Abstract
To determine the association of weight loss with risk of clinical fractures at the hip, spine and pelvis (central body fractures [CBF]) in older men with and without accounting for the competing risk of mortality, we used data from 4,523 men (mean age 77.5 years). Weight change between baseline and follow-up (mean 4.5 years between examinations) was categorized as moderate loss (loss ≥10%), mild loss (loss 5% to <10%), stable (<5% change) or gain (gain ≥5%). Participants were contacted every 4 months after the follow-up examination to ascertain vital status (deaths verified by death certificates) and ask about fractures (confirmed by radiographic reports). Absolute probability of CBF by weight change category was estimated using traditional Kaplan-Meier method and cumulative incidence function accounting for competing mortality risk. Risk of CBF by weight change category was determined using conventional Cox proportional hazards regression and subdistribution hazards models with death as a competing risk. During an average of 8 years, 337 men (7.5%) experienced CBF and 1,569 (34.7%) died before experiencing this outcome. Among men with moderate weight loss, CBF probability was 6.8% at 5 years and 16.9% at 10 years using Kaplan-Meier vs. 5.7% at 5 years and 10.2% at 10 years using a competing risk approach. Men with moderate weight loss compared with those with stable weight had a 1.6-fold higher adjusted risk of CBF (HR 1.59, 95% CI 1.06–2.38) using Cox models that was substantially attenuated in models accounting for competing mortality risk and no longer significant (subdistribution HR 1.16, 95% CI 0.77–1.75). Results were similar in analyses substituting hip fracture for CBF. Older men with weight loss who survive are at increased risk of CBF, including hip fracture. However, ignoring the competing mortality risk among men with weight loss substantially overestimates their longterm fracture probability and relative fracture risk.
Keywords: central body fractures, weight change, mortality, older men
INTRODUCTION
Prospective studies have reported an independent association between weight loss and an increased risk of clinical fractures at the hip, spine and pelvis (central body fractures) in postmenopausal and older women(1–6), but less is known about weight loss late in life and fracture risk in men. Longitudinal studies(7–10) in older men that have measured concurrent changes in weight and hip bone mineral density (BMD) have reported higher rates of bone loss in men losing weight. One study that investigated the association between weight change since middle adulthood and risk of hip fracture in older men(11) noted that weight loss of 10% of more increased hip fracture risk, while weight gain of 10% or more decreased the risk of hip fracture.
Previous studies examining the association of weight loss with fracture outcomes in older adults have utilized traditional Cox proportional hazards regression models to analyze the association of weight change with fracture. These approaches treat mortality as an uninformative censoring event and assume that subjects censored due to death are representative of those still at risk of fracture at that point in time and have the same distribution of time-to-fracture as subjects who experience fracture. However, ignoring mortality may not provide accurate estimates of risk of fracture because weight loss in older adults strongly predicts death(12–15), making mortality a competing risk.(16)
To investigate the impact of the competing risk of death on absolute probability and adjusted risk of central body fractures in older men with weight loss late in life, we used data from 4523 men enrolled in the Osteoporotic Fractures in Men (MrOS) study with measures of weight change and subsequent follow-up for fractures and mortality.
METHODS
Study Population
A total of 5,994 men ≥65 years old were enrolled from 2000 to 2002 in the prospective Osteoporotic Fractures in Men (MrOS) study.(17) Participants were recruited from population-based listings in six regions of the United States.(18) A history of bilateral hip replacement or the inability to walk without the assistance of another person excluded individuals from study participation. The institutional review board at each participating institution approved the study protocol and written informed consent was obtained from all participants. This analysis is limited to 4,523 men who completed both baseline and a follow-up (2nd) examination and had body weight measured at both examinations (Supplemental Figure 1).
Measurement of Weight Change
Body weight (in indoor clothing with shoes removed) was recorded with a scale (calibrated every month) at both baseline and 2nd examinations (mean (SD) 4.5 (0.4) years between examinations). Weight change was calculated by subtracting baseline weight from 2nd examination weight and expressed as a percentage of the baseline value. Weight change was categorized as moderate weight loss (loss ≥10%), mild weight loss (loss 5% to <10%), stable weight (<5% loss or gain) or weight gain (gain ≥5%) based on current beliefs about clinically relevant weight changes in older adults and availability of sufficient numbers of participants and fractures in each category.
Ascertainment of Incident Central Body Fractures and Mortality
Participants were contacted every 4 months after the 2nd examination (99% of follow-up contacts completed) to ascertain vital status and ask about fractures. Self-reported fracture events including fractures of the hip and pelvis were confirmed by radiographic reports.(19) For any self-reported spine fracture, radiographic reports and a copy of the community spinal imaging study (x-rays, CT and/or MRI studies) were obtained. Clinical vertebral fractures were confirmed by the study radiologist who used the semi-quantitative method of Genant(20) to establish that the community imaging study showed a new deformity of higher grade than was present in the same vertebra on the baseline study film. The primary fracture outcome of interest was central body fracture (clinical fracture at the hip, spine or pelvis).(2) Deaths were verified with death certificates. The mean (SD) follow-up after the 2nd examination (baseline for this analysis) was 7.9 (2.9) years for central body fracture and 8.1 (2.8) years for mortality.
Other Measurements
At the 2nd examination, participants completed a questionnaire and were interviewed and asked about smoking status; history of selected medical conditions (i.e. stroke, diabetes, hyperthyroidism, hypothyroidism, Parkinsonism, myocardial infarction, congestive heart failure, chronic obstructive pulmonary disease, non-skin cancer, liver disease, and renal disease); intention to lose weight (whether or not the participant was trying to lose weight in the past 12 months); and falls in the past year. A multimorbidity score was calculated (potential range 0–11) by summing up the self-reported selected medical conditions. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE).(21) Gait speed (time in seconds to walk 6 meters at usual pace [m/s]) was measured. Weight and height (Harpenden stadiometer) measurements were used to calculate body mass index (BMI, kg/m2). Femoral neck bone mineral density (BMD) was measured with dual x-ray absorptiometry (DXA, QDR 4500W, Hologic, Inc., Bedford, MA) using standardized protocols.(8) Information regarding date of birth and race/ethnicity was collected at the baseline examination.
Statistical Analysis
Characteristics of the 4523 participants at the 2nd examination were compared across the four weight change categories using chi-square tests for categorical variables, ANOVA for continuous variables and Kruskal-Wallis non-parametric tests for skewed variables.
We used restricted cubic splines to determine whether the association between weight change and risk of central body fracture was nonlinear. To allow for a wide range of nonlinear functional forms, we chose a spline model with 5 knots placed at the 5th, 25th, 50th, 75th and 95th percentiles of percent weight change. We then plotted the spline curve and 95% confidence interval for the hazards ratio of central body fracture vs. percent weight change. This graph suggested the presence of a non-linear association between weight change and risk of central body fracture confirming that it was appropriate to express weight change as a categorical variable.
To estimate the absolute probability of central body fracture during follow-up by weight change category, we used two approaches: 1) calculating 1-KM where KM is the traditional Kaplan-Meier (KM) survival function that treats mortality as a censored observation and 2) estimating the cumulative incidence function that considers mortality as a competing risk.(22) In addition, the absolute probability of hip fracture during follow-up by weight change category was estimated using traditional survival analysis and the competing risk approach.
To determine adjusted associations of moderate weight loss, mild weight loss and weight gain with risk of central body fracture after the 2nd examination (referent group stable weight), we used conventional Cox proportional hazards regression models that treat mortality as uninformative censoring and subdistribution hazards models proposed by Fine and Gray(23) that consider death as a competing risk. In Fine-Gray subdistribution models, men who died prior to experiencing a central body fracture are not censored. Death is treated as an informative competing event and those who died remain in the risk set after the event contributing person-time until the end of follow-up to allow comparisons between those with central body fractures, those with fracture-free survival and those who died. Subdistribution hazards ratio tend to be smaller in magnitude and more conservative than those from traditional Cox proportional regression since those with the competing event are usually more similar to those with the outcome of interest than those who are event-free.
Using both approaches, associations were initially adjusted for age alone and then further adjusted for, race, smoking, and multimorbidity. Subsequently, potential mediators including physical activity, fall history, gait speed, and femoral neck BMD were added one at a time to the model. We also performed analyses stratifying participants by characteristics measured at the 2nd examination, including BMI (<26.8 kg/m2 (median) vs. ≥26.8 kg/m2) and intention to lose weight (trying vs. not trying to lose weight) and tested for interactions between weight change and these variables. In secondary analyses, Cox proportional hazards models and subdistribution hazards models were used to estimate age-adjusted associations of moderate weight loss, mild weight loss and weight gain with risk of individual fracture types.
RESULTS
Among the 4,523 men who comprised the analytical cohort, mean (SD) participant age at the 2nd examination was 77.5 (5.5) years) and mean (SD) percent weight change between baseline and 2nd examination was −1.6 (5.5). A total of 283 men (6.3%) had moderate weight loss (loss ≥10%), 742 (16.4%) had mild weight loss (loss 5% to <10%), 3,076 (68.0%) had stable weight and 422 (9.3%) had weight gain. Characteristics of the cohort overall and by category of weight change are shown in Table 1.
Table 1.
Characteristics of 4523 Men by Category of Weight Change
| Overall | Weight Loss ≥10% |
Weight Loss 5% to <10% |
Stable Weight (Loss or Gain <5%) |
Weight Gain ≥5% |
p-value | |
|---|---|---|---|---|---|---|
| Characteristic | (N=4,523) | (N=283) | (N=742) | (N=3,076) | (N=422) | p-value |
| Age, yrs, mean (SD) | 77.5 (5.5) | 78.9 (5.8) | 78.9 (5.5) | 77.2 (5.4) | 76.1 (5.2) | <0.001 |
| Caucasian race, n (%) | 4154 (91.8) | 265 (93.6) | 691 (93.1) | 2813 (91.5) | 385 (91.2) | 0.30 |
| Trying to lose weight past 12 months, n (%) | 1599 (35.4) | 87 (30.7) | 217 (29.3) | 1088 (35.4) | 207 (49.1) | <0.001 |
| Current smoker, n (%) | 97 (2.2) | 11 (3.9) | 26 (3.5) | 46 (1.5) | 14 (3.3) | <0.001 |
| Multimorbidity score (0–11)*, mean (SD) | 0.9 (1.0) | 1.4 (1.1) | 1.0 (1.1) | 0.9 (0.9) | 1.0 (1.0) | <0.001 |
| PASE score, mean (SD) | 135.6 (68.8) | 114.0 (65.0) | 131.6 (69.4 | 139.9 (69.0) | 125.7 (64.5) | <0.001 |
| Fall history, n (%) | 1318 (29.2) | 115 (40.6) | 228 (30.7) | 849 (27.6) | 126 (29.9) | <0.001 |
| Gait speed, m/s, mean (SD) | 1.1 (0.2) | 1.0 (0.3) | 1.1 (0.2) | 1.2 (0.2) | 1.1 (0.2) | <0.001 |
| Body mass index, kg/m2, mean (SD) | 27.3 (3.9) | 25.2 (3.6) | 26.0 (3.7) | 27.4 (3.7) | 29.9 (4.7) | <0.001 |
| Femoral neck BMD, g/cm2, mean (SD) | 0.78 (0.13) | 0.76 (0.14) | 0.76 (0.13) | 0.78 (0.13) | 0.80 (0.14) | <0.001 |
| Central body fracture incidence rate per 1,000 person-years† | 9.4 (8.4–10.4) | 16.0 (9.5–22.4) | 10.6 (8.0–13.2) | 8.7 (7.6–9.9) | 8.3 (4.9–11.6) | 0.03 |
| Mortality incidence rate (95% CI) per 1,000 person-years† | 47.6 (45.4–49.8) | 99.4 (83.9–114.9) | 58.3 (51.4–65.3) | 41.4 (38.9–44.0) | 55.0 (42.2–67.9) | <0.001 |
Abbreviations: PASE, Physical Activity Scale for the Elderly; BMD, bone mineral density
multimorbidities include stroke, diabetes, hyperthyroidism, hypothyroidism, Parkinsonism, myocardial infarction, congestive heart failure, chronic obstructive pulmonary disease, non-skin cancer, liver disease, and renal disease
adjusted for age
During an average follow-up of 8 years after the 2nd examination, 337 men (7.5%) experienced a central body fracture and 1569 (34.7%) died prior to experiencing this outcome (Table 2). Average weight change was similar among men who experienced a central body fracture (−2.3%) and those who died (−2.5%), but smaller in magnitude among men who survived without fracture and who were censored at end of follow-up (−1.0%) (p<0.001 for comparisons of men who survived without fracture to men with fracture and to men who died).
Table 2.
Characteristics of Men with Weight Change Measures by Censoring Event
| Characteristic at 2nd Exam | Central body fracture* | Death | End of Follow-up | p-value† | p-value‡ |
|---|---|---|---|---|---|
| (N=337) | (N=1569) | (N=2617) | |||
| Age, yrs, mean (SD) | 79.4 (5.5) | 80.0 (5.7) | 75.7 (4.6) | 0.08 | <0.001 |
| Caucasian race, n (%) | 323 (95.9) | 1472 (93.8) | 2359 (90.1) | 0.15 | 0.001 |
| Trying to lose weight past 12 months, n (%) | 91 (27.0) | 492 (31.4) | 1016 (38.8) | 0.12 | <0.001 |
| Current smoker, n (%) | 8 (2.4) | 42 (2.7) | 47 (1.8) | 0.75 | 0.46 |
| Multimorbidity score (0–11)**, mean (SD) | 1.1 (1.0) | 1.2 (1.1) | 0.8 (0.9) | 0.07 | <0.001 |
| PASE score, mean (SD) | 126.7 (66.6) | 120.1 (68.5) | 146.1 (67.3) | 0.11 | <0.001 |
| Fall history, n (%) | 134 (39.8) | 550 (35.1) | 634 (24.2) | 0.10 | <0.001 |
| Gait speed, m/s, mean (SD) | 1.1 (0.3) | 1.1 (0.2) | 1.2 (0.2) | 0.01 | <0.001 |
| Body mass index, kg/m2, mean (SD) | 26.2 (3.8) | 27.3 (4.4) | 27.4 (3.7) | <0.001 | <0.001 |
| Femoral neck BMD, g/cm2, mean (SD) | 0.69 (0.11) | 0.77 (0.13) | 0.79 (0.13) | <0.001 | <0.001 |
| % weight change since baseline, mean (SD) | −2.3 (5.9) | −2.5 (6.2) | −1.0 (5.0) | 0.69 | <0.001 |
Abbreviations: PASE, Physical Activity Scale for the Elderly; BMD, bone mineral density
defined as clinical fracture at the hip, spine, or pelvis
comparing central body fractures to deaths
comparing central body fractures to end of follow-up
multimorbidities include stroke, diabetes, hyperthyroidism, hypothyroidism, Parkinsonism, myocardial infarction, congestive heart failure, chronic obstructive pulmonary disease, non-skin cancer, liver disease, and renal disease
The age-adjusted incidence rate of central body fracture was 9.4 (95% CI 8.4–10.4) per 1000-person years and the age-adjusted mortality rate was 47.6 (95% CI, 45.4–49.8) per 1000 person-years (Table 1). Men with moderate weight loss had the highest rates of central body fracture and mortality (16.0 and 99.4 per 1000 person-years, respectively). The rate of central body fracture was lower and of similar magnitude among men with stable weight and those with weight gain (8.7 and 8.3 per 1000 person-years, respectively) and intermediate among men with mild weight loss (10.6 per 1000 person-years). A non-linear association between weight change and risk of central body fracture was confirmed in an analysis using restricted cubic spline models that indicated a possible threshold value near zero percent weight change (Figure 1). Fracture risk increased in a graded manner with increasing degree of weight loss, whereas weight gain appeared to have no effect on fracture risk.
Figure 1.
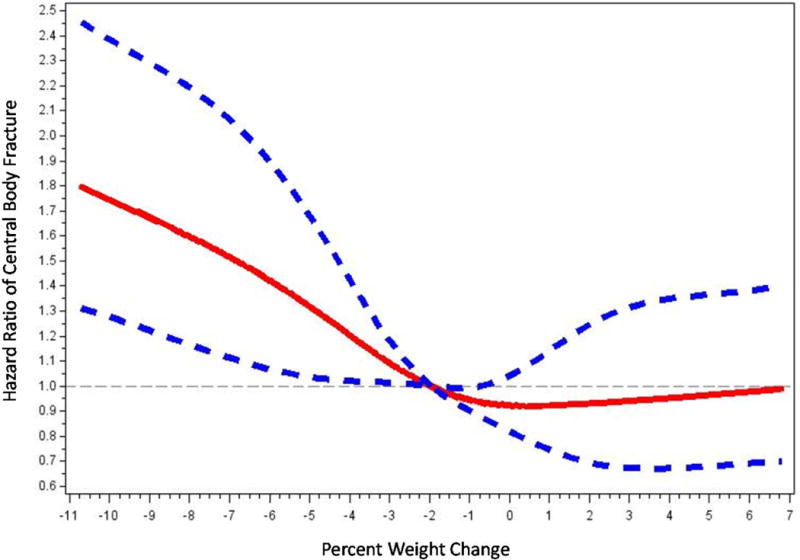
Restricted Cubic Spline Plot of Hazards Ratio for Central Body Fracture by Percent Weight Change
Using traditional survival analysis (Figure 2A) or the competing risk approach (Figure 2B), the absolute probability of central body fracture was higher among the men with moderate weight loss and those with mild weight loss, and lower (and similar in magnitude) among men with stable weight and those with weight gain. Among men with moderate weight loss and those with mild weight loss, the competing risk approach compared with traditional survival analysis resulted in a lower estimate of absolute fracture probability and the difference in estimates was greater as duration of follow-up increased. For example, among men with moderate weight loss, the absolute probability of central body fracture was 6.8% (95% CI 4.0–10.6) at 5 years and 16.9% (95% CI 10.5–24.7) at 10 years using traditional KM survival analysis vs. 5.7% (95% CI 3.4–8.8) at 5 years and 10.2% (95% CI 6.8–14.4) at 10 years using a competing risk approach. Similarly, the absolute probability of hip fracture among men with moderate weight loss was 2.2% (95% CI 0.8–4.8) at 5 years and 10.1% (95% CI 4.8–17.8) at 10 years using traditional KM survival analysis (Figure 3A) vs. 1.8% (95% CI 0.7–3.9) at 5 years and 5.2% (95% CI 2.8–8.7) at 10 years using a competing risk approach (Figure 3B).
Figure 2A.
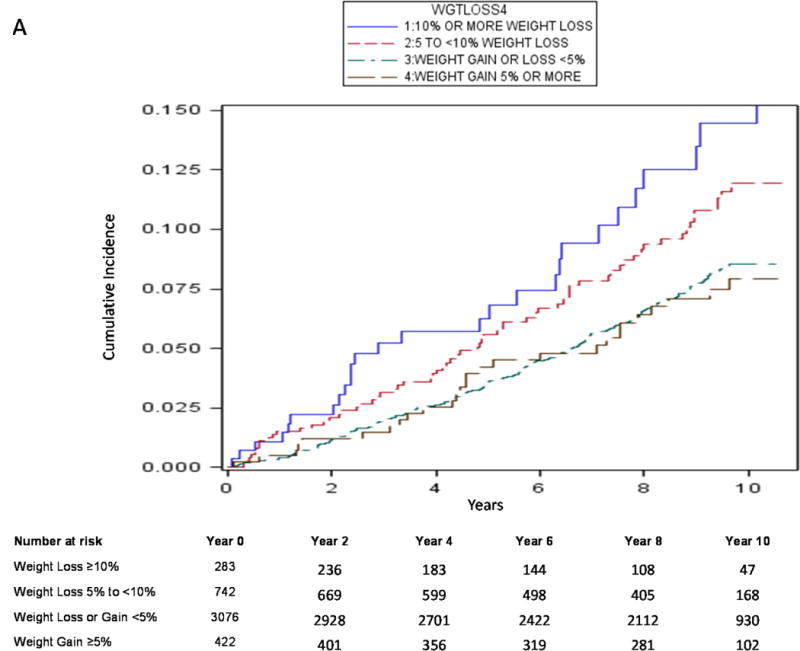
Cumulative Absolute Probability of Central Body Fracture by Weight Change Category using Kaplan-Meier Method
Figure 2B.
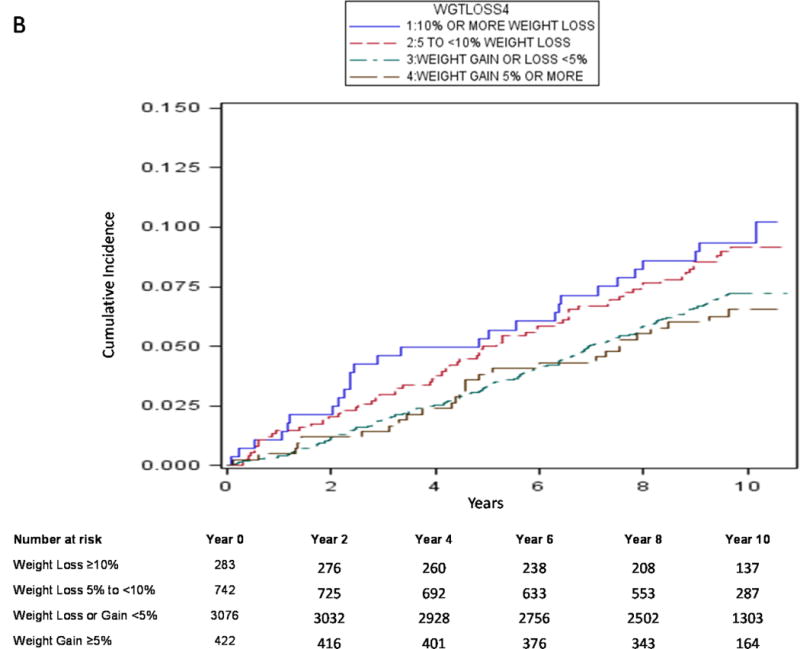
Cumulative Absolute Probability of Central Body Fracture by Weight Change Category using Cumulative Incidence Function (Competing Risk Approach)
Figure 3A.
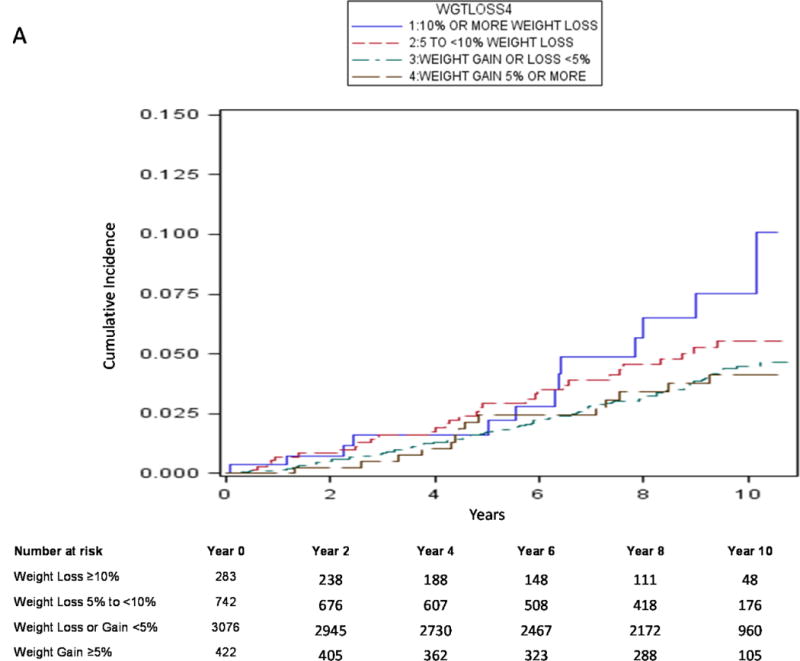
Cumulative Absolute Probability of Hip Fracture by Weight Change Category using Kaplan-Meier Method
Figure 3B.
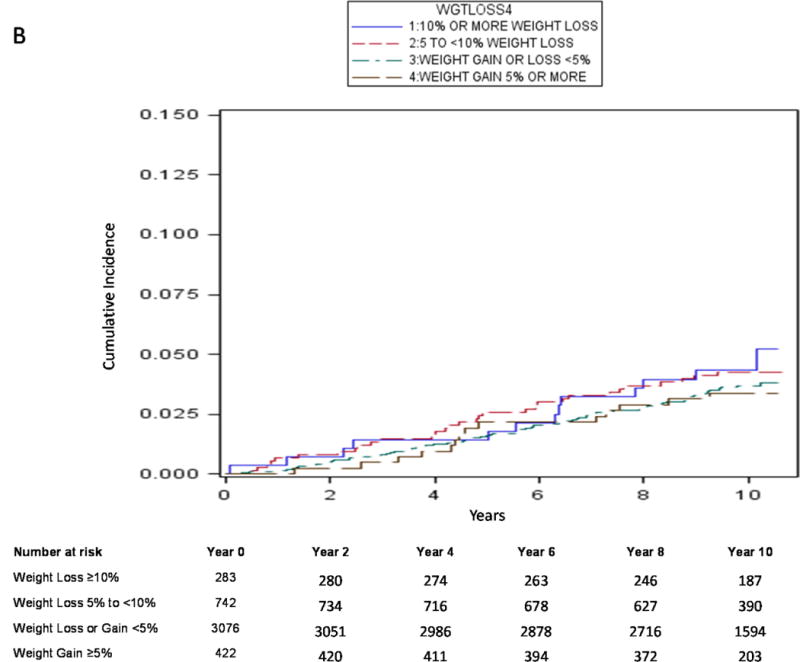
Cumulative Absolute Probability of Hip Fracture by Weight Change Category using Cumulative Incidence Function (Competing Risk Approach)
Men with moderate weight loss compared with those with stable weight had a 1.7-fold higher age-adjusted risk of central body fracture (HR 1.73, 95% CI 1.16–2.59) as calculated by Cox regression (Table 3). Compared to men with stable weight, the age-adjusted risk of central body fracture appeared to be modestly elevated among men with mild weight loss (HR 1.29, 95% CI 0.98–1.70), but the 95% confidence intervals around the point estimate of the association overlapped 1.00. The risk of central body fracture among men with weight gain was similar and not different from that among men with stable weight. In contrast, the age-adjusted risk of central body fracture among men with moderate weight loss was substantially attenuated by 27% in subdistribution models and no longer statistically significant (subdistribution HR 1.24, 95% CI 0.82–1.86). Use of subdistribution models also attenuated the association of mild weight loss with central body fracture, but the effect was smaller in magnitude (subdistribution HR 1.16, 95% CI 0.88–1.53).
Table 3.
Traditional Cox Proportional Hazards Models and Subdistribution Models for Association of Weight Change with Central Body Fracture
| Hazard Ratio (95% CI)
|
||||
|---|---|---|---|---|
| Weight Loss ≥10% |
Weight Loss 5% to <10% |
Stable Weight (Loss or Gain <5%) |
Weight Gain ≥5% | |
| Central body fracture (n=337)* | (N= 283) | (N= 742) | (N= 3,076) | (N= 422) |
| Age-adjusted Model | ||||
| Cox proportional model | 1.73 (1.16–2.59) | 1.29 (0.98–1.70) | 1.00 (referent) | 1.03 (0.69–1.54) |
| Subdistribution model | 1.24 (0.82–1.86) | 1.16 (0.88–1.53) | 1.00 (referent) | 0.96 (0.65–1.44) |
| Base Model† | ||||
| Cox proportional model | 1.59 (1.06–2.38) | 1.25 (0.95–1.65) | 1.00 (referent) | 1.02 (0.68–1.52) |
| Subdistribution model | 1.16 (0.77–1.75) | 1.14 (0.86–1.50) | 1.00 (referent) | 0.96 (0.64–1.43) |
| Base model + PASE | ||||
| Cox proportional model | 1.57 (1.05–2.36) | 1.25 (0.95–1.65) | 1.00 (referent) | 0.99 (0.67–1.49) |
| Subdistribution model | 1.15 (0.77–1.73) | 1.13 (0.86–1.50) | 1.00 (referent) | 0.95 (0.63–1.41) |
| Base model + fall history | ||||
| Cox proportional model | 1.53 (1.02–2.29) | 1.25 (0.95–1.65) | 1.00 (referent) | 1.00 (0.67–1.49) |
| Subdistribution model | 1.12 (0.74–1.69) | 1.13 (0.85–1.49) | 1.00 (referent) | 0.94 (0.63–1.40) |
| Base model + gait speed | ||||
| Cox proportional model | 1.51 (0.99–2.30) | 1.20 (0.90–1.59) | 1.00 (referent) | 1.02 (0.68–1.52) |
| Subdistribution model | 1.18 (0.78–1.80) | 1.11 (0.83–1.47) | 1.00 (referent) | 0.99 (0.66–1.47) |
| Base model + femoral neck BMD | ||||
| Cox proportional model | 1.53 (1.03–2.29) | 1.17 (0.89–1.55) | 1.00 (referent) | 1.01 (0.67–1.51) |
| Subdistribution model | 1.02 (0.67–1.56) | 1.04 (0.78–1.37) | 1.00 (referent) | 1.00 (0.66–1.49) |
defined as hip, clinical vertebral or pelvis fracture
adjusted for age, race, smoking, and multimorbidity score
Further adjustment for potential confounders (race, smoking, multimorbidity burden) or consideration of potential mediators (physical activity, fall history, gait speed, or femoral neck BMD) modestly reduced the association between moderate weight loss and central body fracture in Cox regression models. The impact of consideration of potential confounders and mediators was similar in subdistribution models, but the subdistribution HRs for moderate weight loss were close to and not different from 1.0 in magnitude in all models. We found no evidence using Cox or subdistribution models that the association between weight change and risk of CBF varied by current BMI or intention to lose weight (p interaction terms all ≥0.52 in age and multivariable adjusted models).
In analyses that substituted individual fracture types for central body fracture, there was a similar substantial attenuation in the HR of hip, clinical vertebral or pelvis fracture among men with moderate weight loss compared with those with stable weight when using competing risk vs. traditional Cox regression models (Supplemental Table 1).
DISCUSSION
In this prospective study of community-dwelling older men, men with weight loss late in life who survived were at increased risk of central body fractures, including hip fractures. However, not taking into account the competing mortality risk among men with weight loss markedly overestimated their longterm absolute fracture probability and adjusted facture risk.
Our estimates of the absolute probability and adjusted risk of central body fracture among older men with weight loss derived from traditional analytical approaches (KM method and Cox proportional hazards regression) are in agreement with findings of previous prospective studies examining the association of weight change with fracture risk in middle-aged and older adults. Studies in postmenopausal and older women(1–6) have consistently reported that self-reported or documented weight loss is independently associated with up to a 2-fold increase in risk of fragility fractures including hip, clinical vertebral and pelvis fractures. These studies assessed weight change or weight loss in particular over time periods between 1 and 20 years and subsequently followed participants for fracture outcomes for time periods ranging from nearly 2 to over 10 years. Similarly, a prospective investigation in men aged 67 years and older(11) found that self-reported weight loss of 10% or more since age 50 is associated with a 1.8-fold risk of hip fracture during 8 years of follow-up. We also found evidence of a non-linear association of weight change with central body fracture in our cohort of older men as fracture risk increased in a graded manner with increasing degree of weight loss, while weight gain appeared to have no effect on fracture risk. These findings are similar to those reported in prospective studies of postmenopausal and older women(2,4) that have examined the association of weight change with risk of central body fractures, including hip fractures. However, all previous studies relied solely on conventional analytical approaches that may be inappropriate in the presence of the competing risk of mortality.
Among participants with moderate weight loss in this cohort of older men, the mortality incidence rate was over 6-fold greater than the rate of central body fracture suggesting that mortality was a major competitor to a experiencing a central body fracture event. Our estimates of 5 and 10-year absolute probabilities of central body fracture among men with weight loss were lower using a competing risk approach vs. traditional survival analysis and the magnitude of difference in probabilities calculated by the 2 methods noticeably increased with increasing duration of follow-up. In our analyses estimating the relative risk of central body fracture among men with weight loss after accounting for potential confounders, subdistribution hazards ratios from models treating death as a competing risk were smaller in magnitude and more conservative than those from traditional Cox proportional regression since central body fracture and death share many common risk factors. Consideration of the competing risk of mortality attenuated the magnitude of the point estimate of the association by 27% and it was no longer statistically significant. In addition, our findings regarding the differences in estimates of absolute fracture probability and adjusted fracture risk when using a traditional vs. competing risk approach were similar in analyses substituting hip fracture for central body fracture as the outcome variable.
Thus, our results indicate that it is essential for studies to incorporate statistical approaches that account for the competing mortality risk when evaluating the longterm association of weight loss in old age with fragility fracture risk in order to accurately estimate absolute fracture probability and adjusted fracture risk. Taking into account the competing risk of mortality is of less importance when evaluating the association of weight loss with risk of fracture in studies with shorter follow-up periods. While our investigation provides novel information on the impact of the competing risk of mortality on the relationship of weight loss with fracture, a competing risk approach has been utilized in other investigations in the field including those examining the risk of second fracture events and mortality following osteoporotic fracture(24), evaluating lifetime risk of fracture by age group and BMD category(25), and estimating BMD testing intervals in healthy older adults according to age and BMD category.(26,27)
Weight loss may be a proxy for underlying serious medical conditions, but our results from both conventional and competing risk approaches regarding the association of weight change with central body fracture were not substantially altered by adjustment for multimorbidity burden as defined by number of comorbid medical conditions suggesting that weight loss is a proxy for subclinical illness. On the other hand, greater attenuation of the association may have resulted if our analyses had adjusted for a weighted index of medical conditions specifically developed to predict fracture risk. In addition, we did not find evidence using either approach that the association varied between heavier and thinner men or between men with trying and those not trying to lose weight. Our findings from analyses using the traditional Cox proportional hazards models are consistent with those from previous studies in women(1–6) that have use this approach to evaluate effects of multimorbidity and current body weight on the association between weight change and fragility fracture. While a study in older women(4) of similar age to the men in this study reported that both involuntary and voluntary documented weight loss were associated with an increase in the risk of hip fracture, a study of younger postmenopausal women(2) found that self-reported unintentional weight loss, but not intentional weight loss, was related to higher hip fracture risk.
Our results have implications for fracture risk assessment and clinical decision-making in older men. Some professional societies(28,29) have advocated more aggressive osteoporosis screening, fracture risk assessment and use of drug treatment in older men to prevent fracture. However, these guidelines did not take into account the impact of the competing risk of death. Because of the high competing mortality risk among older men with weight loss, aggressive assessment and management strategies may be not be appropriate as many men in this group will die prior to experiencing a disabling fracture event. Our results also suggest that fracture risk assessment tools that provide longterm individual based estimates of fracture probability might be improved by incorporation of individual patient-based estimates of competing mortality risk as available web-based tools either do not take into account competing mortality risk(30,31) or only account for country-specific death rates.(32)
This study has several strengths. It was comprised of a large cohort of community-dwelling men with objective repeated measures of body weight, prospective longterm fracture follow-up and confirmation of incident fractures. However, this study also has several limitations. The cohort was predominantly older Caucasian community-dwelling men, so results may not be generalizable to other populations, especially those such as younger postmenopausal women where the competing risk of mortality is markedly lower. In addition, there was limited power for specific fractures types. We were unable to examine the association of weight fluctuation with risk of central body fracture because annual measurements of body weight were not performed in the MrOS study.
In conclusion, older men with weight loss late in life who survive have a higher risk of central body fractures, including hip fractures. However, not taking into account the competing mortality risk among men with weight loss greatly overestimates their longterm absolute fracture probability and adjusted facture risk. These findings suggest that among older men with a history of weight loss in old age, a competing risk approach is needed to better inform fracture risk assessment and clinical decision-making.
Supplementary Material
Acknowledgments
Source of Funding:
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System.
Authors’ roles:
Study concept and design: KEE, JAC, CEL, ESO
Data collection: KEE, JAC, CEL, ESO
Data analysis and interpretation: KEE, SLH
Drafting manuscript: KEE
Critical review and final approval of manuscript content: KEE, SLH, JAC, LL, JTS, DMK, MLG, JGL, LF, NN, CJC, CEL, ESO, MLS, PMC
Statistical Analysis: Ms. Stephanie Harrison performed the statistical analyses and is independent of any commercial funder. She had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1.Compston JE, Wyman A, FitzGerald G, et al. Increase in Fracture Risk Following Unintentional Weight Loss in Postmenopausal Women: The Global Longitudinal Study of Osteoporosis in Women. J Bone Miner Res. 2016 doi: 10.1002/jbmr.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crandall CJ, Yildiz VO, Wactawski-Wende J, et al. Postmenopausal weight change and incidence of fracture: post hoc findings from Women’s Health Initiative Observational Study and Clinical Trials. BMJ. 2015;350:h25. doi: 10.1136/bmj.h25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ensrud KE, Cauley J, Lipschutz R, Cummings SR. Weight change and fractures in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1997;157:857–63. [PubMed] [Google Scholar]
- 4.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–7. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 5.Langlois JA, Harris T, Looker AC, Madans J. Weight change between age 50 years and old age is associated with risk of hip fracture in white women aged 67 years and older. Arch Intern Med. 1996;156:989–94. [PubMed] [Google Scholar]
- 6.Langlois JA, Mussolino ME, Visser M, Looker AC, Harris T, Madans J. Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: the NHANES I epidemiologic follow-up study. Osteoporos Int. 2001;12:763–8. doi: 10.1007/s001980170053. [DOI] [PubMed] [Google Scholar]
- 7.Dennison E, Eastell R, Fall CH, Kellingray S, Wood PJ, Cooper C. Determinants of bone loss in elderly men and women: a prospective population-based study. Osteoporos Int. 1999;10:384–91. doi: 10.1007/s001980050244. [DOI] [PubMed] [Google Scholar]
- 8.Ensrud KE, Fullman RL, Barrett-Connor E, et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90:1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- 9.Hannan MT, Felson DT, Dawson-Hughes B, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–20. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 10.Knoke JD, Barrett-Connor E. Weight loss: a determinant of hip bone loss in older men and women. The Rancho Bernardo Study. Am J Epidemiol. 2003;158:1132–8. doi: 10.1093/aje/kwg265. [DOI] [PubMed] [Google Scholar]
- 11.Langlois JA, Visser M, Davidovic LS, Maggi S, Li G, Harris TB. Hip fracture risk in older white men is associated with change in body weight from age 50 years to old age. Arch Intern Med. 1998;158:990–6. doi: 10.1001/archinte.158.9.990. [DOI] [PubMed] [Google Scholar]
- 12.Knudtson MD, Klein BE, Klein R, Shankar A. Associations with weight loss and subsequent mortality risk. Ann Epidemiol. 2005;15:483–91. doi: 10.1016/j.annepidem.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–18. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen ND, Center JR, Eisman JA, Nguyen TV. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J Bone Miner Res. 2007;22:1147–54. doi: 10.1359/jbmr.070412. [DOI] [PubMed] [Google Scholar]
- 15.Wedick NM, Barrett-Connor E, Knoke JD, Wingard DL. The relationship between weight loss and all-cause mortality in older men and women with and without diabetes mellitus: the Rancho Bernardo study. J Am Geriatr Soc. 2002;50:1810–5. doi: 10.1046/j.1532-5415.2002.50509.x. [DOI] [PubMed] [Google Scholar]
- 16.Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–7. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Lewis CE, Ewing SK, Taylor BC, et al. Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res. 2007;22:211–9. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 20.Genant HK, Wu CY, van KC, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 21.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 22.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4:103–12. [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22:781–8. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 25.Bliuc D, Nguyen ND, Nguyen TV, Eisman JA, Center JR. Compound risk of high mortality following osteoporotic fracture and refracture in elderly women and men. J Bone Miner Res. 2013;28:2317–24. doi: 10.1002/jbmr.1968. [DOI] [PubMed] [Google Scholar]
- 26.Gourlay ML, Fine JP, Preisser JS, et al. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225–33. doi: 10.1056/NEJMoa1107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gourlay ML, Overman RA, Fine JP, et al. Time to Osteoporosis and Major Fracture in Older Men: The MrOS Study. Am J Prev Med. 2016;50:727–36. doi: 10.1016/j.amepre.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802–22. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 30.ClinRisk, Ltd. QFracture-2013 risk calculator. http://www.qfracture.org/. Updated: 2015. Accessed 6-1-2016.
- 31.Garvan Institute of Medical Research. Bone fracture risk calculator. http://www.garvan.org.au/bone-fracture-risk. Updated: 2013. Accessed 6-1-2016.
- 32.FRAX WHO Fracture Risk Assessment Tool. http://www.shef.ac.uk/FRAX/. Updated: 2011. Accessed 6-1-2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


