Abstract
Epithelial—mesenchymal transition (EMT) of mammary epithelial cells is important in both normal morphogenesis of mammary glands and metastasis of breast cancer. Cadherin switching from E-cadherin to N-cadherin plays important roles in EMT. We found that cadherin switching is important in bone morphogenetic protein 4 (BMP4)-induced EMT in MCF-10A cells. BMP4 increased the phosphorylation of SMAD proteins in MCF-10A cells. Canonical BMP4 signaling decreased the expression of E-cadherin and disrupted the polarity of the tight junction protein ZO-1 in MCF-10A cells. However, the expression of N-cadherin and SNAI2 was up-regulated in BMP4-treated MCF-10A cells. MCF-10A cells that expressed N-cadherin migrated into type I collagen gels in response to BMP4 when evaluated using three-dimensional culture assays. Thus, active canonical BMP4 signaling is important for the migration and EMT of mammary epithelial cells. Moreover, the decrease in E-cadherin and/or increase in N-cadherin may be required for BMP4-induced migration and EMT.
Keywords: Epithelial mesenchymal transition, Bone morphogenetic protein 4, N-cadherin, Mammary epithelial cell, MCF-10A
Introduction
Bone morphogenetic proteins (BMPs) are a subgroup of the transforming growth factor-β (TGF-β) family and play essential roles in tumor metastasis and normal embryonic developmental processes [1]. BMPs transduce signals through both SMAD-dependent (canonical) and SMAD-independent (non-canonical) pathways [1, 2]. In canonical signal transduction pathways, BMPs facilitate the formation of hetero-oligomeric complexes of BMP receptors. Inside the complex, BMP type II receptors phosphorylate BMP type I receptors. Phosphorylated BMP type I receptors are activated and phosphorylate SMAD1/5/9. Phosphorylated SMAD1/5/9 form a complex with SMAD4, and this SMAD complex enters the nucleus and regulates the expression of BMP downstream genes [3].
TGF-β induces epithelial—mesenchymal transition (EMT) in epithelial cells from various tissues [4]. EMT is an important process that regulates tumor cell metastasis and the development of embryos and organs [5]. Components of epithelial cell polarity such as tight junctions are lost and motility is induced in epithelial cells undergoing EMT [6]. Epithelial cells undergoing EMT are characterized by the loss of cadherin-mediated cell—cell adhesions [6, 5]. Therefore, the regulation of cadherins is important in the EMT process. Down-regulation of E-cadherin is required for EMT of epithelial cells. However, up-regulation of the expression of other types of cadherins is also necessary for EMT [7, 8]. Cadherin switching usually means a switch from expression of E-cadherin to expression of N-cadherin, but it is also able to occur during the cadherin switching process that the expression level of N-cadherin increases in cells without change in the expression level of E-cadherin [7]. Many studies have suggested that cadherin switching (usually from expression of E-cadherin to expression of N-cadherin) is frequently observed in the EMT process and is a fundamental factor in normal development and pathological processes [7, 9]. TGF-β induces motility of MCF-10A cells and cadherin switching (from E-cadherin to N-cadherin) in the cells [10]. However, siRNA-mediated knockdown of N-cadherin inhibits the motility of MCF-10A mediated with TGF-β, and it suggests that the cadherin switching (N-cadherin expression) is required for the motility of MCF-10A [10]. More interestingly, cadherin switching is necessary for BMP signaling-mediated EMT. Cadherin-6B is required for BMP signaling-mediated EMT of neural crest cells, whereas N-cadherin inhibits EMT [8, 11].
TGF-β family signaling is important in both breast tumorigenesis and mammary gland development [12, 13]. The activation both of SMAD-dependent, canonical signaling [12] and SMAD-independent, non-canonical signaling [14] is important in breast tumorigenesis. BMP genes, including BMP4, are expressed during fetal and postnatal mammary gland development [13]. TGF-β family signaling is also important in cadherin switching in breast cancer [15]. However, to date, it has not been reported that BMP4-mediated canonical signaling would induce EMT of mammary epithelial cells and that cadherin switching from E-cadherin to N-cadherin would occur in cells undergoing BMP4-mediated EMT. Therefore, we wanted to test whether BMP4-mediated canonical signaling can regulate the expression level of E-cadherin and N-cadherin in MCF-10A cells to induce EMT as well as whether cadherin switching is important in BMP4-mediated motility of MCF-10A cells.
Materials and methods
Cell culture
MCF-10A cells (ATCC, Manassas, VA, USA) were cultured in DMEM/F12 (Invitrogen, Carlsbad, CA, USA) supplemented with 5 % horse serum (Invitrogen), 0.5 % hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA), 100 ng/ml cholera toxin (Sigma-Aldrich), 10 μg/ml insulin (Sigma-Aldrich), and 20 ng/ml recombinant human epidermal growth factor (Sigma-Aldrich) at 37 °C and 5 % CO2. In some experiments, MCF-10A cells were serum-starved (DMEM/F12 supplemented with 5 % bovine albumin [Sigma-Aldrich]) for 18 h and then treated with 50 ng/ml BMP4 (R&D Systems, Minneapolis, MN, USA).
Western blot analysis
MCF-10A cells were washed three times with ice-cold phosphate-buffered saline (PBS; Invitrogen), and sodium dodecyl sulfate (SDS) buffer (2×) was directly added to the cells. The cell extracts were then completely denatured by boiling at 95 °C for 10 min. Next, proteins were separated by 8 or 10 % SDS-polyacrylamide gel electrophoresis, and Western blotting was carried out using standard procedures. Primary antibodies against phosphorylated SMAD1/5/9 (pSMAD1/5/9, Cell Signaling Technology, Beverly, MA, USA), E-cadherin (BD Biosciences, San Jose, CA, USA), N-cadherin (Invitrogen), SMAD1 (Millipore, Darmstadt, Germany), and α-tubulin (Sigma-Aldrich) were used for immunoblotting. If necessary, band densities of E-cadherin, N-cadherin, or SMAD1 were measured with ImageJ (NIH) and normalized to the control level.
Immunofluorescence
MCF-10A cells were seeded and cultured on fibronectin (Sigma-Aldrich)-coated cover glasses. Cells were fixed with 4 % paraformaldehyde (Electron Microscopy Science, Hatfield, PA, USA) in PBS for 10 min on ice. Then, immunostaining was performed using standard procedures. Primary antibodies against pSMAD1/5/9 (Cell Signaling Technology), E-cadherin (BD Biosciences), N-cadherin (Invitrogen), ZO-1 (Invitrogen), laminin (Sigma-Aldrich), and SNAI2 (Santa Cruz Biotechnology, Dallas, TX, USA) were used. When required, phalloidin-Texas red (Molecular Probes, Carlsbad, CA, USA) was used for ac-tin staining and DRAQ5 (Biostatus, Leicestershire, UK) was used for nucleus staining. Cells were imaged using a Nikon Eclipse TE2000 confocal microscope (Nikon Instruments, Inc., Melville, NY).
Real-time PCR
Real-time PCR was performed to quantitatively determine the messenger RNA level of CDH2 and SNAI2 in MCF-10A. Total RNA was extracted from MCF-10A cells treated with 0 or 50 ng/ml BMP4 for 1 day with TRIzol (Invitrogen), according to the manufacturer’s protocol. Five micrograms of total RNA was used for single-strand cDNA synthesis using Superscript First-Strand cDNA Synthesis System (Invitrogen) according to the manufacturer’s instructions. Then, quantitative RT-PCR was performed using Power SYBR Green PCR Master Mix (Invitrogen). Human ribosomal protein S9 (RPS9) gene was used as an endogenous control. The following primers were used to detect the expression of CDH2 (a gene of human N-cadherin), SNAI2,and RPS9: CDH2 (sense), 5′-CGGGTAATCCTCCAA AATCA-3′, CDH2 (antisense), 5′-CTTTATCCCGGCCTTTCATC-3′; SNAI2 (sense), 5′-AGATGCATATTCGGACCCAC-3′, SNAI2 (antisense), 5′-CTTCATGTTTGTGCAGGACA-3′; and RPS9 (sense), 5′-CTG ACGCTTGATGAGAAGGAC-3′, and RPS9 (anti-sense), 5′-CAGCTTCATCTTGCCCTCAT-3′.
Three-dimensional cell culture assay
Type I collagen matrix solution was prepared as a mixture of type I collagen solution (8.4 mg/ml, BD Biosciences), reconstitution buffer (2.2 g NaHCO3 and 200 mM HEPES in 0.05 N NaOH), PBS (5×), and5 mMCaCl2 (5:1:2:2). When required, 200 ng/ml of BMP4 was added to the matrix solution. Then, the type I collagen matrix solution was dispensed in 12-mm Millicells (Millipore, Billerica, MA, USA) and incubated overnight at 37 °C and 5 % CO2. MCF-10A cells were seeded at over-confluent density onto the gels and cultured for 6 days. The culture medium for the BMP4-treated group was supplemented with 200 ng/ml of BMP4. The medium was exchanged every 2 days. For the preparation of cryosections, gels were fixed with 4 % paraformaldehyde in PBS for 15 min on ice, embedded into OCT compound (Tissue-Tek), and sectioned at 10 μm thickness. Then, immunostaining was performed using standard procedures. Cells were imaged using a Nikon Eclipse TE2000 confocal microscope.
Results
BMP4 activates canonical signaling in MCF-10A cells
It is known that mammary epithelial cells express BMP receptors and SMAD molecules [16]. Thus, we examined whether BMP4 can activate canonical BMP signaling in MCF-10A cells, which are normal mammary epithelial cells. Phosphorylation of SMAD1/5/9 started to occur at 10 min after BMP4 treatment (Fig. 1a). However, the phosphorylation of SMAD1/5/9 gradually decreased within 1h(Fig. 1a) and was slightly maintained 24 h after BMP4 treatment (Fig. 1b) with no change in the expression level of SMAD1 (Fig. 1b, c), which means BMP4 treatment did not induce a mechanism of feedback regulation via the regulation of SMAD expression level. Thus, canonical BMP signaling is activated in MCF-10A cells by treatment with BMP4.
Fig. 1.
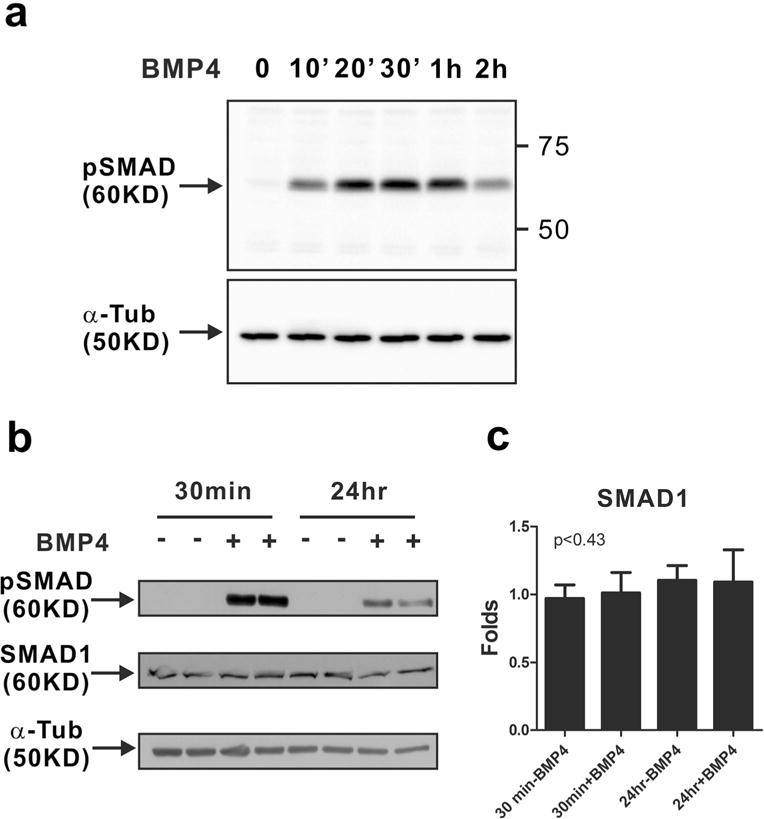
Activation of canonical BMP signaling in MCF-10A cells in response to BMP4. MCF-10A cells were treated with BMP4 (50 ng/ml) after serum starvation. Western blot analysis of phosphorylated SMAD1/5/9 (pSMAD) was performed. a The phosphorylation of SMAD1/5/9 (pSMAD) was detectable 10 min after BMP4 treatment. Alpha-tubulin (α-Tub) was used as an internal control in Western blots of phosphorylated SMAD1/5/9. b The phosphorylation of SMAD1/5/9 was observed 24 h after BMP4 treatment as well as 30 min. Western blot analysis of phosphorylated SMAD1/5/9 (pSMAD) and SMAD1 was performed. α-Tub was used as an internal control in the western blot. c The band intensity of SMAD1 was quantified by ImageJ and normalized to α-tubulin levels. The graph shows fold increase as compared with the levels in the non-treated control group (30 min without BMP4 treatment). Results are shown in mean±SD, and p value was calculated with one-way ANOVA
BMP4 induces EMT in MCF-10A cells
BMP signaling plays important roles in the morphogenesis of mammary glands mediated by the EMT process [13]. Moreover, it has also been suggested that BMP signaling enhances metastasis of breast cancer [17]. Loss of E-cadherin and depolarization of tight junction proteins such as ZO-1 are characteristic for EMT [6]. We found that E-cadherin expression was significantly decreased in cells in which canonical BMP signaling was activated following BMP4 treatment (Fig. 2a, arrows) and the percentage of E-cadherin negative cells increased 5-fold with BMP4 treatment as compared to the control group (Fig. 2b). We also investigated the subcellular localization of ZO-1. We found that ZO-1 was localized to the cell membrane in MCF-10A cells. However, BMP4 induced the redistribution of ZO-1 from the plasma membrane to the cytoplasm (Fig. 2c, arrows). Therefore, these results suggest that BMP4-mediated activation of canonical BMP signaling induces EMT in MCF-10A cells.
Fig. 2.
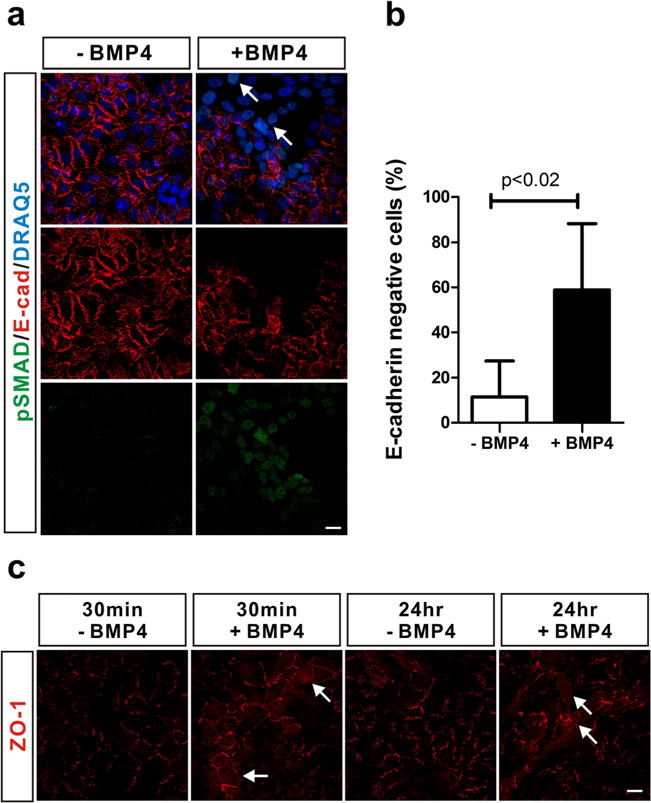
Induction of EMT in MCF-10A cells in response to BMP4. a BMP4 reduced the expression level of E-cadherin in MCF-10A cells. MCF-10A cells were treated with BMP4 (50 ng/ml) for 24 h after serum starvation. Phosphorylated SMAD1/5/9 were stained in green (pSMAD), and E-cadherin was stained in red (E-cad). Nuclei were stained in blue by DRAQ5. White arrows indicate E-cadherin negative cells. b To statistically analyze the percentage of E-cadherin negative cells, more than 323 cells were counted in each experimental group after immunostaining against E-cadherin. Results are shown in mean±SD, and p value was calculated with Student’s t test. c BMP4 induced the depolarization of ZO-1. MCF-10A cells were treated with BMP4 (50 ng/ml) for 30 min or 24 h after serum starvation. ZO-1 was stained in red. White arrows indicate cells in which ZO-1 is depolarized. Scale bars=25 μm
BMP4 induces cadherin switching from E-cadherin to N-cadherin in MCF-10A cells
We investigated BMP4-induced EMT in MCF-10A cells. E-cadherin expression was down-regulated in cells undergoing EMT. Cadherin switching from E-cadherin to N-cadherin is frequently observed during EMT [7]. Thus, we examined the N-cadherin expression in MCF-10A cells treated with BMP4 for up to 24 h. The activation of canonical BMP signaling was observed after BMP4 treatment (Fig. 3a). BMP4 treatment slightly decreased E-cadherin expression 24 h after BMP4 treatment, whereas N-cadherin expression increased by BMP4 treatment (Fig. 3a). Moreover, BMP4 treatment also increased messenger RNA (mRNA) levels of both CDH2, a gene of human N-cadherin (Fig. 3b) and SNAI2, one of EMT markers (Fig. 3c). These results show that canonical BMP4 signaling might induce cadherin switching from E-cadherin to N-cadherin in MCF-10A cells undergoing EMT.
Fig. 3.
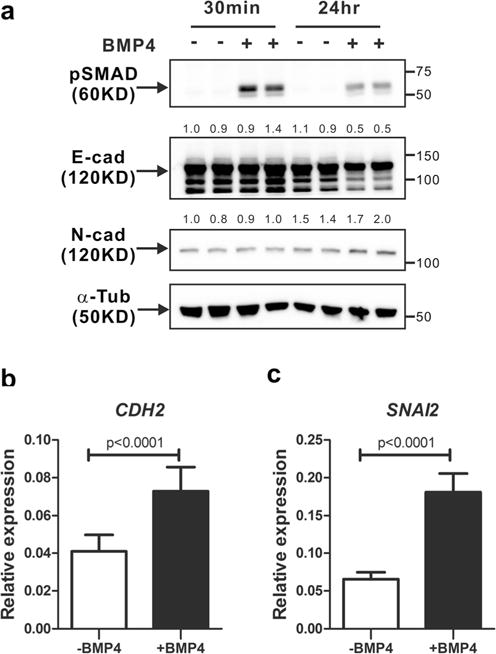
Cadherin switching in MCF-10A cells in response to BMP4. a MCF-10A cells were treated with BMP4 (50 ng/ml) for 30 min or 24 h after serum starvation. Western blot analysis of phosphorylated SMAD1/5/9 (pSMAD), E-cadherin (E-cad), and N-cadherin (N-cad) was performed. Alpha-tubulin (α-Tub) was used as an internal control in the western blot. Quantification of E-cadherin gel bands or N-cadherin was normalized to the alpha-tubulin controls. The numbers indicate values relative to the control (30 min without BMP4 treatment). b, c MCF-10A cells were treated with BMP4 (50 ng/ml) for 24 h after serum starvation and quantitative real-time PCR analysis was performed. The graphs show relative expression levels of CDH2 mRNA (b) and SNM2 mRNA (c). Results are shown in mean±SD, and p values were calculated with Student’s t test
BMP4 induces the migration of MCF-10A cells that undergo cadherin switching
Cadherin switching has been clearly observed in both metastasis of tumor cells and migration of cells during normal developmental processes, e.g., neural crest migration [7]. To examine whether migration of MCF-10A cells is induced by N-cadherin in response to BMP4 treatment, we performed a three-dimensional migration assay on type I collagen gels (Fig. 4a). Interestingly, MCF-10A cells migrated into type I collagen gels following BMP4 treatment (Fig. 4b–f). No cells migrate into type I collagen gels without BMP4 treatment, but ~8 % of cells migrated into the gels with BMP4 treatment (Fig. 4b). Phosphorylation of SMAD1/5/9 proteins was detected in BMP4-treated cells (Fig. 4c). BMP4 treatment induced the expression of SNAI2, which is a master transcription factor to induce EMT [18] (Fig. 4d). The expression of laminin, another marker of EMT [19], was significantly increased in migrating cells (Fig. 4e). The localization of ZO-1 changed in migrating cells. Compared to the control cells, ZO-1 was scattered over the entire cytoplasm in BMP4-treated cells (Fig. 4f). These results are in very good agreement with those shown in Fig. 2b. None of the migrating MCF-10A cells expressed E-cadherin (Fig. 4e, red). However, migrating MCF-10A cells expressed N-cadherin (Fig. 4c, red). Overall, these results suggest that MCF-10A cells undergo cadherin switching and EMT in response to BMP4 and that N-cadherin expression is important in BMP4-mediated migration of MCF-10A cells.
Fig. 4.
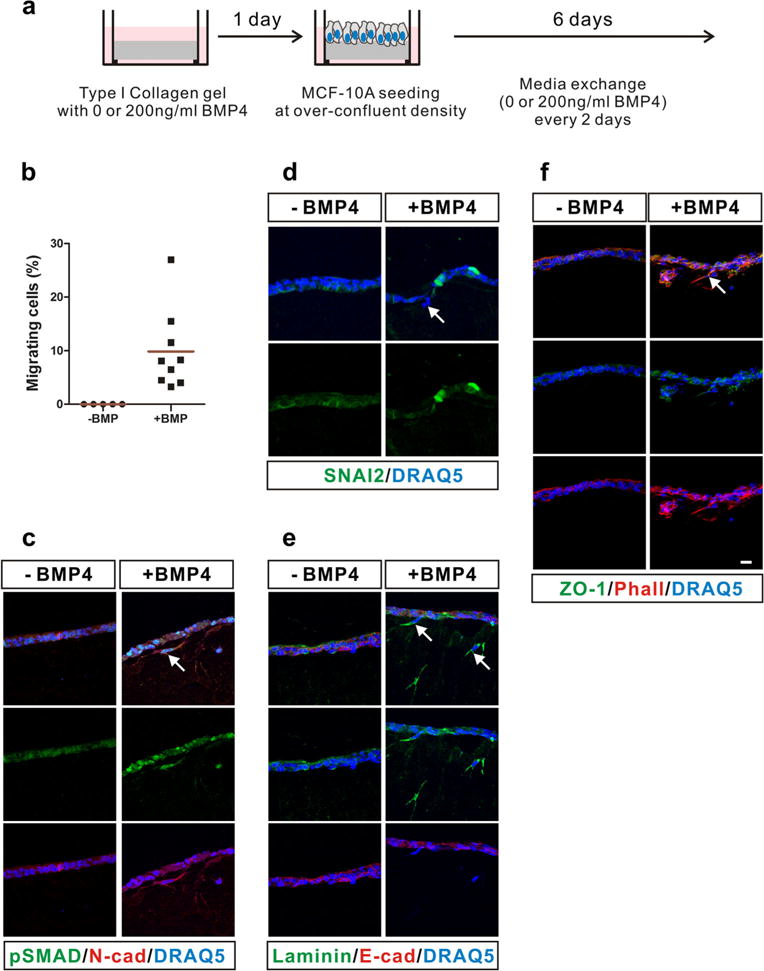
Motility of MCF-10A cells expressing N-cadherin in response to BMP4. a Scheme of the three-dimensional culture of MCF-10A cells on type I collagen gels. MCF-10A cells were treated with BMP4 (200 ng/ml) every 2 for 6 days. b The percentage of the migrating MCF-10A cells is shown. More than 229 cells were counted, and red lines show mean values. c–f Immunostaining was performed with cryosections of the three-dimensional culture of MCF-10A cells. c Phosphorylated SMAD1/5/9 (pSMAD, green), N-cadherin (N-cad, red). d SNAI2 (green). e Laminin (green), E-cadherin (E-cad, red). f ZO-1 (green), actin staining with phalloidin (Phall, red). White arrows indicate migrating cells in response to BMP4. Nuclei were stained blue with DRAQ5. Scale bar=25 μm
Discussion
To our knowledge, our findings are the first to demonstrate that canonical BMP4 signaling induces EMT in MCF-10A cells (a non-transformed breast epithelial cell line) and increases the expression of N-cadherin, which contributes to the motility of MCF-10A cells induced with BMP4. It is known that BMP4 is expressed in mammary epithelial cells undergoing EMT during mammary gland development [13] and that phosphorylation of SMAD1/5/9 was found in patients with breast cancer bone metastasis [17]. BMPs activate canonical BMP signaling in normal and tumor mammary epithelial cells [20], and the canonical BMP signaling mediates migration of normal mammary epithelial cells and invasion of tumor mammary epithelial cells [17, 20]. Moreover, the canonical BMP signaling regulates the cellular proliferation both of normal mammary epithelial cells including MCF-10A cells and tumor mammary epithelial cells [21, 22]. However, it is not well understood whether cadherin switching contributes to the biological processes that are mediated via BMP signaling.
Here, we showed that the expression of E-cadherin is reduced in MCF-10A cells in response to canonical BMP signaling activated by BMP4. In contrast, the expression of N-cadherin is increased in MCF-10A cells following BMP4 treatment. Moreover, BMP4 induces the motility of MCF-10A cells that express N-cadherin. These results suggest that cadherin switching is important for the motility of mammary epithelial cells during normal mammary gland development and metastasis of breast cancer in response to canonical BMP signaling.
Snail superfamily members (e.g., SNAI1 and SNAI2) and TWIST1 have been shown to play a critical role(s) in EMT [18]. It has been well documented that BMP signaling induces EMT following the induction of SNAI2 during various biological processes, e.g., the migration of neural crest cells during normal embryonic development [23]. SNAI2 is a zinc-finger transcription factor that mediates the transcriptional repression of E-cadherin to induce EMT [24]. Although N-cadherin expression is down-regulated by both transcription-dependent [25] and transcription-independent [26] mechanisms, Mani et al. found that N-cadherin expression decreased in SNAI2-expressing cells [27]. We found that BMP4-mediated activation of canonical BMP signaling induced cadherin switching (N-cadherin from E-cadherin) in MCF-10A cells. What is the underlying mechanism(s) of cadherin switching in these cells? Activation of canonical BMP signaling also induces the expression of SNAI2 in MCF-10A cells undergoing EMT. In turn, up-regulation of SNAI2 expression might induce cadherin switching in these cells (inhibition of E-cadherin expression and induction of N-cadherin). However, it is also possible that the stability of N-cadherin proteins is regulated by BMP type I/II receptors activated by a BMP4 ligand through an interaction between BMP type I/II receptors and N-cadherin. For example, N-cadherin inhibits fibroblast growth factor 2 (FGF-2) ligand-mediated internalization of FGF receptors through direct interaction between N-cadherin and FGF receptors, resulting in increased stability of FGF receptors [28]. In contrast, BMP4 stimulates destabilization of N-cadherin via an ADAM-10-dependent mechanism [29]. It would be worthwhile to study whether a BMP4 ligand initiates the interaction between BMP type I/II receptors and N-cadherin, resulting in the stabilization of N-cadherin in MCF-10A cells.
The polarized distribution and/or expression of tight junction proteins such as ZO-1, claudins, and occludin is lost in epithelial cells undergoing EMT and SNAI2 plays an important role(s) in the regulation of tight junctions during EMT [30]. The expression of claudins and occludin is inhibited and ZO-1 translocates via SNAI2 [30]. We found that BMP4 treatment induced the depolarization of ZO-1 in MCF-10A cells after 30 min as well as 24 h. This finding suggests that BMP4 is able to induce depolarization of ZO-1 independent of the transcriptional activation of EMT mediators. The rapid depolarization of ZO-1 could be explained by the BMP4 ligand-mediated activation of BMP type I/II receptors, which destabilizes polarized tight junction proteins and cell polarity proteins such as Par6. A recent study showed that TGF-β-mediated activation of type II receptors directly results in phosphorylation of Par6, which in turn forms a complex with Smurf1 in TGF-β-dependent EMT. The Par6/Smurf1 complex induces depolarization of tight junctions through localized degradation of RhoA [4]. It would be interesting to further investigate whether BMP4-mediated activation of BMP type I/II receptors mediates the disruption of the polarity of ZO-1.
We also found that only MCF-10A cells that expressed N-cadherin migrated into type I collagen gels in response to BMP4. These results suggest that N-cadherin mediates the cellular motility of MCF-10A cells. It has been shown that N-cadherin promotes cell motility of breast cancer cells through FGF receptor signaling, regardless of E-cadherin expression [31, 32]. It has also been reported that N-cadherin enhances breast cancer metastasis via ERK MAPK signaling [33]. We observed that ERK MAPK was activated in MCF-10A cells in response to BMP4 treatment (data not shown). It would be very important and interesting to study the mechanism(s) of N-cadherin-mediated migration of MCF-10A cells in further studies.
Acknowledgments
This work was financially supported by the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1479 to Ki-Sook Park), the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2012R1A1A2042265 to Ki-Sook Park), a grant from Kyung Hee University in 2014 (KHU-20140698 to Ki-Sook Park), and National Institutes of Health grant R01 (GM-28140 to Barry Gumbiner).
Footnotes
Conflicts of interest None
Contributor Information
Ki-Sook Park, Email: kisookpark@khu.ac.kr, East-West Medical Research Institute/College of Medicine, Kyung Hee University, Seoul 130-701, Korea.
Maria Jose Dubon, Graduate School of Biotechnology, Kyung Hee University, Yong-In 446-701, Korea.
Barry M. Gumbiner, Department of Cell Biology, University of Virginia School of Medicine, Charlottesville, VA 22908, USA
References
- 1.Massague J. TGFbeta in Cancer. Cell. 2008;134(2):215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 4.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307(5715):1603–9. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 7.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121(Pt 6):727–35. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 8.Park KS, Gumbiner BM. Cadherin 6B induces BMP signaling and de-epithelialization during the epithelial mesenchymal transition of the neural crest. Development. 2010;137(16):2691–701. doi: 10.1242/dev.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schafer G, Narasimha M, Vogelsang E, Leptin M. Cadherin switching during the formation and differentiation of the Drosophila mesoderm: implications for epithelial mesenchymal transitions. J Cell Sci. 2014;127(Pt 7):1511–22. doi: 10.1242/jcs.139485. [DOI] [PubMed] [Google Scholar]
- 10.Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118(Pt 5):873–87. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- 11.Liu JP, Jessell TM. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development. 1998;125(24):5055–67. doi: 10.1242/dev.125.24.5055. [DOI] [PubMed] [Google Scholar]
- 12.Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA. 2005;102(39):13909–14. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, et al. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122(9):2729–37. doi: 10.1242/dev.122.9.2729. [DOI] [PubMed] [Google Scholar]
- 14.Pal A, Huang W, Li X, Toy KA, Nikolovska-Coleska Z, Kleer CG. CCN6 modulates BMP signaling via the Smad-independent TAK1/p38 pathway, acting to suppress metastasis of breast cancer. Cancer Res. 2012;72(18):4818–28. doi: 10.1158/0008-5472.CAN-12-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews JL, Kim AC, Hens JR. The role and function of cadherins in the mammary gland. Breast Cancer Res. 2012;14(1):203. doi: 10.1186/bcr3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi M, Otsuka F, Miyoshi T, Otani H, Goto J, Yamashita M, et al. Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 mitogen-activated protein kinase activation. J Endocrinol. 2008;199(3):445–55. doi: 10.1677/JOE-08-0226. [DOI] [PubMed] [Google Scholar]
- 17.Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27(49):6322–33. doi: 10.1038/onc.2008.232. [DOI] [PubMed] [Google Scholar]
- 18.Nieto MA. The snail superfamily ofzinc-fingertranscriptionfactors. Nat Rev Mol Cell Biol. 2002;3(3):155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 19.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatza CE, Elderbroom JL, Oh SY, Starr MD, Nixon AB, Blobe GC. The balance of cell surface and soluble type III TGF-beta receptor regulates BMP signaling in normal and cancerous mammary epithelial cells. Neoplasia. 2014;16(6):489–500. doi: 10.1016/j.neo.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montesano R. Bone morphogenetic protein-4 abrogates lumen formation by mammary epithelial cells and promotes invasive growth. Biochem Biophys Res Commun. 2007;353(3):817–22. doi: 10.1016/j.bbrc.2006.12.109. [DOI] [PubMed] [Google Scholar]
- 22.Ampuja M, Jokimaki R, Juuti-Uusitalo K, Rodriguez-Martinez A, Alarmo EL, Kallioniemi A. BMP4 inhibits the proliferation of breast cancer cells and induces an MMP-dependent migratory phenotype in MDA-MB-231 cells in 3D environment. BMC Cancer. 2013;13:429. doi: 10.1186/1471-2407-13-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sela-Donenfeld D, Kalcheim C. Regulation of the onset of neural crest migration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development. 1999;126(21):4749–62. doi: 10.1242/dev.126.21.4749. [DOI] [PubMed] [Google Scholar]
- 24.Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116(Pt 3):499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- 25.Alexander NR, Tran NL, Rekapally H, Summers CE, Glackin C, Heimark RL. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res. 2006;66(7):3365–9. doi: 10.1158/0008-5472.CAN-05-3401. [DOI] [PubMed] [Google Scholar]
- 26.Palma-Nicolas JP, Lopez-Colome AM. Thrombin induces slug-mediated E-cadherin transcriptional repression and the parallel up-regulation of N-cadherin by a transcription-independent mechanism in RPE cells. J Cell Physiol. 2013;228(3):581–9. doi: 10.1002/jcp.24165. [DOI] [PubMed] [Google Scholar]
- 27.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells withproperties of stem cells. Cell. 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2(4):301–14. doi: 10.1016/s1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 29.Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delam-ination. Development. 2007;134(3):491–501. doi: 10.1242/dev.02742. [DOI] [PubMed] [Google Scholar]
- 30.Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116(Pt 10):1959–67. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- 31.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147(3):631–44. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148(4):779–90. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hulit J, Suyama K, Chung S, Keren R, Agiostratidou G, Shan W, et al. N-cadherin signaling potentiates mammary tumor metastasis via enhanced extracellular signal-regulated kinase activation. Cancer Res. 2007;67(7):3106–16. doi: 10.1158/0008-5472.CAN-06-3401. [DOI] [PubMed] [Google Scholar]


