell or tumor proliferation.29 In keloid tissue, excessive cell proliferation and collagen synthesis can happen for a long period of time up to months or years since the formation of the wound,30 and it is assumed that prolonged hypoxia occurs. Proliferation of normal cells should occur in shorter period of time, i.e. it takes place in 5–7 days after wound formation.28,29
6.1. Conclusion
Expression of collagen I and III plays roles in hypoxic keloid tissue, which is characterized by increased expression. The expression of collagen I and III is associated with stable HIF-1α in keloid tissue.
6.2. Suggestions
Based on results of our study, we suggest further studies on:
Cell line culture of fibroblast with controlled hypoxia in order to reveal the association between collagen I and III activity with HIF-1α.
The role of collagen I, collagen III and HIF-1α in chronic fibrosis.
REFERENCES
- 1.Morris PJ, Wood WC. Text Book of Surgery. 2nd Ed. Oxford University Press; New York: 2000. [Google Scholar]
- 2.Saed GM, Ladin D, Olson J, Han X, Hou Z, Fivenson D. Analysis of p53 Gene Mutations in Keloids Using Polymerase Chain Reaction–Based Single-Strand Conformational Polymorphism and DNA Sequencing. Arch dermatol. 1998;134:963–71. doi: 10.1001/archderm.134.8.963. [DOI] [PubMed] [Google Scholar]
- 3.Lei ZX, Yin JD, Chang WJ, Li LJ, Zhong LZ, Long CJ. Transforming growth factor-α1 phage model peptides isolated from a phage display 7-mer peptide library can inhibit the activity of keloid fibroblasts. Chin Med J. 2011;124(3):429–435. [PubMed] [Google Scholar]
- 4.Tuan TL, Hwu P, Ho W, Yiu P, Chang R, Wysocki A, et al. Adenoviral Overexpression and Small Interfering RNA Suppression Demonstrate That Plasminogen Activator Inhibitor-1 Produces Elevated Collagen Accumulation in Normal and Keloid Fibroblasts. Am J Pathol. 2008;173(5):124–8. doi: 10.2353/ajpath.2008.080272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel R, Papaspyros SC, Javangula KC, Nair U. Presentation and management of keloid scarring following median sternotomy: a case study. J Cardiothorac Surg. 2010;5:122–9. doi: 10.1186/1749-8090-5-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith JC, Boone BE, Opalenik SR, Williams SM, Russel SB. Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated. J Invest Dermatol. 2008;128(5):1298–1310. doi: 10.1038/sj.jid.5701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabiston DC, Jr, Lyerly HK. Essentials of surgery. 2nd ed. WB Saunders; Philadelphia: 1994. [Google Scholar]
- 8.Vincent S, Phan TT, Mukhopadhyay A, Lim HY, Halliwell B, Wong KP. Human Skin Keloid Fibroblasts Display Bioenergetics of Cancer Cells. J Invest Dermatol. 2008;128:702–709. doi: 10.1038/sj.jid.5701107. [DOI] [PubMed] [Google Scholar]
- 9.Park TH, Seo SW, Kim JK, Chang CH. Management of chest keloids. J Cardiothorac Surg. 2011;6:49–52. doi: 10.1186/1749-8090-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syed F, Ahmadi E, Iqbal SA, Singh S, McGrouther DA, Bayat A. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: clinical implications for lesional site-directed therapy. Br J Dermatol. 2011;164(1):83–96. doi: 10.1111/j.1365-2133.2010.10048.x. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Manda SM, Sikder D, Birrer MJ, Rothermel BA, Garry DJ, Mammen PP. Calcineurin Activates Cytoglobin Transcription in Hypoxic Myocytes. J Biol Chem. 2009;284(16):10409–21. doi: 10.1074/jbc.M809572200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 6th Ed. Churchill Livingstone; 2007. [Google Scholar]
- 13.Wick MR. Diagnostic Histochemistry. Cambridge University Press; New York: 2008. [Google Scholar]
- 14.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14(6):430–9. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barara M, Mendiratta V, Chander R. Cryotherapy in treatment of keloids: evaluation of factors affecting treatment outcome. J Cutan Aesthet Surg. 2012;5(3):185–9. doi: 10.4103/0974-2077.101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh JG, Khurana RN, Lai MM, Rodriguez A, Rao NA. Keloid of the Conjunctiva Simulating a Conjunctival Malignancy. Br J Ophthalmol. 2007;91(9):1251–2. doi: 10.1136/bjo.2006.112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelse K, Pöschl E, Aigner T. Collagen-structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–46. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Rokowski R, Cutroneo KR, Guzman NA, Fallon A, Cardinale GJ. In Vitro Synthesis of Collagen Prolyl Hydroxylase. J Biol Chem. 1981;256(3):1340–5. [PubMed] [Google Scholar]
- 19.Pataridis S, Eckhardt A, Mikulíková K, Sedláková P, Miksík I. Identification of collagen types in tissues using HPLC-MS/MS. J Sep Sci. 2008;31:3483–8. doi: 10.1002/jssc.200800351. [DOI] [PubMed] [Google Scholar]
- 20.Shoulders MD, Raines RT. Collagen Structure and Stability. Annu Rev Biochem. 2009;78:929–58. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng LY, Huang MJ, Ye XQ, Fan MW, Bian Z. Increased expression of collagen prolyl 4-hydroxylases in Chinese patients with hereditary gingival fibromatosis. Arch Oral Biol. 2007;52:1209–14. doi: 10.1016/j.archoralbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Geesin JC, Darr D, Kaufman R, Murad S, Pinnell SR. Ascorbic Acid Spesifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J Invest Dermatol. 1988;90:420–4. doi: 10.1111/1523-1747.ep12460849. [DOI] [PubMed] [Google Scholar]
- 23.Cheng W, Yan-hua R, Fang-gang N, Guo-an Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr J Biotechnol. 2011;10(13):2524–9. [Google Scholar]
- 24.Nagy MA. HIF-1 is the Commander of Gateways to Cancer. J Cancer Sci Ther. 2011;3(2):35–40. [Google Scholar]
- 25.Baek JH, Reiter CE, Manalo DJ, Buehler PW, Hider RC, Alayash AI. Induction of hypoxia inducible factor (HIF-1α) in rat kidneys by iron chelation with the hydroxypyridinone, CP94. Biochim Biophys Acta. 2011;1809:262–8. doi: 10.1016/j.bbagrm.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res. 2007;40:285–91. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- 27.Glikes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor-1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288(15):10819–29. doi: 10.1074/jbc.M112.442939. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Powis G, Kirkpatrick L. Hypoxia inducible factor-1α as a cancer drug target. Mol Cancer Ther. 2004;3:647–654. [PubMed] [Google Scholar]
- 29.Fawcett DA. Text Book of Histology. 12th ed. Vol. 963. Chapman & Hall; 1994. pp. 813–5. [Google Scholar]
- 30.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and Current and emerging treatment strategies. Mol Med. 2011;17(1–2):113–25. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]