Abstract
Inflammatory bone resorption mediated by osteoclasts is a major cause of morbidity and disability in many inflammatory disorders, including rheumatoid arthritis (RA). The mechanisms that regulate osteoclastogenesis and bone resorption in inflammatory settings are complex and have not been well elucidated. In this study, we identify the immunoregulator Def6 as a novel inhibitor of osteoclastogenesis in both physiological and inflammatory conditions. Def6 deficiency in Def6−/− mice enhanced the sensitivity of osteoclast precursors to the physiological osteoclastogenic inducer RANKL, and Def6−/− osteoclasts formed actin rings. Furthermore, Def6 deficiency markedly increased TNF-α-induced osteoclastogenesis both in vitro and in vivo and enhanced bone resorption in an inflammatory osteolysis mouse model. TNF-α serum levels negatively correlated with Def6 expression levels in osteoclast precursors obtained from RA patients and the osteoclastogenic capacity of the osteoclast precursors was significantly inversely correlated with their Def6 expression levels, indicating that Def6 functions as an inhibitor of excessive osteoclast formation and bone destruction in RA. Mechanistically, Def6 suppressed osteoclastogenesis and the expression of key osteoclastogenic factors NFATc1, Blimp1 and c-Fos by regulating an endogenous IFN-β mediated autocrine feedback loop. The Def6-dependent pathway may represent a novel therapeutic target to prevent pathological bone destruction.
Introduction
The adult skeleton undergoes constant and dynamic remodeling. The bone homeostasis is tightly regulated by osteoclast-mediated bone resorption and associated osteoblast/osteocyte-mediated bone formation (1–3). Recent studies from the osteoimmunology field highlight the critical role of the immune system in the regulation of bone homeostasis in both physiological and pathological conditions (4–8). Chronic inflammation such as occurs in rheumatoid arthritis and periodontitis is one of the most common pathological conditions associated with excessive bone loss. Abnormally enhanced generation and/or function of osteoclasts lead to inflammatory bone resorption in these settings (9–12). Osteoclasts, which are derived from monocyte/macrophage precursors, are the exclusive cell type responsible for bone resorption. Osteoclastogenesis is effectively induced by the major osteoclastogenic cytokine receptor activator of nuclear factor-κB ligand (RANKL), which acts in concert with macrophage colony-stimulating factor (M-CSF) and immunoreceptor tyrosine-base activation motif (ITAM)-mediated co-stimulatory signals. Binding of RANKL to its receptor RANK activates a broad range of signaling cascades, including canonical and non-canonical NF-κB pathways; mitogen-activated kinase (MAPK) pathways leading to the activation of AP-1 and CREB transcription factors; and calcium signaling, to induce the expression of key transcription factors Blimp1 and NFATc1 for osteoclast differentiation (13–17). In inflammatory diseases, osteoclastogenesis and osteoclast-mediated bone resorption are increased. We and other groups have identified several factors that contribute to the de-regulated pathological osteoclastogenesis (14, 16, 18, 19). Nonetheless, the mechanisms that regulate inflammatory osteoclastogenesis and bone resorption are complex and far from well understood.
Tumor necrosis factor (TNF) -α is a pleiotropic cytokine important for immunity and inflammation. In addition to driving chronic inflammation, TNF-α plays a key role in inflammatory bone destruction such as occurs in inflammatory arthritis and periodontitis. TNF-α promotes inflammatory bone resorption by acting on osteoblasts and tissue stromal cells to induce RANKL production. It also acts directly on osteoclast precursors, often in synergy with RANKL, to promote osteoclastogenesis (12, 18, 20–25). In contrast to RANKL, however, TNF-α alone does not effectively induce osteoclast differentiation. Although recent studies by our group and others have identified several factors involved in the direct osteoclastogenic properties of TNF-α (26, 27), the feedback mechanisms regulating TNF-induced osteoclastogenesis during inflammatory osteoclast formation have not been well defined.
Type I IFNs, IFN-α and IFN-β, are secreted by many cell types, including lymphocytes, fibroblasts, macrophages and endothelial cells, mainly in response to viral infection and antigens (28). Recent studies have identified additional factors and mechanisms involved in the regulation of interferon production. For example, TNF-α induces macrophages to express IFN-β that acts on the cells in an autocrine manner to maintain a chronic TNF-α response and sustained inflammation (29). Type I IFNs have also been implicated in the suppression of bone resorption. IFN-β expressed by osteoclast precursors functions as a negative-feedback regulator to suppress osteoclast differentiation by decreasing c-Fos expression (14, 30). Although the production of IFN-β by macrophages or osteoclast precursors in response to RANKL or TNF-α is much less compared to other stimuli such as TLR or virus infection, its biological effect is evident in the maintenance of inflammation in macrophages and inhibition of osteoclast differentiation (29, 30). Type I IFN gene expression is tightly controlled by interferon regulator factor (IRF) family members, including the major regulators IRF3 and 7, as well as IRF1 and 5, depending on cell types and specific stimuli (28). Despite the important regulatory role of IFN-β in bone homeostasis and osteoclast differentiation, it remains unclear how the IFN-β-mediated autocrine inhibitory loop is regulated in response to chronic TNF-α stimulation during inflammatory osteoclastogenesis.
Differentially expressed in FDCP 6 homolog (Def6), also known as IRF4-binding protein (IBP) or SWAP-70-like adaptor protein of T cells (SLAT), was originally identified as a novel type of guanine nucleotide exchange factors (GEFs) that is expressed predominantly in T cells and regulates T cell development and activation/function (31, 32). Def6 functions through its GEF activity to activate Rho GTPases Rac and Cdc42 in T cells. In addition to its GEF activity, accumulating evidence has shown that Def6 can play diverse roles via different mechanisms (31–37). For example, Def6 physically binds to and sequesters IRF4, preventing it from activating the expression of IL-17 and IL-21 in T cells (34, 37) and association of Def6 with IP3 receptor 1 regulates calcium signaling in T cells (36). These studies indicate that Def6 acts as a multifunctional protein, whose roles are not limited to GEF activity. Def6 harbors, from its N to C terminus, a potential calcium binding EF-hand domain; an immunoreceptor tyrosine-based activation motif-like sequence; a PI(3,4,5)P3-binding pleckstrin-homology (PH) domain; and a Dbl-homology (DH) domain exhibiting GEF activity (32–34). This domain organization is opposite to the other GEFs in which the GEF component is on the N terminus of the PH domain. The unique molecular structure and domain organization of Def6 shows limited homology with other classical Rho-family GEFs, which may explain its diverse functions and unique downstream effector mechanisms that differ from the other classical GTPases. Def6 exhibits significant sequence homology to only one other molecule, SWAP-70, a novel type of Rac activator (38). Swap-70 plays various roles in different cell types. In bone biology, SWAP-70 regulates osteoclast function but not differentiation (39). Previous studies show that Def6 is also expressed in myeloid cells and regulates innate immunity (40). Def6 deficient mice crossed with TCR transgenic DO11.10 mice develop rheumatoid arthritis-like joint disease with bone erosion (37). Although the mechanisms that contribute to the bone resorption in these mice with complex autoimmunity have not been fully elucidated, these data suggest potential involvement of Def6 in pathologic bone resorption. Def6 was reported to modulate RANKL-induced osteoclast differentiation in vitro (41). However, the role of Def6 in vivo especially bone erosion in inflammatory conditions and the underlying molecular mechanisms have not been delineated.
In the present study, we found that Def6 plays an important inhibitory role in osteoclastogenesis both in vitro and in vivo. Def6 deficiency enhances the sensitivity of osteoclast precursors to the major osteoclastogenic inducer RANKL. Importantly, Def6 deficiency enables TNF-α alone to induce osteoclastogenesis in the absence of RANKL and markedly enhances TNF-α-induced osteoclastogenesis and bone resorption. Moreover, we found a significant downregulation of Def6 expression by TNF-α in human macrophages and a close correlation of Def6 expression levels in osteoclast precursors with serum TNF-α levels and RA disease activity. Anti-TNF treatment in RA patients significantly increased Def6 levels in osteoclast precursors, further supporting a role for Def6 in modulating the effects of TNF-α on osteoclastogenesis. Mechanistically, we show that Def6 suppresses the expression of key osteoclastogenic regulators NFATc1, Blimp1 and c-Fos by regulating an autocrine feedback loop mediated by endogenous RANKL- or TNF-α-induced IFN-β, identifying Def6 as a novel negative regulator of osteoclastogenesis in both physiological and inflammatory conditions.
Materials and Methods
Mice and analysis of bone phenotype
Def6−/− mice have been described previously (42). Def6−/− mice used in this study have been backcrossed with C57/BL6 mice for more than 10 times. Def6 deficient mice on the C57/BL6 background do not develop autoantibodies or spontaneous autoimmunity disease. We thus took advantage of the Def6 KO mice on the C57/BL6 background to study osteoclastogenesis and inflammatory bone resorption to avoid the complex impact of the autoimmune disease severity and pattern that occur in other mouse strains. Gender and age matched Def6−/− mice and C57/BL6 mice as the wild type (WT) controls were used for in vitro experiments. Eight to twelve week old Def6−/− mice and littermate control mice were used for the in vivo experiments. We used the protocol for in vivo analysis of TNF-α-induced inflammatory bone resorption as described previously (27). When the mice were sacrificed, serum was collected to determine the TRAP concentration using a Mouse TRAP Assay Kit (immunodiagnostic systems) according to the manufacturer’s instructions. Calvarial bones and femur bones were subjected to μCT scanning and analysis. Femur and calvarial bones were subjected to sectioning, TRAP staining, and histological analysis (43). Osteomeasure software was used for bone histomorphometry. All mouse experiments were approved by Institutional Animal Care and Use Committee of the Hospital for Special Surgery.
Reagents
Murine or human M-CSF, Murine or human TNF-α and sRANKL were purchased from Peprotech. Recombinant mouse IFN-β was from PBL technology. The control IgG (rabbit) was obtained from Santa Cruz Biotechnology, and IFN-β neutralizing antibody (rabbit polyclonal antibody against mouse interferon beta) was from PBL technology.
Cell culture
Murine bone marrow cells were harvested from tibiae and femora, and cultured overnight in Petri dishes (BD Biosciences) in α-MEM medium (Invitrogen) with 10% FBS (Invitrogen). Except where stated, CMG14-12 supernatant, which contained the equivalent of 20 ng/ml of recombinant M-CSF, were used as a source of M-CSF as described (43) in experiments. Non-adherent cells were then re-plated into tissue culture dishes and cultured in the same medium for 3 days to obtain bone marrow derived macrophages (BMMs), which are capable of differentiating to osteoclasts, and thus were used as osteoclast precursors. The attached BMMs were scraped, seeded at a density of 4.5 × 104/cm2 and cultured in α-MEM medium with 10% FBS and CMG14-12 supernatant for 1 day. The cells were then treated without or with RANKL, TNF-α, IFN-β, control IgG or IFN-β neutralizing antibody for indicated times as shown in the figure legends. Culture media were exchanged every three days. TRAP staining was performed with an acid phosphatase leukocyte diagnostic kit (Sigma-Aldrich) in accordance with the manufacturer’s instructions. The relative area of TRAP positive multinucleated cells (MNCs)/osteoclasts to the culture well was measured using osteomeasure software. BMMs (4.5 × 104/cm2) were cultured on the surface of bovine bone surfaces in the 10% FBS and CMG14-12 supernatant with TNF-α. Bone resorptive pits were stained by wheat germ agglutinin. The BMM proliferation was assessed using a BrdU proliferation kit following the manufacturer’s instructions (Roche). The relative BrdU incorporation was measured by the absorbance (A370 nm – A492 nm). The osteoclast apoptosis was assessed using a Cell Death Detection ELISA kit following the manufacturer’s instructions (Roche). The relative cytoplasmic histone-associated DNA fragments were measured by the absorbance (A405nm-A490nm). For actin ring staining, the cells were fixed with 4% paraformaldehyde for 10 min at room temperature (RT), washed with PBS and permeabilized with 0.1% Triton X-100 in PBS for 5 min at RT, and then stained with 0.2 μM rhodamine phalloidin (Sigma-Aldrich) for 60 min at 37°C (27). Peripheral blood mononuclear cells (PBMCs) from whole blood of healthy volunteers or RA patients were isolated by density gradient centrifugation using Ficoll (Invitrogen Life Technologies, Carlsbad, CA). CD14+ cells were purified from fresh PBMCs using anti-CD14 magnetic beads (Miltenyi Biotec, Auburn, CA) as recommended by the manufacturer. Human monocytes were cultured α-MEM medium with 10% FBS in the presence of M-CSF (20 ng/ml; PeproTech, Rocky Hill, NJ) for 2 days to obtain monocyte-derived macrophages. Experiments with human cells were approved by the Hospital for Special Surgery Institutional Review Board and University of Erlangen-Nuremberg (Germany).
In vitro gene silencing
Antisense inhibition using locked nucleic acid (LNA) technology from Exiqon was applied to silence gene expression in vitro. LNA oligonucleotides specifically targeting Lgr4 and nontargeting control LNAs were from Exiqon and were transfected into murine BMMs using TransIT-TKO transfection reagent (Mirus) in accordance with the manufacturer’s instructions.
Reverse transcription and real-time PCR
Reverse transcription and real-time PCR were performed as previously described (43). The primers for real-time PCR were as follows: Nfatc1: 5′-CCCGTCACATTCTGGTCCAT-3′ and 5′-CAAGTAACCGTGTAGCTCCACAA-3′; Prdm1: 5′-TTCTTGTGTGGTATTGTCGGGACTT-3′ and 5′-TTGGGGACACTCTTTGGGTAGAGTT-3′; Acp5: 5′-ACGGCTACTTGCGGTTTC-3′ and 5′-TCCTTGGGAGGCTGGTC-3′; Ctsk: 5′-AAGATATTGGTGGCTTTGG-3′ and 5′-ATCGCTGCGTCCCTCT-3′; Itgb3: 5′-CCGGGGGACTTAATGAGACCACTT-3′ and 5′-ACGCCCCAAATCCCACCCATACA-3′; Gapdh: 5′-ATCAAGAAGGTGGTGAAGCA-3′ and 5′-AGACAACCTGGTCCTCAGTGT-3′.
Immunoblot analysis
Total cell extracts were obtained using lysis buffer containing 20 mM HEPES (pH 7.0), 300 mM NaCl, 10 mM KCl, 1 mM MgCl2, 0.1% Triton X-100, 0.5 mM DTT, 20% glycerol, and 1 x proteinase inhibitor cocktail (Roche). The cell membrane-permeable protease inhibitor Pefabloc (1 mM) was added immediately before harvesting cells. The protein concentration of extracts was quantified using the BCA protein assay kit (Pierce). Cell lysates (10 μg/sample) were fractionated on 7.5% SDS-PAGE, transferred to Immobilon-P membranes (Millipore) and incubated with specific antibodies. Western Lightning plus-ECL (PerkinElmer) was used for detection. Densitometry was performed using ImageJ software (National Institutes of Health). Def6 antibody was produced by the Dr. Pernis lab (32). c-Fos, Blimp1, GAPDH and p38α antibodies were from Santa Cruz Biotechnology. The NFATc1 antibody was from BD Biosciences, and p-STAT1 (Y701), p-STAT1 (S727), p-STAT3 (Y705), p-IκB, IκB, p100, p52, p-p38, p-ERK and ERK were purchased from Cell Signaling.
Statistical analysis
Statistical analysis was performed using Graphpad Prism® software. Student’s t-test was applied if there are only 2 groups of samples. In the case of more than 2 groups of samples with one condition, one-way ANOVA followed by Tukey’s post hoc test was used to calculate difference between any groups of samples. In the case of more than 2 groups of samples with more than one condition/treatment, two-way ANOVA followed by Sidak’s multiple comparisons were used. p value <0.05 was taken as statistically significant; *p value < 0.05 and **p value < 0.01 and all data are presented as the mean ± SD.
Results
Def6 regulates macrophage responsiveness to RANKL
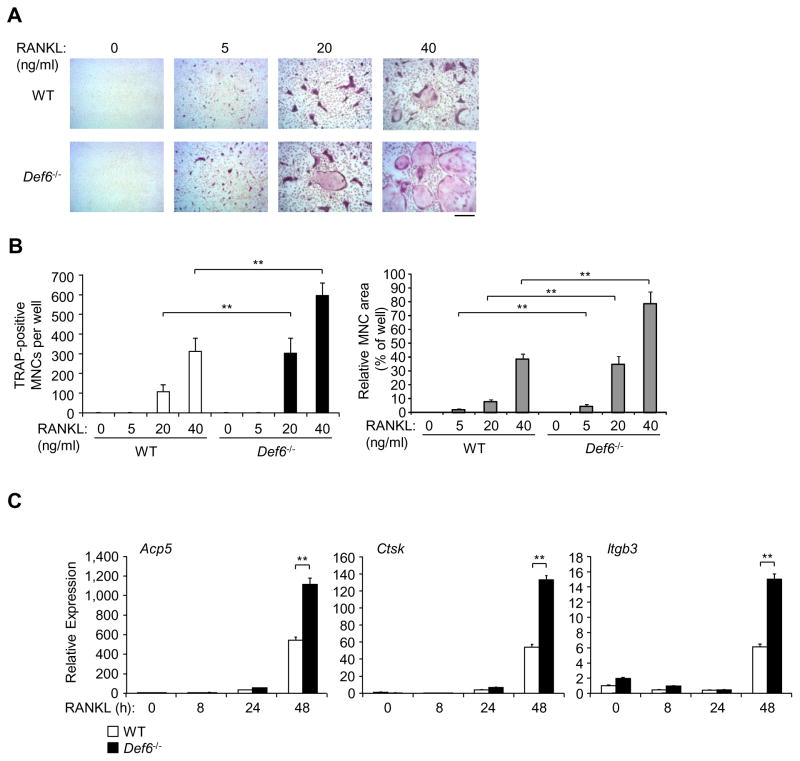
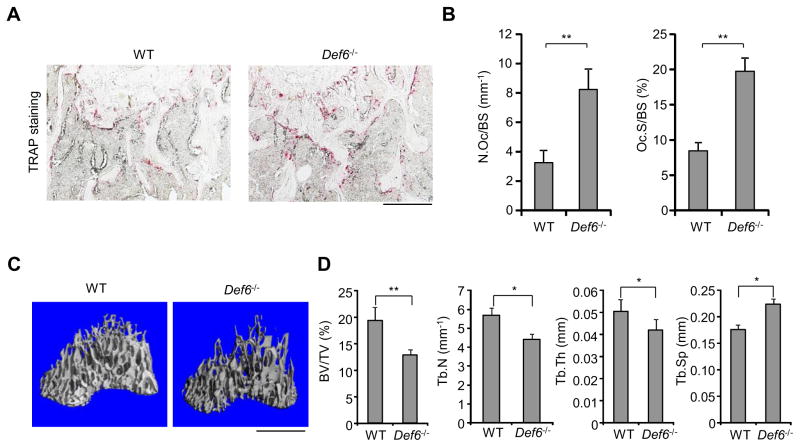
Based on the observation that TCR transgenic Def6 deficient mice develop a rheumatoid arthritis-like joint disease with bone erosion (37), we wished to characterize the role of Def6 in osteoclastogenesis and pathological bone resorption and to define its mechanism of action. We first used bone marrow derived macrophages (BMMs) as osteoclast precursors to examine in vitro osteoclast differentiation in response to RANKL, the master osteoclastogenic inducer. We found that Def6−/− derived BMMs exhibited an increased responsiveness to RANKL, determined by an increased number and area of TRAP-positive multinucleated osteoclasts (Fig. 1A, B). Both of wild type (WT) and Def6−/− derived osteoclasts formed actin rings (Supplementary Fig. 1A). Neither M-CSF dependent BMM proliferation nor osteoclast apoptosis was significantly different between Def6−/− and wild-type cell cultures (Supplementary Fig. 1B, C). These results indicate that Def6 regulates the differentiation of osteoclasts but not the proliferation of precursors or the survival of mature osteoclasts. In parallel with increased osteoclast formation, the expression of osteoclast marker genes Acp (encoding TRAP), Itgb3 (encoding b3) and Ctsk (encoding cathepsin K) was significantly enhanced by RANKL in Def6−/− BMM cultures compared to the BMMs cultured from wild-type controls (Fig. 1C). Moreover, the osteoclast numbers and surfaces in Def6−/− mice were dramatically increased (Fig. 2A, B). Def6−/− mice exhibit osteoporotic phenotype indicated by decreased trabecular bone volume, number, and thickness and increased trabecular bone spacing (Fig. 2C, D). These data collectively indicate that Def6 acts as a negative regulator of RANKL-induced osteoclast differentiation both in vitro and in vivo.
Figure 1. Def6 deficiency enhances macrophage responsiveness to RANKL.
(A) BMMs derived from wild-type (WT) control and Def6−/− mice were stimulated with RANKL at the indicated doses for 4 days. TRAP staining was performed (A) and the number (B, left panel) as well as the area (B, right panel) of TRAP-positive MNCs relative to per well was determined as described in the “Materials and Methods”. TRAP-positive cells appear red in the photographs. Scale bar, 100μm. Data are representative of at least five independent experiments. *P<0.05; **P<0.01. (C) Quantitative real-time PCR analysis of mRNA expression of Acp5 (encoding TRAP), Ctsk (encoding cathepsin K) and Itgb3 (encoding β3) in BMMs from the WT control and Def6−/− mice treated with RANKL (40 ng/ml) for the indicated times. Data are representative of at least three independent experiments. **P<0.01.
Figure 2. Def6−/− mice exhibit osteoporotic phenotype and enhanced osteoclast formation.
(A) TRAP staining and (B) histomorphometric analysis of the histological sections obtained from the metaphysis region of distal femurs of the twelve week old male Def6−/− mice and their littermate control mice, N. Oc./BS, number of osteoclasts per bone surface; Oc. S/BS, osteoclast surface per bone surface. n = 5 per group. **p < 0.01. Scale bar: 100 μm. Microcomputed tomography (μCT) images (C) and the bone morphometric analysis (D) of trabecular bones of the distal femurs isolated from the twelve week old male Def6−/− mice and their littermate control mice. Scale bar: 1 mm. BV/TV, bone volume per tissue volume; Tb. N, trabecular number; Tb. Th, trabecular bone thickness; Tb. Sp, trabecular bone spacing. n = 5 per group. *p < 0.05; **p < 0.01.
Def6 inhibits TNF-α-induced osteoclast formation
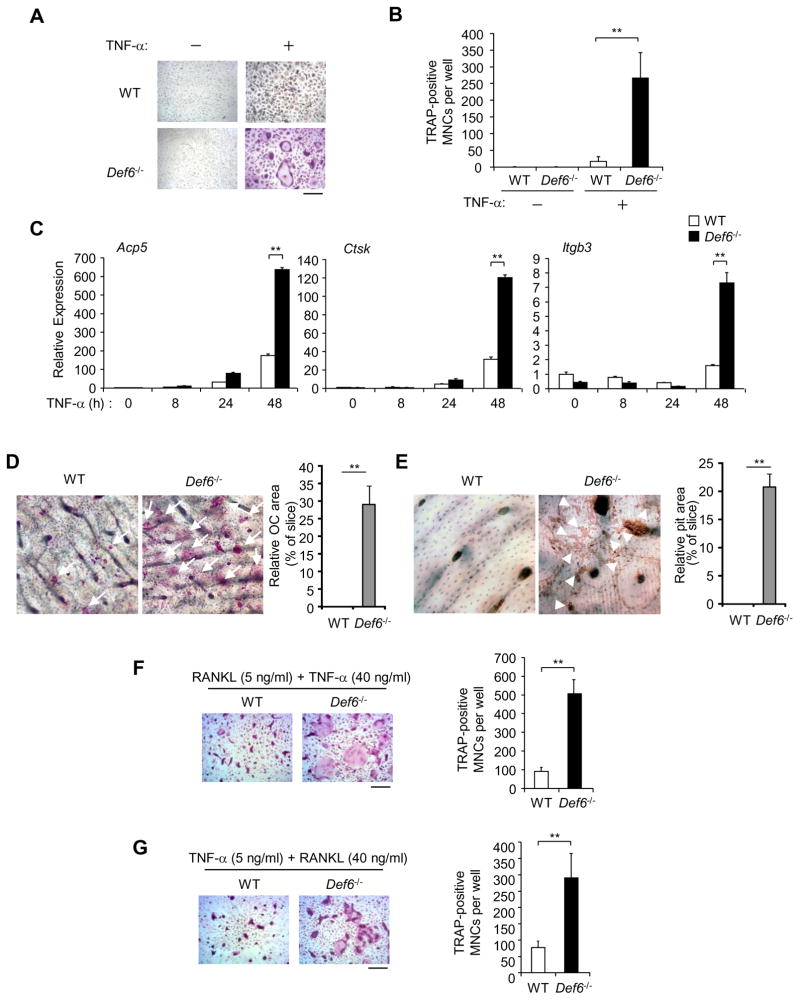
As TNF-α plays a major role in pathological bone resorption in inflammatory arthritis, we next examined whether Def6 regulates TNF-mediated osteoclastogenesis. We found that TNF-α alone induced a limited number of small TRAP positive osteoclasts in wild-type BMM cells (Fig. 3A, B), which is consistent with other published reports (22, 26, 27, 43, 44). Strikingly, the absence of Def6 markedly enhanced the formation of multinucleated osteoclasts with well-spread cell morphology in response to TNF-α (Fig. 3A, B). In parallel with the increased osteoclast formation, the expression of osteoclast marker genes Acp5, Ctsk and Itgb3 was dramatically enhanced in TNF-treated Def6−/− cells compared to cells from wild-type controls (Fig. 3C). We next examined whether Def6 deficient osteoclasts induced by TNF-α stimulation were functional by culturing BMMs on bovine bone slices during TNF-induced osteoclastogenesis. Similar to the results obtained using plastic tissue culture dishes, Def6−/− cell cultures exhibited markedly increased formation of TRAP positive multinucleated cells on the bone slices compared to the wild type controls (Fig. 3D). Furthermore, only the bone slices cultured with Def6−/− osteoclasts showed significant bone resorption, as determined by positive wheat germ agglutinin staining (Fig. 3E). These data demonstrate that TNF-induced Def6−/− osteoclasts possess bone resorptive capability. These results demonstrate that in the absence of Def6, RANKL-induced osteoclastogenesis is enhanced (Fig. 1), and importantly that TNF-α is sufficient to induce robust osteoclast differentiation and activity (Fig. 3A–E), even in the absence of RANKL. TNF-induced osteoclast differentiation is highly dependent on synergy with or pretreatment of RANKL (RANKL priming) in most in vitro culture systems. In inflammatory settings, osteoclast precursors are generally influenced by both RANKL and inflammatory cytokines, such as TNF-α that promotes osteoclast formation by priming osteoclast precursors or acting in synergy with RANKL. The mechanisms that regulate the priming or synergic effects between RANKL and TNF-α are not well understood. Here, we found that Def6 deficiency dramatically enhanced osteoclast formation in the RANKL (Fig. 3F) or TNF-α (Fig. 3G) priming conditions, indicating that Def6 contributes an important inhibitory role in the synergy between RANKL- and TNF-induced osteoclastogenesis.
Figure 3. Def6 deficiency increases TNF-α-induced osteoclast formation.
(A) BMMs derived from WT control and Def6−/− mice were treated with TNF-α (40 ng/ml) for 3 days. TRAP staining was performed (A) and the number of TRAP-positive MNCs per well was counted (B). TRAP-positive cells appear red in the photographs. Scale bar, 100μm. Data are representative of at least five independent experiments. **P<0.01. (C) Quantitative real-time PCR analysis of mRNA expression of Acp5 (encoding TRAP), Ctsk (encoding cathepsin K) and Itgb3 (encoding β3) in BMMs from the WT control and Def6−/− mice treated with TNF-α (40 ng/ml) for the indicated times. Data are representative of at least three independent experiments. **P<0.01. (D) TRAP staining of bone slices (left panel) on which the WT control and Def6−/− BMMs were cultured with TNF-α for 5 days. Osteoclasts formed on the bone slices appeared red by TRAP-staining, and were also indicated by white arrows. Right panel: the relative area of the osteoclasts (OCs) to the slice (%). **P<0.01. (E) Bone resorption indicated by yellow-red stained pits on the bovine bone slices by wheat germ agglutinin staining, which was also indicated by white arrowheads. Right panel: the relative area of the pits to the slice (%). **P<0.01. (F) The WT control and Def6−/− BMMs were treated with RANKL (5 ng/ml) for 1 day followed by co-stimulation with RANKL (5 ng/ml) and TNF-α (40 ng/ml) for 2 days. (G) The WT control and Def6−/− BMMs were treated with TNF-α (5 ng/ml) for 1 day followed by co-stimulation with TNF-α (5 ng/ml) and RANKL (40 ng/ml) for 2 days. Osteoclast formation was determined by TRAP staining (left panels in F and G) and the number of TRAP-positive MNCs per well (right panels in F and G). **P<0.01.
Def6 modulates TNF-α-induced osteolysis
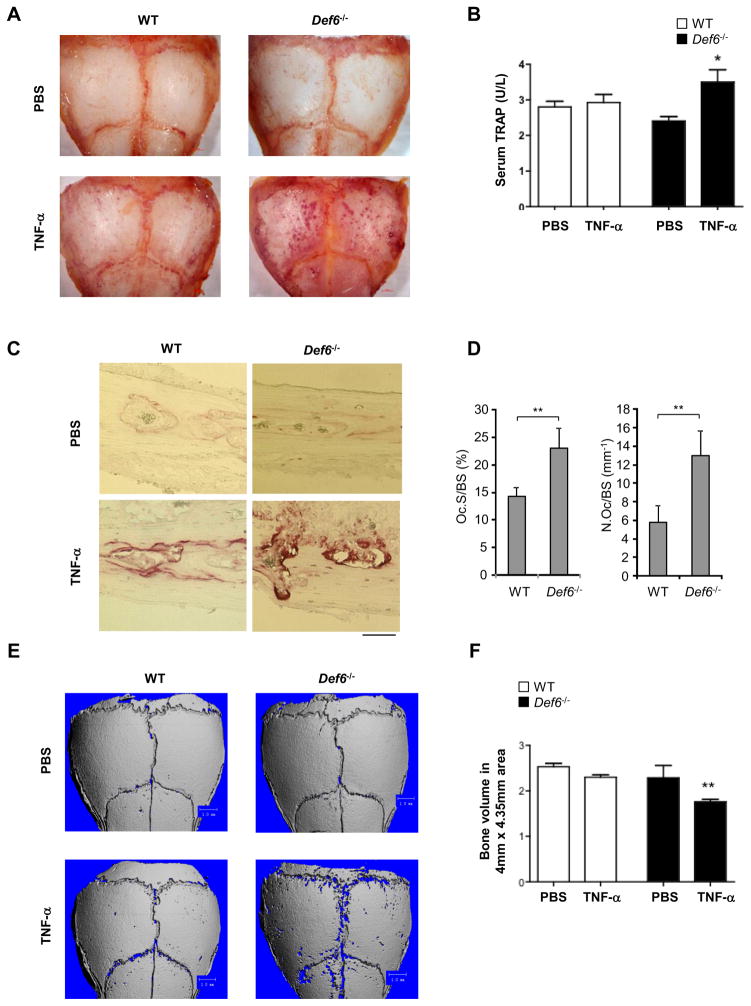
Based on our in vitro studies demonstrating that Def6 negatively controls TNF-induced osteoclast differentiation, we next explored the role of Def6 in modulating inflammatory osteoclastogenesis and bone resorption in vivo. We utilized a well-established TNF-α-induced inflammatory bone resorption mouse model (27, 43, 45) involving the injection of mouse recombinant TNF-α into the subcutaneous space over the calvarial bones of wild-type and Def6−/− mice. As a control, equal volumes of phosphate buffered saline (PBS) were injected. After 5 days, the calvarial bones were isolated and subjected to histological staining for the osteoclast marker TRAP (Fig. 4A). PBS injection did not induce TRAP positive osteoclastic erosions on the surface of the calvarial bones in wild type or Def6−/− mice. Wild-type mice receiving TNF showed, as expected, only minor erosions, identifiable by purple-red stained pits on the surface of the calvarial bones. Def6−/− mice, however, exhibited a marked increase in TNF-induced osteoclastic erosions, showing many purple stained pits on the surface of the calvarial bones (Fig. 4A). In parallel, we determined the serum TRAP levels after the treatments, which serve as a serological marker of osteoclast-mediated bone resorption. Consistent with the calvarial TRAP staining data, only Def6−/− mice receiving TNF-α injections showed a significant increase in serum TRAP levels (Fig. 4B). Furthermore, histological analysis of the calvarial bones showed significantly increased osteoclast numbers and surfaces in response to TNF-α treatment in the Def6 deficient mice (Fig. 4C, D), indicating that Def6 deficiency also enhances TNF-induced osteoclast formation in vivo. To assess the magnitude of bone erosion, we performed μCT scanning of the calvarial bones (Fig. 4E) and quantified bone resorption by evaluating the volume of bone loss within the resorption pits (Fig. 4F). Both of wild type and Def6−/− mice receiving PBS injections did not show evidence of erosions. Administration of TNF-α to the wild type mice induced only a small number of resorptive pits (Fig. 4E) with minimal volumetric bone loss (Fig. 4F). In contrast, Def6−/− mice exhibited markedly enhanced resorptive pit formation on the surface of the calvarial bones (Fig. 4E) and significant bone loss in response to TNF-α stimulation compared with wild type mice (Fig. 4F). These data confirm that Def6 deficiency enhances osteoclastogenesis and bone resoption in response to TNF-α in vivo and indicate that Def6 functions as a negative regulator of osteoclastogenesis providing a protective role in TNF-mediated inflammatory bone resorption.
Figure 4. Def6 deficiency promotes TNF-α induced bone erosion.
TRAP staining of calvarial bones (A), of calvarial histological sections (C) and concentration of serum TRAP (B) obtained from the WT and Def6−/− mice after five day application of TNF-α or PBS to the calvarial periosteum. (D) Histomorphometric analysis of calvarial slices. N.Oc/BS, number of osteoclasts per bone surface; Oc.S/BS, osteoclast surface per bone surface. *p<0.05. **p<0.01. n=5 in each group. Scale bar: 100μm. (E) μCT images of the surface of whole calvaria obtained from the indicated mice. (F) Quantification of bone volumes of the calvaria obtained from the indicated mice after five day application of PBS or TNF-α to the calvarial periosteum. n=5 in PBS group. n= 5 in TNF-α group. **p<0.01.
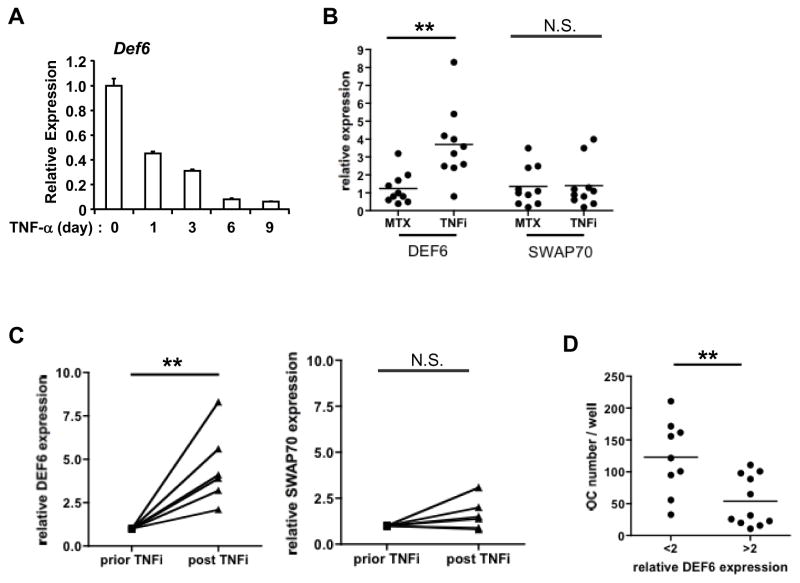
TNF-α regulates Def6 expression in human monocytes/macrophages
Having established that Def6 modulates the effects of TNF-α on osteoclast formation and bone resorption, we wished to examine whether TNF-α stimulation affects Def6 expression. We found that Def6 protein levels in mouse BMMs were decreased after TNF-α treatment or during the time course of RANKL-induced osteoclastogenesis (Supplementary Fig. 2). Moreover, TNF-α treatment also decreased DEF6 protein levels in human osteoclast precursors derived from CD14 (+) peripheral blood monocytes (PBMCs) from healthy donors (Fig. 5A). The TNF-induced down-regulation of Def6 results in the loss of a constraint on osteoclast differentiation and suggests that this effect contributes to the pathogenic mechanism by which TNF-α enhances inflammatory bone resorption.
Figure 5. DEF6 expression is regulated by TNF-α in human monocytes/macrophages.
(A) Quantitative real-time PCR analysis of mRNA expression of DEF6 in human CD14 (+) PBMC-derived macrophages treated by TNF-α (40 ng/ml) at the indicated times. (B) Quantitative real-time PCR analysis of mRNA expression of DEF6 or SWAP70 in CD14 (+) PBMCs isolated from RA patients treated with MTX (Methotrexate, 20 mg per week) or TNFi (TNF inhibitor adalimumab, 40 mg every two weeks). n=10 for each group. **P<0.01. (C) Quantitative real-time PCR analysis of mRNA expression of DEF6 or SWAP70 in PBMCs isolated from the same RA patient prior and post TNFi (TNF inhibitor adalimumab, 40 mg every two weeks). Results were normalized to prior TNFi condition for each individual patient. n=6. **P<0.01. (D) Osteoclast differentiation of human PBMCs obtained from two groups of RA patients with relative Def6 expression level (based on qPCR results) smaller than 2 (n=9) or greater than 2 (n=11). Human CD14 (+) PBMC-derived macrophages were treated with TNF-α (40 ng/ml) for five days and TRAP-positive osteoclast numbers were counted. **P<0.01.
Given the importance of TNF-α in the pathogenesis of rheumatoid arthritis and the resounding success of TNF blockade therapy (TNFi) in the treatment of rheumatoid arthritis, we next investigated the expression levels of Def6 in the PBMCs from patients with RA treated with either methotrexate (MTX) or a humanized antibody that specifically blocks TNF activity (adalimumab) (TNFi therapy) (Fig. 5B). We found that TNFi treatment resulted in a 4-fold increase in DEF6 expression levels in PBMCs compared to the MTX therapy. As a control, we also determined the relative expression levels of SWAP70, a homolog molecule of DEF6, the levels of which did not differ in the two treatment groups, consistent with a direct and specific effect of TNF-α on Def6 expression. Moreover, we determined the relative expression of DEF6 in PBMCs from the same RA patient prior to and after TNFi therapy. Strikingly, we found that the therapeutic reduction of TNF activity lead to an up to 7-fold increase in DEF6 mRNA expression levels (Fig. 5C). Collectively, these data show that the blockade of TNF-α in vivo results in a significant increase in DEF6 expression levels in PBMCs. Taken together with the in vitro data that TNF-α stimulation downregulates DEF6 expression, these results indicate that there is a strong inverse correlation between the expression levels of TNF-α and DEF6 and support the concept that Def6 provides a negative feedback mechanism that constrains the osteoclastogenic effect of TNF-α and protects hosts from TNF-dependent pathological bone loss (Figs 3–4). DEF6 expression levels in PBMC osteoclast precursors thus could serve as an indicator of the capacity of TNF-α to enhance osteoclastogenesis in a given patient with RA. To test this hypothesis, we divided the RA patients into two groups according to their relative expression level of Def6 in PBMCs (>2 or < 2 fold induction by Real Time PCR). We then performed ex vivo osteoclastogenesis studies using the PBMCs from these two patient groups. Our results demonstrate that the patients with a relative expression level of DEF6 smaller than two exhibited an almost 2-fold increase in osteoclast numbers when compared to those with a relative expression level of DEF6 greater than two (Fig. 5D), supporting the idea that DEF6 levels negatively correlate with the osteoclastogenic capacity.
Def6 suppresses the expression of the c-Fos-Blimp1/NFATc1 axis by regulating an endogenous IFN-β-mediated autocrine inhibitory loop
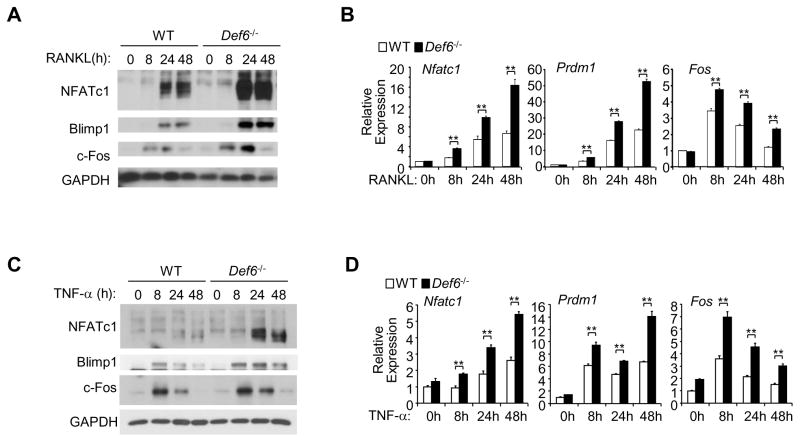
We next explored the mechanisms by which Def6 inhibits osteoclast differentiation. Def6 is reported as a novel type of Rho GTPase activators that plays a role in the activation of downstream effectors Rac and Cdc42 in T cells (32, 46) and small GTPases, including Rac and Cdc42, have been shown to regulate RANKL-induced osteoclastogenesis (47, 48). We therefore tested whether the inhibitory effects of Def6 on osteoclastogenesis were dependent on its GEF activity. We found that TNF-α stimulation for 24 hours did increase the amount of Rac1-GTP in mouse BMMs using pull-down of the Rac1-GTP bound effector protein p21 activated kinase (PAK), but the difference between wild-type and Def6−/− cells was not consistent or significant (data not shown), potentially suggesting that Def6 may exert its regulatory role independent of its GEF activity during TNF-α-induced osteoclastogenesis. We next examined the expression levels of NFATc1, a key transcription factor involved in regulation of osteoclast differentiation. TNF-α minimally increased NFATc1 expression in WT BMMs. In contrast, Def6 deficiency dramatically enhanced both RANKL and TNF-α-induced NFATc1 expression levels (Fig. 6A–D). Previous studies have shown a reciprocal positive regulation between NFATc1 and Blimp1 expression during osteoclastogenesis (27, 49, 50). Consistent with this, Blimp1 was more highly induced by RANKL or TNF-α treatment in Def6 deficient cells than WT cells, similar to the effects of these treatments on NFATc1 expression (Fig. 6A–D). In order to identify the mechanisms by which Def6 controls NFATc1 induction, we explored the upstream signaling pathways that regulate NFATc1/Blimp1 expression. We did not observe significant or consistent differences in NF-κB or MAPK signaling pathways induced by TNF-α in WT or Def6 deficient cell cultures (Supplementary Fig. 3). ITAM-mediated costimulation is essential for calcium signaling-induced NFATc1 induction and osteoclastogenesis and prior studies have shown that Def6 regulates calcium signaling and NFATc1 translocation and activation in T cells (46, 51). Although we observed a trend of an increase in the activity of PLCγ2 and downstream calcium oscillation in Def6 deficient cells in response to TNF-α when compared to WT cells, the difference was not significant (data not shown). However, c-Fos, another key upstream regulator of NFATc1, was significantly enhanced in Def6 deficient cells during both RANKL- or TNF-induced osteoclast differentiation (Fig. 6A–D). Furthermore, Def6 deficiency significantly increased RANKL- or TNF-α-induced mRNA expression levels of NFATc1, Blimp1 and c-Fos (Fig. 6B, D). Thus, Def6 negatively regulates c-Fos-mediated NFATc1/Blimp1 axis.
Figure 6. Def6 inhibits the induction of c-Fos, Blimp1 and NFATc1 by TNF-α or RANKL during osteoclastogenesis.
(A) Immunoblot analysis of the expression of NFATc1, Blimp1 and c-Fos induced by TNF-α (40 ng/ml) or RANKL (40 ng/ml) in the indicated time course. GAPDH was used as a loading control. (B) Quantitative real-time PCR analysis of the mRNA expression of Nfatc1 (encoding NFATc1), Prdm1 (encoding Blimp1) and Fos (encoding c-Fos). **P<0.01.
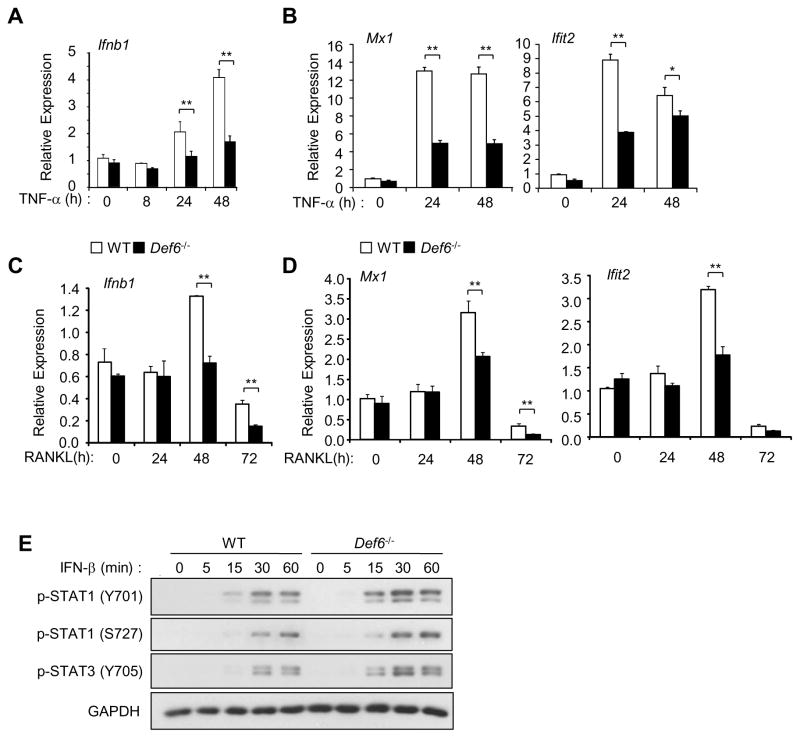
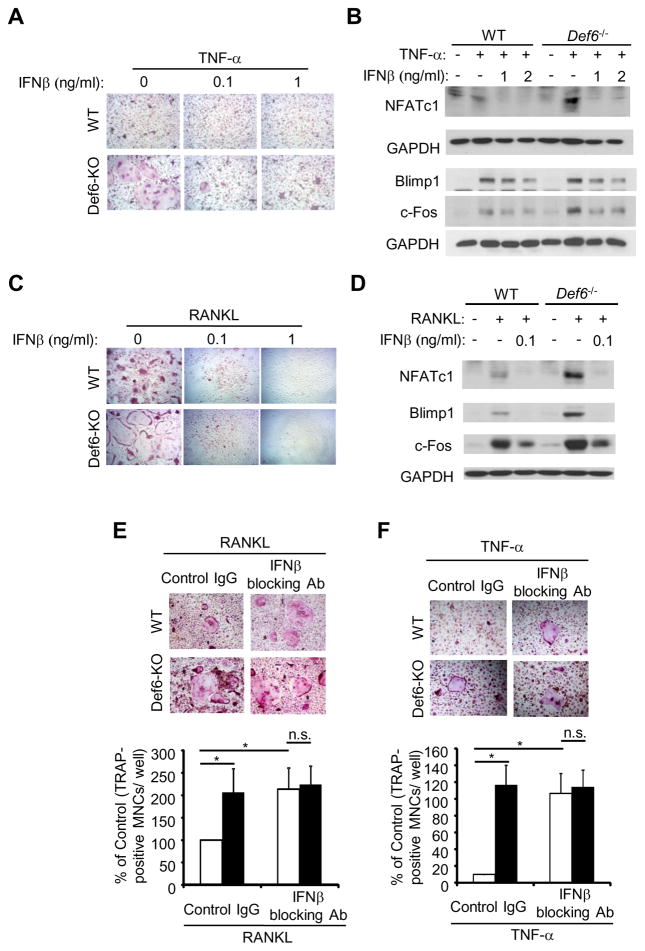
As endogenous IFN-β produced by macrophages/osteoclast precursors is a strong feed-back mechanism that restrains osteoclast differentiation and c-Fos expression (30). We therefore asked whether the inhibitory effects of Def6 on the c-Fos/NFATc1/Blimp1 axis involved IFN-β. Previous studies have demonstrated that RANKL or TNF-α treatment can induce type I IFN expression in macrophages/osteoclast precursors. Although the magnitude of type I IFNs induction by RANKL or TNF-α is small (approximately 10 pg/ml after 24 hour stimulation) (29, 30) when compared with other stimuli such as Toll Like Receptor (TLR) stimulation, type I IFNs show significant effects on the inhibition of osteoclast differentiation. Consistent with these observations (29, 30), we found that both TNF-α and RANKL induced IFN-β expression in WT BMMs and that Def6 deficiency significantly attenuated IFN-β induction induced by these treatments (Fig. 7A, C). The attenuation of IFN expression by Def6 deficiency was further corroborated by the demonstration that Def6 deficiency also attenuated the expression of IFN responsive gene expressions of Mx1 and Ifit2 after TNF-α or RANKL treatment (Fig. 7B, D). The expression of IFN responsive genes can be influenced by the IFN-β expression level and/or the cellular response to IFN-β. We examined the latter possibility by comparing the activation of IFN signaling pathways using a low amount of exogenous IFN-β to stimulate the wild-type and Def6 deficient BMMs. We found that very similar or slightly higher levels of phospho-stat1, 2 and 3 were induced by IFN-β in Def6 deficient cells compared to WT cells (Fig. 7E), indicating that Def6 does not significantly alter cell responsiveness to IFN-β but instead affects the expression of IFN-β (Fig. 7A, C). To test whether this is a prominent mechanism controlling the Def6-mediated osteoclast differentiation program, we first added low amounts of exogenous IFN-β to the TNF-α or RANKL-induced Def6 KO BMMs. We found that very small amounts of IFN-β prevented the enhanced osteoclast formation induced by TNF-α (Fig. 8A). Furthermore, treatment with small amounts of IFN-β also reversed the TNF-enhanced c-Fos and downstream NFATc1 and Blimp1 expression in Def6 deficient cells (Fig. 8B) resulting in expression levels comparable to the TNF-treated WT cultures. The same amount of IFN-β almost completely blocked RANKL-induced osteoclast differentiation as well as Def6 deficiency enhanced expression of c-Fos, NFATc1 and Blimp1 (Fig. 8C, D). Furthermore, we blocked the inhibitory function of endogenous IFN-β on osteoclastogenesis using an IFN-β neutralizing antibody. Consistent with previous findings, blocking of endogenous IFN-β enhanced RANKL- or TNF-induced osteoclastogenesis in WT cell cultures (Fig. 8E and F). The effect of Def6 deficiency on enhanced osteoclastogenesis, however, was abrogated by blocking IFN-β, as evidenced by the data that Def6 deletion increased osteoclastogenesis in the presence of the control IgG, but not in the presence of the IFN-β neutralizing antibody (Fig. 8E and F). These data collectively indicate that Def6-regulated endogenous IFN-β production contributes to the control of osteoclast differentiation by RANKL or TNF-α and also indicate that Def6 suppresses c-Fos and NFATc1/Blimp1 axis at least partially via regulating an endogenous IFN-β mediated inhibitory loop during osteoclastogenesis.
Figure 7. Def6 deficiency suppresses endogenous IFN-β expression induced by TNF-α or RANKL.
Quantitative real-time PCR analysis of the expression of Ifnb1 (encoding IFN-β) (A, C) and Mx1 (encoding Mx1) and Ifit2 (encoding Ifit2) (B, D) in WT control and Def6−/− BMMs stimulated with TNF-α (40 ng/ml) or RANKL (40 ng/ml) during osteoclastogenesis. (E) Immunoblot analysis of the induction of p-STAT1 and 3 in WT control and Def6−/− BMMs stimulated by mouse recombinant IFN-β (100 U/ml) at the indicated time points. GAPDH was used as a loading control.
Figure 8. IFN-β levels regulate the Def6 effects on osteoclastogenesis and osteoclast gene expression.
(A, C) Osteoclast differentiation induced by TNF-α (40 ng/ml) or RANKL (40 ng/ml) for 3 days in the absence or presence of a serial doses of mouse recombinant IFN-β in the WT control and Def6−/− BMMs. (B, D) Immunoblot analysis of the expression of NFATc1, Blimp1 and c-Fos induced by TNF-α (40 ng/ml) or RANKL (40 ng/ml) without or with IFN-β in the WT control and Def6−/− BMMs. (E, F) BMMs were treated with the control IgG (10 U/ml) or IFN-β neutralizing antibody (10 U/ml) in the presence of RANKL (40 ng/ml) or TNF-α (40 ng/ml) for 4 days. Upper panel: TRAP staining; Lower panel: quantification of the number of TRAP+ MNCs (≥3 nuclei/cell) per well relative to the control.
Discussion
Bone destruction is a major cause of morbidity and disability in many inflammatory skeletal diseases, such as rheumatoid arthritis (RA), psoriatic arthritis, periodontitis and peri-prosthetic loosening (9–12). The mechanisms that regulate inflammatory osteoclastogenesis and bone resorption are not fully understood. In this study, we identified Def6 as a novel negative regulator of osteoclastogenesis in both physiological and inflammatory conditions. Our results indicate that Def6 plays a protective role in TNF-α-induced osteolysis and suppresses osteoclastogenesis by regulating an endogenous IFN-β-mediated autocrine inhibitory loop. The role of Def6 in osteoclastogenesis and the close correlation between Def6 and TNF-α levels in RA patients suggest that the Def6-pathway represents an attractive therapeutic target to prevent bone destruction in inflammatory diseases associated with enhanced bone resorption.
Def6 exhibits significant sequence homology to only one other molecule Swap70, a novel type of GEF. Def6 is abundantly expressed in lymphoid tissues, thymocytes, B and T cells in which it regulates immunological synapse formation, cytoskeletal reorganization and cytokine production while SWAP70 is highly expressed in B cells and mast cells, in which it functions as an adapter in signal transduction and a GEF for the small GTPases Rac and cdc42. In contrast, in myeloid lineage cells, including macrophages, dendritic cells and osteoclast precursors, both Def6 and Swap70 are expressed. Interestingly, however, Def6 and Swap70 play different roles in osteoclast biology although they are highly homologous in sequences and structural domain arrangement. Swap70 does not significantly affect the differentiation of osteoclasts, but was found by Jessberger group (39) and also our group (unpublished data) to promote osteoclast resorptive function by regulating F-actin rearrangement. In this study, we revealed the inhibitory role of Def6 in osteoclast differentiation. Unlike the Swap70 knockout cells, the Def6 deficient osteoclasts are able to form actin rings. Notably, Swap-70 has an actin binding motif at its extreme C terminus, which is required for actin ring formation and bone resorption in osteoclasts (39, 52, 53). This F-actin binding motif, however, is absent in Def6. This small but important structural difference would thus at least partially explain their distinct effects on actin ring organization as well as resorptive function in osteoclasts. In addition, the expression patterns of Def6 and Swap70 are different during osteoclastogenesis. Def6 expression is down regulated at an early stage of osteoclast differentiation, while Swap70 is gradually induced and reaches high levels when osteoclasts become mature ((39) and Zhao B, unpublished data). Thus, the osteoclastogenic signaling induced by RANKL or TNF-α seems to confine the expression of Def6 and Swap70 to different stages of osteoclastogenesis. The expression kinetics of Def6 and Swap70 correlate with their functional activity at different stages of osteoclastogenesis. Def6 regulates osteoclast differentiation at an early phase, and Swap70 mainly controls osteoclast function at late stage. This phenomenon is similar to the differential roles of Def6 and Swap70 in distinct stages of B cell differentiation, in which Def6 appears to primarily control the early phase while Swap70 regulates terminal differentiation (54, 55). These findings collectively indicate that Def6 and Swap70 are not functionally redundant but work coordinately to regulate B cell differentiation as well as osteoclast differentiation and functional activity.
NFATc1 plays a central role in TCR responsiveness and regulation of cytokine production in T cells (56). During osteoclastogenesis, NFATc1 also functions as a master regulator of osteeoclast differentation (57). Interestingly, NFATc1 is differentially regulated by Def6 in T cells and osteoclast precursors. In unstimulated T cells, Def6 does not exhibit GEF activity due to an inhibitory interaction between its N- and C-termini. Upon TCR engagement, Def6 is phosphorylated via Lck, which disrupts the autoinhibitory interaction and allows Def6 to translocate to the immunological synapse to activate NFATc1 in a Rho-GTPases Rac and Cdc42 dependent manner (34, 46). The phosphorylation of Def6, activation of Rho-GTPases and NFATc1 by Def6 occur rapidly upon TCR stimulation. In T cells, NFATc1 is expressed abundantly at basal levels and Def6 is required mainly for inducing its activity but not for inducing its expression. In contrast, NFATc1 expression levels are almost undetectable in macrophages and osteoclast precursors, and are induced to high levels approximately two to three days after RANKL stimulation. Different from TCR responsiveness, RANK activation by RANKL or TNFR activation by TNF-α in macrophages or osteoclast precursors does not activate Lck (data not shown). Furthermore, the regulation of NFATc1 expression by Def6 during osteoclastogenesis requires long term stimulation by RANKL or TNF-α. These differences can explain at least partially the differential regulation of NFATc1 by Def6 in distinct cell types.
In this study, we identified a novel mechanism by which Def6 inhibits osteoclast differentiation and NFATc1 expression by promoting RANKL- or TNF-induced endogenous IFN-β production, thereby contributing to the type I IFN-mediated autocrine inhibition of osteoclast differentiation (30). In response to strong IFN-β inducers, such as LPS, Def6 alone does not significantly affect IFN-β production in peritoneal macrophages (40). However, the lack of both Def6 and Swap70 results in a hyper-responsiveness in bone marrow derived dendritic cells to LPS stimulation and leads to increased IFN-β expression (58). The differential regulation of IFN-β by Def6 in these distinct settings suggest that Def6 plays a role in fine tuning IFN-β expression in a cellular and specific context dependent manner. Transcription of Ifnb1 involves, in most cases, the activation of NF-κB and various interferon regulatory factors (IRFs), depending on the cell types and context of the treatment (28). We did not observe differences in NF-κB signaling between WT and Def6−/− BMMs, but found significantly decreased nuclear IRF1 levels in TNF-α-treated Def6−/− BMMs during osteoclastogenesis (Zhao B, unpublished data). TNF-α–induced IFN-β expression in monocytes/macrophages is mainly dependent on IRF1 (29). Thus, the decreased IRF1 level in Def6 KO cells contributes to the lower IFN-β expression, and also indicates that Def6 regulates Ifnb1 transcription at least in part via modulation of IRF1 nuclear expression level. Future studies are required to fully dissect the underlying molecular mechanisms by which Def6 regulates IFN-β expression. A recent finding shows that Lgr4 is a new receptor for RANKL and the signal through Lgr4 suppresses osteoclastogenesis (60). In our experiments, we found that the RANKL-regulated Def6 expression was not affected by the knockdown of Lgr4 expression (Supplementary Fig. 4A, B), suggesting that RANKL regulates Def6 expression independently of Lgr4 receptor. Collectively, the diverse roles and differential mechanisms by which Def6 regulates cellular activities demonstrate that Def6 is a multifunctional regulator that plays specific roles in a cell and context dependent manner.
The importance of Def6 was first revealed by the evidence obtained from animal models. Def6 deficient DO11.10 mice on the Balb/c background with TCR activation developed a RA-like joint disease with bone erosion (37). In addition, Def6 deficient female mice on a mixed 129/BL6 background develop a lupus-like syndrome (34, 42). Most recent studies identify Def6 as a new systemic lupus erythematosus (SLE) risk variant (59), thus revealing the importance of Def6 in human diseases. In the present study, we not only uncovered a key role of Def6 in inhibiting osteoclast formation and bone resorption, but also provided a new link between Def6 and human diseases and demonstrated a significant negative correlation between Def6 expression levels in osteoclast precursors and TNF-α activity in vivo in patients with RA. Our data identify Def6 as a novel negative regulator of osteoclastogenesis in both physiological and inflammatory conditions, acting via novel mechanism driven by the Def6-IFN-β axis and suggest that this Def6 pathway represents a novel therapeutic target to prevent pathological bone destruction in inflammatory disorders.
Supplementary Material
Acknowledgments
G.S. was supported by the Deutsche Forschungsgemeinschaft (SPP1468-IMMUNOBONE, CRC1181), the Bundesministerium für Bildung und Forschung (BMBF; project Metharthros), the Marie Curie project OSTEOIMMUNE and the IMI funded project BTCure. This work was supported by grants from the National Institutes of Health AR062047 and AR068970 (B.Z.).
We thank Mahmoud Elguindy for valuable discussion and technical assistance.
Abbreviations
- BMMs
bone marrow derived macrophages
- RANK
receptor activator for NF-κB
- RANKL
receptor activator for NF-κB ligand
- siRNA
small interfering RNA
- Blimp1
B lymphocyte-induced maturation protein-1
- Def6
Differentially expressed in FDCP 6 homolog
- Irf8
interferon regulatory factor
- MNCs
multinucleated cells
- TRAP
tartrate resistant acid phosphatase
- RA
rheumatoid arthritis
Footnotes
Disclosures: The authors have no financial conflict of interest.
References
- 1.Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3:481. doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473:201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285:25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takayanagi H. SnapShot: Osteoimmunology. Cell Metab. 2015;21:502, e501. doi: 10.1016/j.cmet.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Goldring SR. Osteoimmunology and bone homeostasis: relevance to spondyloarthritis. Curr Rheumatol Rep. 2013;15:342. doi: 10.1007/s11926-013-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schett G. Osteoimmunology in rheumatic diseases. Arthritis Res Ther. 2009;11:210. doi: 10.1186/ar2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenzo J, Choi Y. Osteoimmunology. Immunol Rev. 2005;208:5–6. doi: 10.1111/j.0105-2896.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 8.Pacifici R. Osteoimmunology and its implications for transplantation. Am J Transplant. 2013;13:2245–2254. doi: 10.1111/ajt.12380. [DOI] [PubMed] [Google Scholar]
- 9.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldring SR, Purdue PE, Crotti TN, Shen Z, Flannery MR, Binder NB, Ross FP, McHugh KP. Bone remodelling in inflammatory arthritis. Ann Rheum Dis. 2013;72(Suppl 2):ii52–55. doi: 10.1136/annrheumdis-2012-202199. [DOI] [PubMed] [Google Scholar]
- 11.Teitelbaum SL. Osteoclasts; culprits in inflammatory osteolysis. Arthritis Res Ther. 2006;8:201. doi: 10.1186/ar1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyce BF, Schwarz EM, Xing L. Osteoclast precursors: cytokine-stimulated immunomodulators of inflammatory bone disease. Curr Opin Rheumatol. 2006;18:427–432. doi: 10.1097/01.bor.0000231913.32364.32. [DOI] [PubMed] [Google Scholar]
- 13.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B, Ivashkiv LB. Negative regulation of osteoclastogenesis and bone resorption by cytokines and transcriptional repressors. Arthritis Res Ther. 2011;13:234. doi: 10.1186/ar3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 16.Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92:860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu Rev Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 18.Schett G, Teitelbaum SL. Osteoclasts and arthritis. J Bone Miner Res. 2009;24:1142–1146. doi: 10.1359/jbmr.090533. [DOI] [PubMed] [Google Scholar]
- 19.Regard JB, Zhong Z, Williams BO, Yang Y. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, Yeh WC, Lee SK, Lorenzo JA, Choi Y. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202:589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Z, Li P, Zhang Q, Schwarz EM, Keng P, Arbini A, Boyce BF, Xing L. Tumor necrosis factor-alpha increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J Biol Chem. 2006;281:11846–11855. doi: 10.1074/jbc.M512624200. [DOI] [PubMed] [Google Scholar]
- 26.Boyce BF, Xiu Y, Li J, Xing L, Yao Z. NF-kappaB-Mediated Regulation of Osteoclastogenesis. Endocrinol Metab (Seoul) 2015;30:35–44. doi: 10.3803/EnM.2015.30.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao B, Grimes SN, Li S, Hu X, Ivashkiv LB. TNF-induced osteoclastogenesis and inflammatory bone resorption are inhibited by transcription factor RBP-J. J Exp Med. 2012;209:319–334. doi: 10.1084/jem.20111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitha PM, Kunzi MS. Type I interferon: the ever unfolding story. Curr Top Microbiol Immunol. 2007;316:41–70. doi: 10.1007/978-3-540-71329-6_4. [DOI] [PubMed] [Google Scholar]
- 29.Yarilina A, Park-Min KH, Antoniv T, Hu X, Ivashkiv LB. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol. 2008;9:378–387. doi: 10.1038/ni1576. [DOI] [PubMed] [Google Scholar]
- 30.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka Y, Bi K, Kitamura R, Hong S, Altman Y, Matsumoto A, Tabata H, Lebedeva S, Bushway PJ, Altman A. SWAP-70-like adapter of T cells, an adapter protein that regulates early TCR-initiated signaling in Th2 lineage cells. Immunity. 2003;18:403–414. doi: 10.1016/s1074-7613(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 32.Gupta S, Fanzo JC, Hu C, Cox D, Jang SY, Lee AE, Greenberg S, Pernis AB. T cell receptor engagement leads to the recruitment of IBP, a novel guanine nucleotide exchange factor, to the immunological synapse. J Biol Chem. 2003;278:43541–43549. doi: 10.1074/jbc.M308960200. [DOI] [PubMed] [Google Scholar]
- 33.Mavrakis KJ, McKinlay KJ, Jones P, Sablitzky F. DEF6, a novel PH-DH-like domain protein, is an upstream activator of the Rho GTPases Rac1, Cdc42, and RhoA. Exp Cell Res. 2004;294:335–344. doi: 10.1016/j.yexcr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Biswas PS, Bhagat G, Pernis AB. IRF4 and its regulators: evolving insights into the pathogenesis of inflammatory arthritis? Immunol Rev. 2010;233:79–96. doi: 10.1111/j.0105-2896.2009.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altman A, Becart S. Does Def6 deficiency cause autoimmunity? Immunity. 2009;31:1–2. doi: 10.1016/j.immuni.2009.06.013. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 36.Fos C, Becart S, Canonigo Balancio AJ, Boehning D, Altman A. Association of the EF-hand and PH domains of the guanine nucleotide exchange factor SLAT with IP(3) receptor 1 promotes Ca(2)(+) signaling in T cells. Sci Signal. 2014;7:ra93. doi: 10.1126/scisignal.2005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Q, Yang W, Gupta S, Biswas P, Smith P, Bhagat G, Pernis AB. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, Gotoh Y, Ihara S, Nagata S, Itoh H, Fukui Y, Jessberger R. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature. 2002;416:759–763. doi: 10.1038/416759a. [DOI] [PubMed] [Google Scholar]
- 39.Garbe AI, Roscher A, Schuler C, Lutter AH, Glosmann M, Bernhardt R, Chopin M, Hempel U, Hofbauer LC, Rammelt S, Egerbacher M, Erben RG, Jessberger R. Regulation of bone mass and osteoclast function depend on the F-actin modulator SWAP-70. J Bone Miner Res. 2012;27:2085–2096. doi: 10.1002/jbmr.1670. [DOI] [PubMed] [Google Scholar]
- 40.Chen Q, Gupta S, Pernis AB. Regulation of TLR4-mediated signaling by IBP/Def6, a novel activator of Rho GTPases. J Leukoc Biol. 2009;85:539–543. doi: 10.1189/jlb.0308219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youn BU, Kim K, Kim JH, Lee J, Moon JB, Kim I, Park YW, Kim N. SLAT negatively regulates RANKL-induced osteoclast differentiation. Mol Cells. 2013;36:252–257. doi: 10.1007/s10059-013-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fanzo JC, Yang W, Jang SY, Gupta S, Chen Q, Siddiq A, Greenberg S, Pernis AB. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. J Clin Invest. 2006;116:703–714. doi: 10.1172/JCI24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Miller CH, Giannopoulou E, Hu X, Ivashkiv LB, Zhao B. RBP-J imposes a requirement for ITAM-mediated costimulation of osteoclastogenesis. J Clin Invest. 2014;124:5057–5073. doi: 10.1172/JCI71882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Z, Xing L, Boyce BF. NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J Clin Invest. 2009;119:3024–3034. doi: 10.1172/JCI38716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL. M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest. 2005;115:3418–3427. doi: 10.1172/JCI26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becart S, Balancio AJ, Charvet C, Feau S, Sedwick CE, Altman A. Tyrosine-phosphorylation-dependent translocation of the SLAT protein to the immunological synapse is required for NFAT transcription factor activation. Immunity. 2008;29:704–719. doi: 10.1016/j.immuni.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weivoda MM, Oursler MJ. The Roles of Small GTPases in Osteoclast Biology. Orthop Muscular Syst. 2014:3. doi: 10.4172/2161-0533.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Touaitahuata H, Blangy A, Vives V. Modulation of osteoclast differentiation and bone resorption by Rho GTPases. Small GTPases. 2014;5:e28119. doi: 10.4161/sgtp.28119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishikawa K, Nakashima T, Hayashi M, Fukunaga T, Kato S, Kodama T, Takahashi S, Calame K, Takayanagi H. Blimp1-mediated repression of negative regulators is required for osteoclast differentiation. Proc Natl Acad Sci U S A. 2010;107:3117–3122. doi: 10.1073/pnas.0912779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyauchi Y, Ninomiya K, Miyamoto H, Sakamoto A, Iwasaki R, Hoshi H, Miyamoto K, Hao W, Yoshida S, Morioka H, Chiba K, Kato S, Tokuhisa T, Saitou M, Toyama Y, Suda T, Miyamoto T. The Blimp1-Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J Exp Med. 2010;207:751–762. doi: 10.1084/jem.20091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becart S, Charvet C, Canonigo Balancio AJ, De Trez C, Tanaka Y, Duan W, Ware C, Croft M, Altman A. SLAT regulates Th1 and Th2 inflammatory responses by controlling Ca2+/NFAT signaling. J Clin Invest. 2007;117:2164–2175. doi: 10.1172/JCI31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ihara S, Oka T, Fukui Y. Direct binding of SWAP-70 to non-muscle actin is required for membrane ruffling. J Cell Sci. 2006;119:500–507. doi: 10.1242/jcs.02767. [DOI] [PubMed] [Google Scholar]
- 53.Pearce G, Audzevich T, Jessberger R. SYK regulates B-cell migration by phosphorylation of the F-actin interacting protein SWAP-70. Blood. 2011;117:1574–1584. doi: 10.1182/blood-2010-07-295659. [DOI] [PubMed] [Google Scholar]
- 54.Biswas PS, Gupta S, Stirzaker RA, Kumar V, Jessberger R, Lu TT, Bhagat G, Pernis AB. Dual regulation of IRF4 function in T and B cells is required for the coordination of T-B cell interactions and the prevention of autoimmunity. J Exp Med. 2012;209:581–596. doi: 10.1084/jem.20111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quemeneur L, Angeli V, Chopin M, Jessberger R. SWAP-70 deficiency causes high-affinity plasma cell generation despite impaired germinal center formation. Blood. 2008;111:2714–2724. doi: 10.1182/blood-2007-07-102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naito T, Tanaka H, Naoe Y, Taniuchi I. Transcriptional control of T-cell development. Int Immunol. 2011;23:661–668. doi: 10.1093/intimm/dxr078. [DOI] [PubMed] [Google Scholar]
- 57.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–237. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 58.Manni M, Gupta S, Nixon BG, Weaver CT, Jessberger R, Pernis AB. IRF4-Dependent and IRF4-Independent Pathways Contribute to DC Dysfunction in Lupus. PLoS One. 2015;10:e0141927. doi: 10.1371/journal.pone.0141927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun C, Molineros JE, Looger LL, Zhou XJ, Kim K, Okada Y, Ma J, Qi YY, Kim-Howard X, Motghare P, Bhattarai K, Adler A, Bang SY, Lee HS, Kim TH, Kang YM, Suh CH, Chung WT, Park YB, Choe JY, Shim SC, Kochi Y, Suzuki A, Kubo M, Sumida T, Yamamoto K, Lee SS, Kim YJ, Han BG, Dozmorov M, Kaufman KM, Wren JD, Harley JB, Shen N, Chua KH, Zhang H, Bae SC, Nath SK. High-density genotyping of immune-related loci identifies new SLE risk variants in individuals with Asian ancestry. Nat Genet. 2016;48:323–330. doi: 10.1038/ng.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo J, Yang Z, Ma Y, Yue Z, Lin H, Qu G, Huang J, Dai W, Li C, Zheng C, Xu L, Chen H, Wang J, Li D, Siwko S, Penninger JM, Ning G, Xiao J, Liu M. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med. 2016;22:539–546. doi: 10.1038/nm.4076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.