Abstract
In Schizosaccharomyces pombe, Ssp2, an ortholog of AMP-activated protein kinase (AMPK), is critical for cell growth at restrictive temperatures and under glucose depletion as well as sexual differentiation under nitrogen depletion. To identify genes genetically related to Ssp2, we performed a genetic screening to search for the genes whose overexpression rescued the growth defects in Δssp2 cells at restrictive temperatures, and identified 35 cosmids as multicopy suppressor genes. In Southern blot analyses, 22 out of these cosmids were hybridized to an ssp2+ probe. Using nucleotide sequencing, we identified the gsk3+ gene in one of the cosmids, and the remaining 12 cosmids were hybridized to a gsk3+ probe. Overexpression of the gsk3+ gene or the gsk31+ gene, another GSK3 member, rescues defective growth of Δssp2 cells at restrictive temperatures and under glucose depletion as well as sexual differentiation under nitrogen depletion. Δgsk3Δgsk31 double knockout cells, but neither Δgsk3 nor Δgsk31 single knockout cells, phenocopy Δssp2 cells. The deletion of the gsk3+ or gsk31+ gene augments the phenotypes of Δssp2 cells. These findings suggest that Gsk3 and Gsk31 are critical and interact with Ssp2 in multiple cellular functions.
Keywords: AMPK, Glycogen synthase kinases, Glucose depletion, Fission yeast,
INTRODUCTION
AMP-activated protein kinase (AMPK) is a highly conserved heterotrimeric (α/β/γ) complex that plays crucial roles in the regulation of energy homeostasis by balancing ATP consumption and generation at both cellular and whole body levels (Mihaylova and Shaw 2011 ; Hardie 2011). Ssp2 is a Schizosaccharomyces pombe (S. pombe) ortholog of α subunit of AMPK in mammalian cells. Previous studies have shown that the cells lacking ssp2+ cannot grow at restrictive temperatures or under substitution of glucose by alternative carbon sources (Matsuzawa et al. 2012). Under glucose or nitrogen depletion, S. pombe CaMKKβ ortholog, Ssp1 or Ppk34, respectively, phosphorylates Ssp2 on its Thr189 residue, thereby leading to its activation and nuclear localization (Davie et al. 2015 ; Valbuena and Moreno 2012). Under nitrogen depletion, Ssp2 activation induces nuclear localization of Ste11, a transcription factor, and consequently leads to sexual differentiation (Valbuena and Moreno 2012). Under glucose depletion, Ssp2 activation induces phosphorylation and nuclear export of Scr1, a transcriptional repressor, and consequently relieves transcription of various metabolizing enzymes from glucose repression (Matsuzawa et al. 2012).
Glycogen synthase kinase 3 (GSK3) is a highly conserved serine/threonine kinase. In mammals, GSK3 is encoded by GSK3α and GSK3β. It was first discovered as a regulatory kinase for glycogen synthase (Embi et al. 1980), and then has been identified to phosphorylate over 40 different substrates for regulating cellular proliferation, migration and glucose regulation (Frame and Cohen 2001 ; Rayasam et al. 2009). In S. pombe, GSK3 orthologs are encoded by the gsk3+/skp1+ gene (SPAC1687.15) and the gsk31+ gene (SPBC8D2.01). Gsk3/Skp1 overexpression suppresses the defective cytokinesis of cdc14 mutant, and gsk3 deletion exhibits defects in heat shock and sporulation (Plyte et al. 1996). It has also been reported that Gsk3 overexpression suppresses defective chromosomal segregation due to mis12 mutation and HIV Vpr2-induced cell death in fission yeast (Goshima et al. 2003 ; Huard et al. 2008). By contrast, the functions of the gsk31+ gene in S. pombe have not been examined.
To identify genes genetically related to Ssp2 for cell growth at restrictive temperatures, we performed a genetic screening to search for the genes whose overexpression rescued the growth defects in Δssp2 cells at restrictive temperatures, and identified the gsk3+ gene as a multicopy suppressor gene. Overexpression of the gsk3+ gene also rescued growth defects in Δssp2 cells under glucose depletion and defective sexual differentiation under nitrogen depletion. Overexpression of the gsk31+ gene also rescued the phenotypes of Δssp2 cells. The loss of the gsk3+ and gsk31+ genes in combination, but not either of these genes, phenocopied Δssp2 cells, suggesting that these GSK3 family members function redundantly. Furthermore, deletion of the gsk3+ or gsk31+ gene alone augmented the phenotypes of Δssp2 cells. Therefore, the present study suggests genetic interactions between Ssp2 and Gsk3 or Gsk31 in multiple cellular functions.
MATERIALS AND METHODS
Yeast strains, growth media, drugs and general methods
The S. pombe strains used in this study are listed in Table I. The complete medium YPD (yeast-extract–peptone–dextrose) and the minimal medium EMM (Edinburgh minimal medium) were described previously (Toda et al. 1996). Sporulation agar (SPA) plates were made as described previously (Smith 2009). Gluconate plates were made based on EMM recipe, except that 3% sodium-D-gluconate (Nacalai) was used instead of 2% D-glucose. EMM plates were supplemented with 225 mg/liter leucine when necessary. Gene disruptions are indicated by gene symbols preceded by Δ (for example, Δssp2). Proteins are denoted by Roman letters with only the first letter capitalized (for example, Ssp2). Database searches were performed using Pombe community database PomBase (http://www.pombase.org).
Table I.
Fission yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| HM123 | h− leu1-32 | Our stock |
| KP207 | h+ his2 leu1-32 | Our stock |
| HM528 | h+ his2 | Our stock |
| KP928 | h+ his2 leu1-32 ura4-D18 | Our stock |
| KP1737 | h− leu1-32 ura4-D18 gsk3::ura4+ gsk31::KanMX6 | Our stock |
| KP1813 | h− leu1-32 ura4-D18 gsk3::ura4+ | Our stock |
| KP2310 | h− leu1-32 ura4-D18 gsk31::KanMX6 | Our stock |
| KP3089 | h− leu1-32 ura4-D18 ssp2::ura4+ | This study |
| KP4275 | h− leu1-32 ura4-D18 ssp2::ura4+ gsk31::KanMX6 ura4+ | This study |
| KP4283 | h− leu1-32 ura4-D18 ssp2::ura4+ gsk3::ura4+ | This study |
Gene deletion
A one-step gene disruption by homologous recombination was performed as previously described (Rothstein 1983). The ssp2::ura4+ disruption was constructed as follows. The genomic DNA including the ssp2+ gene was amplified by polymerase chain reaction (PCR) using the sense primer (1151) 5′-CGG GAT CCA TGC AAC CGC AGG AGG TTG ATT TAA TG-3′, and the antisense primer (1152) 5′-GGA ATT CAT GCA GAA AAT AAC TTG CAC-3′. The BamHI/EcoRI fragment containing ssp2+ was subcloned into the BamHI/EcoRI site of pGEM-7Zf (+) (Promega). Then, a HindIII fragment containing ura4+ was inserted into the HindIII site of the previous construct. The fragment containing the disrupted ssp2+ gene was transformed into the diploid cells. Stable integrants were selected on EMM plates lacking uracil, and disruption of the gene was checked by genomic Southern hybridization (data not shown).
Isolation of the multicopy suppressor genes for the ssp2 deletion using temperature-sensitive growth defect
To screen for dosage-dependent suppressors for the ssp2 deletion, the Δssp2 cells were transformed with a fission yeast genomic DNA library, and the Leu+ transformants were replica-plated onto both YPD plates at 27°C and 37°C. The 35 cosmids that rescued the temperature-sensitive phenotype were recovered from the cells, and were characterized using Southern blotting and nucleotide sequencing, as described in the Results section in details.
Plasmids
For ectopic expression of proteins, we used the thiamine repressible nmt1 promoter (Maundrell 1993). Expression was repressed by the addition of 4 μM thiamine to EMM, and was induced by washing and incubating the cells in EMM lacking thiamine. To avoid excessive expression of exogenous proteins, we used leaky expression by this promoter with 4 μM thiamine in this study. The complete open reading frame (ORF) of ssp2+ gene was amplified by PCR with the genomic DNA of wild-type cells as a template. The sense primer was (3750) 5′-CGG GAT CCT ATG CAA CCG CAG GAG G-3′, and the antisense primer was (3751) 5′-CGG GAT CCT TCA TGC AGA AAA TAA CTT GC-3′. The BamHI fragment containing ssp2+ ORF was subcloned into the BamHI site of the pREP1 vector to construct pREP1-Ssp2 (pKB8182). A plasmid expressing Ssp2T189A, an Ssp2 mutant in which Thr189 critical for its kinase activity is replaced by Ala, was generated using the Quick Change mutagenesis kit (Stratagene, La Jolla, CA, USA). In the amplification reaction for site-directed mutagenesis, the sense primer was (3756) 5′-GGT AAT TTC CTA AAG GCC TCC TGT GGC AGT C-3′, and the antisense primer was (3757) 5′-GAC TGC CAC AGG AGG CCT TTA GGA AAT TAC C-3′.
The plasmid expressing Gsk3 was generated as follows. The cosmid pKB7166 that rescued the temperature-sensitive phenotype of Δssp2 cells was digested with BglII, and subcloned into the BamHI site of a yeast replication plasmid (pKB1037) to construct a sublibrary. A 4.5 kb BglII fragment containing the promoter and ORF of the gsk3+ gene was confirmed to rescue the temperature-sensitive phenotype of Δssp2 cells, and registered as pKB7166 that was used in the present study.
The plasmid expressing Gsk31 was generated as follows. The gsk31+ gene was amplified by PCR with the genomic DNA of wild-type cells as a template. The sense primer was (3976) 5′-CGG GAT CCG AGT TCG GTA TGG AGG AAG-3′ and the antisense primer was (3977) 5′-CGG GAT CCT TAT GAG TCT GCG TCA GCC-3′. The resultant PCR fragment containing the gsk31+ gene containing its promoter and ORF was digested with BamHI and subcloned into the BamHI site of a yeast replication plasmid (pKB1037), and the resulted construct was used to express the gsk31+ gene in fission yeast cells.
Differential interference contrast (DIC) imaging
After overnight culture to log phase, wild-type and mutant cells (h−) were mated with wild-type cells (KP207, h+) on SPA medium, and then incubated at 27°C for 20 hours. Then the resulted cells were diluted on glass slides with distilled water, covered by cover glasses and subjected to microscopic analysis using an Axioskop 2 Plus microscope (Carl Zeiss, Inc., Germany) equipped with an alpha Plan-Fluor 100x/N.A.1.45 oil objective (Carl Zeiss, Inc.). DIC images were taken using a Visualix VTCH1.4ICE digital camera in combination with the ISCapture software version 4.0.1 (Xintu Photonics). The images were processed using Adobe Photoshop CS6 only for illustrative purposes.
RNA extraction and real-time RT-PCR
Total RNA was extracted using the RNeasy Mini kit (Qiagen) with on-column deoxyribonuclease digestion (RNase-Free DNase Set; Qiagen). cDNA was synthesized from the resultant total RNA using the High Capacity cDNA Reverse Transcription Kit (ABI) and subjected to real-time PCR with the SYBR Green PCR Master Mix (ABI). The primers used for real-time PCR are as follows: for ssp2+, 5′-TGC TAG TGC TGT GGA TAC GA-3′ and 5′-TTT GGA ACG GTA AAC TGA GC-3′; for gsk3+, 5′-CAG CCT CTT TCA CGA GTG TT-3′ and 5′-ACA ATT CGG GTA AAT GAC GA-3′; for gsk31+, 5′-TAT GTT CCG TCC AAG CGT AT-3′ and 5′-TCG TAA ACA TGA GGG GGT AA-3′. The primers for act1+ were described previously (Ma et al. 2015). Signals were detected and analyzed as described previously (Ma et al. 2015).
Statistical analyses
Comparison between two groups was analyzed with unpaired t-test. Comparison of more than two groups was analyzed with one-way ANOVA followed by Tukey’s multiple comparison test to evaluate pairwise group differences, unless otherwise noted. The P values less than 0.05 are considered to be significant. Statistical analyses were performed with Prism 6 (GraphPad). Data are shown as mean ± SEM.
RESULTS
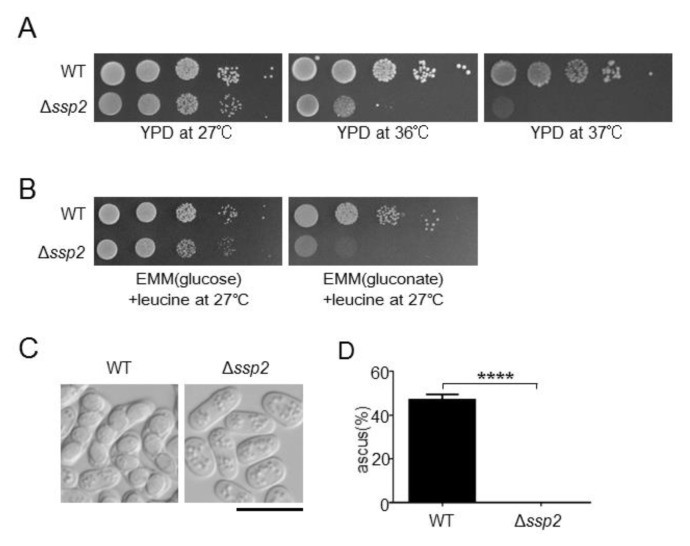
To perform a screening for identifying Ssp2-related genes in S. pombe, we generated Δssp2 cells and examined their phenotypes. First we examined temperature sensitivity of these cells. Wild-type cells and Δssp2 cells were serially diluted by 10 fold and spotted on YPD plate at restrictive temperatures 36°C or 37°C, as well as at permissive temperature 27°C. Both wild-type cells and Δssp2 cells grew normally at 27°C. Whereas wild-type cells grew at both restrictive temperatures, the growth of Δssp2 cells was partially and fully inhibited at 36°C and 37°C, respectively (Figure 1A). Then we examined sensitivity to glucose depletion in these cells. To maintain cell growth of wild-type cells under glucose depletion, we used gluconate as an alternative non-glucose carbon source. Both wild-type cells and Δssp2 cells grew normally in the presence of 2% glucose. By contrast, the growth of Δssp2 cells was markedly inhibited with 3% gluconate instead of glucose (Figure 1B). As previously reported (Matsuzawa et al. 2012 ; Valbuena and Moreno 2012), Δssp2 cells also showed a defect in sexual differentiation under nitrogen depletion (Figures 1C and 1D). After wild-type cells of opposite sexes were mixed and cultured under nitrogen depletion for 20 h, the asci, each of which contained four spores, were observed. By contrast, neither zygotes nor asci were observed in Δssp2 cells mixed with wild-type cells of the opposite sex (Figures 1C and 1D). Notably, overexpression of wild-type ssp2+ rescued defective growth at restrictive temperatures and under glucose depletion in Δssp2 cells (Figures 2A and 2B, + ssp2+) as well as their defective sexual differentiation under nitrogen depletion (Figures 2C and 2D, + ssp2+). These findings confirmed successful generation of Δssp2 cells for subsequent genetic screening.
Figure 1.
The loss of Ssp2 abolishes cell growth at restrictive temperatures and under glucose depletion and causes defective sexual differentiation under nitrogen depletion. (A) Wild-type cells (WT) and Δssp2 cells (KP3089) were cultured to log phase, and adjusted to 0.3 in OD660. The cells were serially diluted by 10 fold and spotted onto YPD plates, and were then incubated at 27°C for 4 days and at 36°C or 37°C for 3 days. Representative images out of 3 independent experiments are shown. (B) Wild-type cells (WT) and Δssp2 cells (KP3089) were prepared as described in Figure 1A, and were spotted onto EMM containing 2% glucose (EMM(glucose)) or EMM with 3% gluconate instead of glucose (EMM(gluconate)). Since the cells in this experiment are leucine auxotrophic, the medium was supplemented with 250 mg/liter leucine (+ leucine). The plates were incubated at 27°C for 4 days onto EMM(glucose) plates or for 6 days onto EMM(gluconate) plates. Representative images out of 3 independent experiments are shown. (C) and (D) Wild-type cells (WT) and Δssp2 cells (KP3089) were prepared as described in Figure 1A, and were mated with wild-type cells of the opposite sex (KP207) on SPA medium for nitrogen depletion and then incubated at 27°C for 20 h. DIC images were taken under light microscopy. Scale bar, 10 μm. The percentage of ascus to the total cell number is averaged from 3 independent experiments, and more than 100 cells were counted in each experiment. ****P<0.0001 compared with wild-type cells by unpaired t-test.
Figure 2.
Overexpression of the ssp2+ gene rescues the phenotypes of Δssp2 cells in a manner dependent on phosphorylation at Thr189 residue. Wild-type cells (WT) transformed with pREP1 vector (+vector) and Δssp2 cells transformed with pREP1 vector (+vector), pREP1-Ssp2 (+ssp2+) or pREP1-Ssp2T189A (+ssp2T189A) were cultured to log phase. (A) The cells were serially diluted by 10 fold, and were spotted and incubated onto YPD plates, as described in Figure 1A. Representative images out of 3 independent experiments are shown. (B) The cells were serially diluted by 10 fold, and were spotted and incubated onto EMM containing 2% glucose (EMM(glucose)) or EMM with 3% gluconate instead of glucose (EMM(gluconate)), as described in Figure 1B. To avoid excessive expression of the transformed genes, the plates were supplemented with 4 μM thiamine. Since the transformed plasmids carry the LEU2 gene, the cells grew on EMM plates in the absence of leucine. Representative images out of 3 independent experiments are shown. (C) and (D) The cells were mated with wild-type cells of the opposite sex (KP207) on SPA medium, and were incubated and analyzed from 3 independent experiments, as described in Figures 1C and 1D. Scale bar, 10 μm. ****P<0.0001 compared with wild-type cells and ####P<0.0001 compared with Δssp2 cells transformed with the vector by one-way ANOVA followed by Tukey’s multiple comparison test.
We then examined whether Ssp2 phosphorylation at Thr189 is involved in cell growth at restrictive temperatures and under glucose depletion. For this purpose, we employed an inactive ssp2 mutant, in which the conserved threonine 189 residue in the activation loop is mutated to alanine (ssp2T189A) (Valbuena and Moreno 2012). Overexpression of ssp2T189A mutant resulted in mRNA expression to the level similar to overexpression of wild-type ssp2+ (data not shown). However, in contrast to wild-type ssp2+, overexpression of ssp2T189A did not rescue cell growth in Δssp2 cells at restrictive temperatures or under glucose depletion (Figures 2A and 2B, + ssp2T189A). This ssp2T189A mutant did not rescue defective sexual differentiation in Δssp2 cells under nitrogen depletion, either (Figures 2C and 2D, + ssp2T189A). These findings suggest an important role of Ssp2 phosphorylation at Thr189 residue.
Using Δssp2 cells generated above, we performed a screen using cosmid library for multicopy suppressors that could rescue the growth defect in Δssp2 cells at the restrictive temperature of 37°C, and isolated 35 cosmids. First, we performed Southern blotting and found that 22 cosmids were hybridized with an ssp2+ probe. Then, we selected one of the remaining 13 cosmids for nucleotide sequencing, and found that this cosmid contained the gsk3+ gene. Using Southern blotting, we found that the remaining 12 cosmids were hybridized with a gsk3+ probe. There are two members of Gsk3 family, namely Gsk3/Skp1 (SPAC1687.15) and Gsk31 (SPBC8D2.01), in S. pombe. Since a high degree of nucleotide sequence homology (more than 50%) exists between these two Gsk3 members, the above 12 cosmids could contain either of the gsk3+ or gsk31+ genes. This prompted us to subclone the gsk3+ and gsk31+ genes and compared their respective complementary abilities. Using real-time RT-PCR, we confirmed that overexpression of the gsk3+ or gsk31+ gene increases respective mRNA levels (data not shown). Overexpression of the gsk3+ or gsk31+ gene similarly rescued the growth defect at the restrictive temperature of 36°C (Figure 3A) and under glucose depletion (Figure 3B). At the restrictive temperature of 37°C, overexpression of the gsk31+ gene rescued the growth defect better than that of the gsk3+ gene. Overexpression of the gsk3+ or gsk31+ gene rescued the defective sexual differentiation of Δssp2 cells, though the recoveries were partial (Figures 3C and 3D).
Figure 3.
Overexpression of the gsk3+ or gsk31+ gene rescues the phenotypes of Δssp2 cells. Wild-type cells (WT) transformed with the control vector (+vector) and Δssp2 cells transformed with the control vector (+vector) or the plasmid containing the gsk3+ gene or the gsk31+ gene were cultured to log phase. (A) The cells were serially diluted by 10 fold, and were spotted and cultured onto YPD plates, as described in Figure 2A. Representative images out of 3 independent experiments are shown. (B) The cells were serially diluted and were spotted onto EMM containing 2% glucose (EMM(glucose)) or EMM containing 3% gluconate instead of glucose (EMM(gluconate)), and incubated as described in Figure 2B. Representative images out of 3 independent experiments are shown. (C) and (D) The cells were mated with wild-type cells of the opposite sex (KP207) on SPA medium, and were incubated and analyzed from 3 independent experiments, as described in Figures. 1C and 1D. Scale bar, 10 μm. ****P<0.0001 compared with wild-type cells and #### P<0.0001 and ##P<0.01 compared with Δssp2 cells transformed with the vector by one-way ANOVA followed by Tukey’s multiple comparison test.
To examine whether Gsk3 and/or Gsk31 are involved in cell growth at restrictive temperatures and under glucose depletion, we examined the phenotypes of Δgsk3 or Δgsk31 single knockout cells as well as Δgsk3Δgsk31 double knockout cells. Δgsk3 cells or Δgsk31 cells appeared to grow normally at restrictive temperatures and under glucose depletion, compared with wild-type cells (Figures 4A and 4B, Δgsk3 and Δgsk31). By contrast, Δgsk3Δgsk31 double knockout cells showed partially defective cell growth at 36°C, and abolished cell growth at 37°C and under glucose depletion (Figures 4A and 4B, Δgsk3Δgsk31). The sexual differentiation under nitrogen depletion was partially defective in Δgsk3 and Δgsk31 single knockout cells and completely defective in Δgsk3Δgsk31 double knockout cells (Figures 4C and 4D). Overexpression of the gsk3+ or gsk31+ gene rescued the growth defect in Δgsk3Δgsk31 double knockout cells at restrictive temperatures and under glucose depletion (Figures 5A and 5B) as well as the defective sexual differentiation under nitrogen depletion (Figures 5C and 5D). These findings suggest that Gsk3 and Gsk31 are redundant but critical for cell growth at restrictive temperatures and under glucose depletion as well as sexual differentiation under nitrogen depletion.
Figure 4.
The loss of Gsk3 and Gsk31 in combination phenocopies Δssp2 cells, and the loss of each of these genes augments the phenotypes of Δssp2 cells. Wild-type cells, Δssp2 cells, Δgsk3 cells and Δgsk31 cells as well as Δgsk3Δssp2, Δgsk31Δssp2 and Δgsk3Δgsk31 double knockout cells were cultured to log phase. (A) The cells were serially diluted by 10 fold, and were spotted and cultured onto YPD plates, as described in Figure 1A. Representative images out of 3 independent experiments are shown. (B) The cells were serially diluted and were spotted onto EMM containing 2% glucose (EMM(glucose)) or EMM containing 3% gluconate instead of glucose (EMM(gluconate)), and incubated as described in Figure 1B. Representative images out of 3 independent experiments are shown. (C) and (D) The cells were mated with wild-type cells of the opposite sex (KP207) on SPA medium, and were incubated and analyzed from 3 independent experiments, as described in Figures 1C and 1D. Scale bar, 10 μm. ****P<0.0001 compared with wild-type cells and ####P<0.0001 compared with Δgsk3Δgsk31 cells by one-way ANOVA followed by Tukey’s multiple comparison test.
Figure 5.
Overexpression of the gsk3+ or gsk31+ gene rescues the phenotypes of Δgsk3Δgsk31 cells. Wild-type cells (WT) transformed with the control vector (+vector) and Δgsk3Δgsk31 cells transformed with the control vector (+vector) or the plasmids expressing the gsk3+ or gsk31+ gene were cultured to log phase. (A) The cells were serially diluted by 10 fold, and were spotted and cultured onto YPD plates, as described in Figure 2A. Representative images out of 3 independent experiments are shown. (B) The cells were serially diluted and were spotted onto EMM containing 2% glucose (EMM(glucose)) or EMM containing 3% gluconate instead of glucose (EMM(gluconate)), and incubated as described in Figure 2B. Representative images out of 3 independent experiments are shown. (C) and (D) The cells were mated with wild-type cells of the opposite sex (KP207) on SPA medium, and were incubated and analyzed from 3 independent experiments, as described in Figures 1C and 1D. Scale bar, 10 μm. ****P<0.0001 compared with wild-type cells and ####P<0.0001 compared with Δgsk3Δgsk31 cells by one-way ANOVA followed by Tukey’s multiple comparison test.
To address the relationship betwee the ssp2+ gene and the gsk3+ or gsk31+ gene, we constructed Δgsk3Δssp2 cells and Δgsk31Δssp2 cells. As described above, Δgsk3 cells or Δgsk31 cells did not show apparent growth defects at restrictive temperatures 36°C or under glucose depletion. By contrast, the growth of Δgsk3Δssp2 cells and Δgsk31Δssp2 cells were completely and partially inhibited at 36°C, and were slower than that of Δssp2 cells (Figure 4A). The growth of Δgsk3Δssp2 cells and Δgsk31Δssp2 cells was similarly inhibited under glucose depletion (Figure 4B), indicating the synthetic growth defect in Δgsk3Δssp2 and Δgsk31Δssp2 cells. We then examined the effect of ssp2+ overexpression in Δgsk3Δgsk31 double knockout cells. Overexpression of the ssp2+ gene failed to rescue the growth defects in Δgsk3Δgsk31 cells at restrictive temperatures or under glucose depletion (Figures 6A and 6B) as well as defective sexual differentiation (Figures 6C and 6D). These findings suggest that Gsk3 and Gsk31 act downstream of or in parallel with Ssp2 in S. pombe.
Figure 6.
Overexpression of the ssp2+ gene fails to rescue the phenotypes of Δgsk3Δgsk31 cells. Wild-type cells (WT) transformed with the control vector (+vector) and Δgsk3Δgsk31 cells transformed with the control vector (+vector) or ssp2+ gene were cultured to log phase. (A) The cells were serially diluted by 10 fold, and were spotted and cultured onto YPD plates, as described in Figure 2A. Representative images out of 3 independent experiments are shown. (B) The cells were serially diluted and were spotted onto EMM containing 2% glucose (EMM(glucose)) or EMM containing 3% gluconate instead of glucose (EMM(gluconate)), and incubated as described in Figure 2B. Representative images out of 3 independent experiments are shown. (C) and (D) The cells were mated with wild-type cells of the opposite sex (KP207) on SPA medium, and were incubated and analyzed from 3 independent experiments, as described in Figures 1C and 1D. Scale bar, 10 μm. ****P<0.0001 compared with wild-type cells by one-way ANOVA followed by Tukey’s multiple comparison test.
DISCUSSION
Previous studies have shown that Ssp2, an S. pombe ortholog of AMPK, plays critical roles in cell growth at restrictive temperatures and under glucose depletion as well as sexual differentiation under nitrogen depletion (Matsuzawa et al. 2012 ; Valbuena and Moreno 2012). To identify molecules genetically related to these Ssp2 functions, we performed genetic screen to isolate the multicopy suppressor genes using temperature sensitive phenotype of Δssp2 cells, and found that overexpression of Gsk3 rescues the growth defect of Δssp2 cells at restrictive temperatures. Overexpression of Gsk31, another member of Gsk3 family, also rescues this phenotype, and the loss of Gsk3 and Gsk31 in combination, but neither the loss of Gsk3 nor Gsk31, causes the defective growth at restrictive temperatures, similarly to the loss of Ssp2. Furthermore, the loss of Gsk3 or Gsk31 augments the defective growth induced by the loss of Ssp2 at restrictive temperatures. Gsk3 and Gsk31 are also critical and genetically interact with Ssp2 for cell growth under glucose depletion and sexual differentiation under nitrogen depletion. Therefore, this study has identified a novel genetic interaction between Ssp2 and Gsk3 or Gsk31 in multiple cellular functions.
In S. pombe, there are two GSK3 members, Gsk3 and Gsk31, and previous studies have suggested that Gsk3 is involved in cell viability after heat shock and nitrogen starvation-induced sporulation (Plyte et al. 1996). The present study has found that loss of Gsk3 or Gsk31 alone partially impairs sexual differentiation under nitrogen depletion, but neither cell growth at restrictive temperatures nor under glucose depletion. Notably, loss of Gsk3 and Gsk31 in combination abolishes cell growth at restrictive temperatures and under glucose depletion as well as sexual differentiation under nitrogen depletion. Therefore, Gsk3 and Gsk31 play redundant roles in these functions, and Gsk3 or Gsk31 alone is sufficient to sustain cell growth at restrictive temperatures and under glucose depletion. We also found that overexpression of Gsk31 rescues the growth defect of Δssp2 cells better than that of Gsk3 at 37°C. By contrast, overexpression of Gsk3 and Gsk31 similarly rescues the growth defect of Δssp2 cells under glucose depletion and the defective sexual differentiation of these cells under nitrogen depletion. Gsk3 and Gsk31 may have partially overlapping substrates with different affinity, or their respective expression may be differently regulated across multiple experimental conditions.
So far, the mechanism underlying the genetic interactions between Ssp2 and Gsk3 or Gsk31 remains unknown. In mammalian cells, AMPK phosphorylates TSC2, a negative regulator for mTOR, at Ser1387, which primes TSC2 phosphorylation by GSK3 and suppresses mTOR activity (Inoki et al. 2006 ; Ma and Blenis 2009). In S. pombe, although neither of these phosphorylation sites is conserved, Ssp2 also suppresses Tor2, mTOR ortholog, through Tsc2 for promoting cell growth with reduced cell size under nitrogen depletion (Davie et al. 2015). However, Tor2 appears not to be involved in the growth defect of Δssp2 cells under glucose depletion, since tor2-287 mutant failed to rescue this phenotype (data not shown). In mammals, a variety of substrates have been identified for AMPK and GSK3 (Rayasam et al. 2009 ; Mihaylova and Shaw 2011). In S. pombe, Ssp2 mediates nitrogen starvation-induced sporulation through Ste11 transcription factor (Valbuena and Moreno 2012), and switches metabolic pathways under glucose depletion through transcription repressor Scr1 (Matsuzawa et al. 2012). Whether Gsk3 or Gsk31 might be involved in these pathways and whether Ssp2, Gsk3 and Gsk31 exert these functions through common substrates remain to be determined. Gsk3 contains LSRVFS sequence, which is the classic AMPK substrate motif (LXRXXS/T), and both Gsk3 and Gsk31 contain the non-classic AMPK substrate motif ΦXXS/TXXXΦ, in which Φ is a hydrophobic residue and X is any amino acid residue. Notably, PInt (Pombe Interactome), a tool that predicts fission yeast protein interactions, suggests a possible physical interaction between Ssp2 and Gsk3 or Gsk31. The possibility that Ssp2 directly interacts with and phosphorylates Gsk3 and Gsk31 also awaits further investigation.
ACKNOWLEDGMENTS
We thank Ms. Misako Takizawa for secretarial assistance. This study was supported by a Grant-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science to Y.M. (No. 26460339).
REFERENCE
- 1.Davie E, Forte GM, Petersen J. Nitrogen regulates AMPK to control TORC1 signaling. Curr Biol. 2015;25(4):445–454. doi: 10.1016/j.cub.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107(2):519–527. [PubMed] [Google Scholar]
- 3.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359(Pt 1):1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goshima G, Iwasaki O, Obuse C, Yanagida M. The role of Ppe1/PP6 phosphatase for equal chromosome segregation in fission yeast kinetochore. EMBO J. 2003;22(11):2752–2763. doi: 10.1093/emboj/cdg266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huard S, Chen M, Burdette KE, Fenyvuesvolgyi C, Yu M, Elder RT, Zhao RY. HIV-1 Vpr-induced cell death in Schizosaccharomyces pombe is reminiscent of apoptosis. Cell Res. 2008;18(9):961–973. doi: 10.1038/cr.2008.272. [DOI] [PubMed] [Google Scholar]
- 7.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 8.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Ma N, Liu Q, Qi Y, Manabe R, Furuyashiki T. Tor Signaling Regulates Transcription of Amino Acid Permeases through a GATA Transcription Factor Gaf1 in Fission Yeast. PLoS One. 2015;10(12):e0144677. doi: 10.1371/journal.pone.0144677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuzawa T, Fujita Y, Tohda H, Takegawa K. Snf1-like protein kinase Ssp2 regulates glucose derepression in Schizosaccharomyces pombe. Eukaryot Cell. 2012;11(2):159–167. doi: 10.1128/EC.05268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plyte SE, Feoktistova A, Burke JD, Woodgett JR, Gould KL. Schizosaccharomyces pombe skp1+ encodes a protein kinase related to mammalian glycogen synthase kinase 3 and complements a cdc14 cytokinesis mutant. Mol Cell Biol. 1996;16(1):179–191. doi: 10.1128/mcb.16.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156(6):885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith GR. Genetic analysis of meiotic recombination in Schizosaccharomyces pombe. Methods Mol Biol. 2009;557:65–76. doi: 10.1007/978-1-59745-527-5_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toda T, Dhut S, Superti-Furga G, Gotoh Y, Nishida E, Sugiura R, Kuno T. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol Cell Biol. 1996;16(12):6752–6764. doi: 10.1128/mcb.16.12.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valbuena N, Moreno S. AMPK phosphorylation by Ssp1 is required for proper sexual differentiation in fission yeast. J Cell Sci. 2012;125(Pt 11):2655–2664. doi: 10.1242/jcs.098533. [DOI] [PubMed] [Google Scholar]