Abstract
Background
Rb1 is a ginsenoside steroid glycoside found exclusively in the plant Panax ginseng. In an earlier report, we showed that Rb1 increased cell proliferation and reduced VEGF (vascular endothelial growth factor) secretion by human retinal pigment epithelial (ARPE19) cells.
Objective
In the present study, we hypothesized that chemical modification of Rb1 changes the level of VEGF secretion by ARPE19 cells.
Method
Three derivatives of Rb1 were chemically synthesized by hydrogenation (Rb1-H2), acetylation (Rb1-Acyl), and epoxidation (Rb1-Epoxy). Structural modifications were confirmed by 1H Nuclear Magnetic Resonance (NMR) spectra and Mass Spectrometry (MS). To test the biological activity, chemically modified compounds were added to cell culture media and incubated for 72 hours at a concentration of 250 nM at 37°C. Conditioned media were collected and cells were harvested/counted after treatment. Viable cell numbers were determined by the trypan blue dye exclusion method and VEGF levels by Enzyme-Linked Immunosorbent Assays (ELISA).
Results
Consistent with the prior report, results of the present study show Rb1 increased cell proliferation and decreased VEGF secretion. Similar to Rb1’s effect on cell proliferation, treatment with Rb1-H2, Rb1-Acyl and Rb1-Epoxy resulted in an increase in cell numbers. In contrast to Rb1-induced decrease in VEGF secretion, treatment with Rb1-H2, Rb-Acyl and Rb1-Epoxy resulted in increased VEGF levels.
Conclusion
Chemical modifications of the ginsenoside Rb1 significantly affect the biological activity of VEGF secretion by ARPE19 cells. Additional SAR (Structure Activity Relationship) experiments will be conducted to study the detailed mechanisms by which how specific modifications of Rb1 functional groups alter biological activities.
Keywords: Cell viability, cytokine release, ginsenoside, structure activity relationship
INTRODUCTION
Ginsenosides or panaxosides are a class of steroid glycosides, and triterpene saponins, found exclusively in the plant genus Panax (ginseng). Although ginsenosides occur as a wide array of structurally related analogues, only a few of the major components have received attention due to trace occurrence and difficulty in isolation. Only major ginsenoside components have been the target of research, as they are viewed as the active compounds behind the claims of ginseng’s therapeutic efficacy. Individual ginsenosides appear to have distinct effects [1]. Whereas one particular ginsenoside stimulates the central nervous system, another sedates the central nervous system. Other ginsenosides produce different effects as balancing metabolic processes, decreasing blood sugar, improving muscle tone, stimulating the endocrine system and maintaining proper hormone levels. Research has shown that ginseng is effective in maintaining and even restoring cellular functions and therefore may reduce a number of symptoms of old-age [1]. Because ginsenosides appear to affect multiple pathways, their mechanisms of action are complex and yet to be fully elucidated.
Some ginsenosides have been isolated to purity by column chromatography. Based on structural differences, they are categorized into two main groups: the Rb1 group (characterized by the presence of protopanaxadiol: Rb1, Rb2, Rc and Rd) and the Rg1 group (characterized by the presence of protopanaxatriol: Rg1, Re, Rf, and Rg2) [2]. Recent studies have shown that acetyl modifications to the protopanaxadiol saponin compounds have notable activity against cancer cells [3]. In the present study, chemical modifications of Rb1 by hydrogenation (Rb1-H2), acylation (Rb1-Acyl), and epoxidation (Rb1-Epoxy) were performed and these Rb1 derivatives were tested (in ARPR-19 cells) to determine how their biological activities were changed in comparison to the parent Rb1 compound.
Macular degeneration is a progressive eye disease affecting as many as 15 million Americans and around 196 million globally. This disease attacks the macula of the eye, where our sharpest central vision occurs. Although it rarely results in complete blindness, it depletes the individual of all but the outermost, peripheral vision, leaving only dim images at the center of vision. AMD (Age-related Macular Degeneration) is characterized by angiogenesis, the abnormal growth of new blood vessels [4], which resulted in the leakage of fluid and blood into the retina, inducing scar formation and destroying central vision. AMD is a slow progressive disease associated with genetic as well as environmental risk factors including cigarette smoking, diet and prolong intense light exposure [5, 6].
Diabetic retinopathy is the result of retinal microvascular changes. Hyperglycemia-induced intramural pericyte apoptosis and thickening of the basement membrane [7]. These damages render retinal blood vessels more permeable. Pericyte death is associated with “hyperglycemia persistently activates protein kinase C-3 and p38 Mitogen-Activated Protein Kinase (MAPK), increasing the expression of PKC-3 signaling” [8, 9]. This signaling cascade leads to Platelet-Derived Growth Factor (PDGF) receptor-dephosphorylation and a reduction in downstream signaling from this receptor, resulting in pericyte apoptosis. An over accumulation of glucose and/or fructose damages blood capillaries in the retina with fluid and lipids leakage to the macula, the region of the retina with high visual acuity. The fluid accumulation results in the swelling (or edema) of the macula, which blurs vision [10].
The retinal pigment epithelium (RPE) plays a critical role in the development and maintenance of adjacent photoreceptors in the vertebrate retina. The RPE and Bruch’s Membrane (BM) endure significant damage over one’s lifetime and such age-related changes are attributable to the development of AMD in certain individuals [11–13]. The RPE is also responsible for phagocytosis and degradation of shed-photoreceptors’ outer segments [14]. The disruption of this process may lead to retinal degradation, and the initiation or progression of AMD in humans [15]. The RPE secretes angiogenic (VEGF) and antiangiogenic (PEDF) cytokines, and therefore plays a key role in chorodial neovascularization, which can lead to AMD and possibly other eye diseases. [16–18].
Vascular endothelial growth factor (VEGF) is a cell signaling protein that stimulates vasculogenesis and angiogenesis. It is part of the negative feedback loop that restores the oxygen supply to tissues when blood is circulating. Serum concentration of VEGF is high in bronchial asthma and diabetes mellitus [12]. VEGF’s normal function is to create new blood vessels during embryonic development, new blood vessels after injury, muscle following exercise, and new vessels (collateral circulation) to bypass blocked vessels. Solid cancers cannot grow beyond a limited size without an adequate blood supply. However, cancers express VEGF to increase blood supply by neovascularlization. Overexpression of VEGF and other proteins cause vascular diseases such as diabetic retinopathy [12]. Drugs, such as bevacizumab, inhibit VEGF to control or halt disease development [4].
VEGF is member of the PDGF family of cysteine-knot growth factors. They are important signaling proteins involved in both vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature) [19]. In a previous study, we reported that Rb1 increases ARPE cell proliferation and inhibits VEGF release. In the present study, we observed that ARPE-19 cells treated by chemically modified Rb1 also increased cell proliferation but they increase VEGF secretion. The molecular mechanism of such drug action is not known.
MATERIALS AND METHODS
Rb1
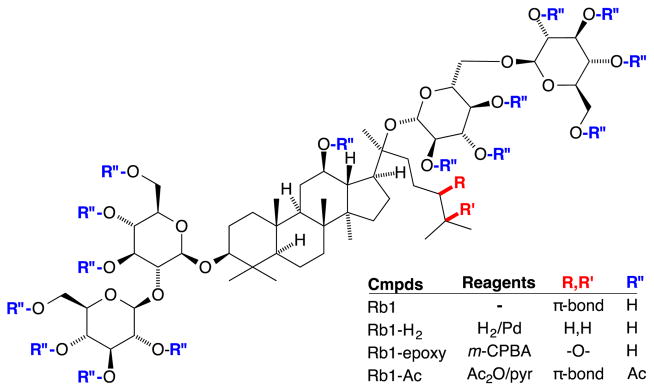
Rb1 was purchased from Fleton Natural Products (Chengdu, China) and was certified by Fleton to be chemically pure (99.8% pure by HPLC analysis) with the ability to be used as raw materials in food, drink, nutrition supplement and cosmetics field. The 1H NMR spectrum of commercial Rb1 was identical to that appearing in the literature [6]. Chemical modifications of Rb1 were carried out in the laboratory by standard organic reactions (Fig. 1) and are described in the following paragraphs. Each of the compounds prepared in these studies was synthesized as previously reported, with modifications indicated below. The Pd catalyst for the hydrogenation was prepared as described by Felpin [20] and used without further purification. The m-CPBA oxidant was purified via liquid-liquid extraction using Et2O and NaHCO3 (aq) prior to use. Acetic anhydride and pyridine were purchased from Acros (Pittsburgh, PA) and used without purification. All reaction conditions were performed in the absence of light. All other solvents and reagents were used without purification as obtained from the supplier.
Fig. (1).
Structure of Ginsenoside, Rb1 showing functional groups with chemical modification.
Rb1-H2
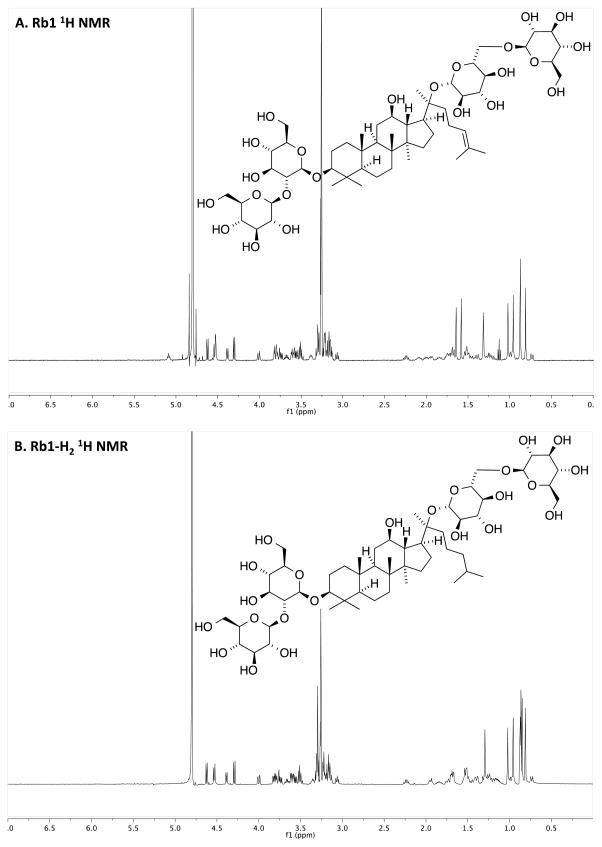
To a stirred solution of Rb1 (17.9 mg, 16.1 μmol) in MeOH (1.0 mL) was added 6 wt% of Pd(OAc)2:10% Pd/C catalyst mixture (5:95) [20] in a reaction flask. Residue on glassware was rinsed with MeOH (0.5 mL) and transferred to the reaction flask. The flask was filled with N2 and placed on a Parr Shaker Hydrogenation Apparatus under H2 (30 psi) at room temperature for 16 hours. The mixture was filtered through a filter paper on sintered glass and the filtrate was evaporated under reduced pressure. The product, which was obtained in quantitative yield, exhibited a 1H NMR spectrum identical to that of the known Rb1-H2 [21], including the expected diagnostic loss of the triplet at δ 5.1 for the tri-substituted olefin proton of Rb1 (Fig. 2A and B). The LCMS trace exhibited one major peak that was shown to have expected m/z for Rb1-H2: MS (m/z) calcd for C54H93O23− [M-H] − 1109.6, found 1109.6.
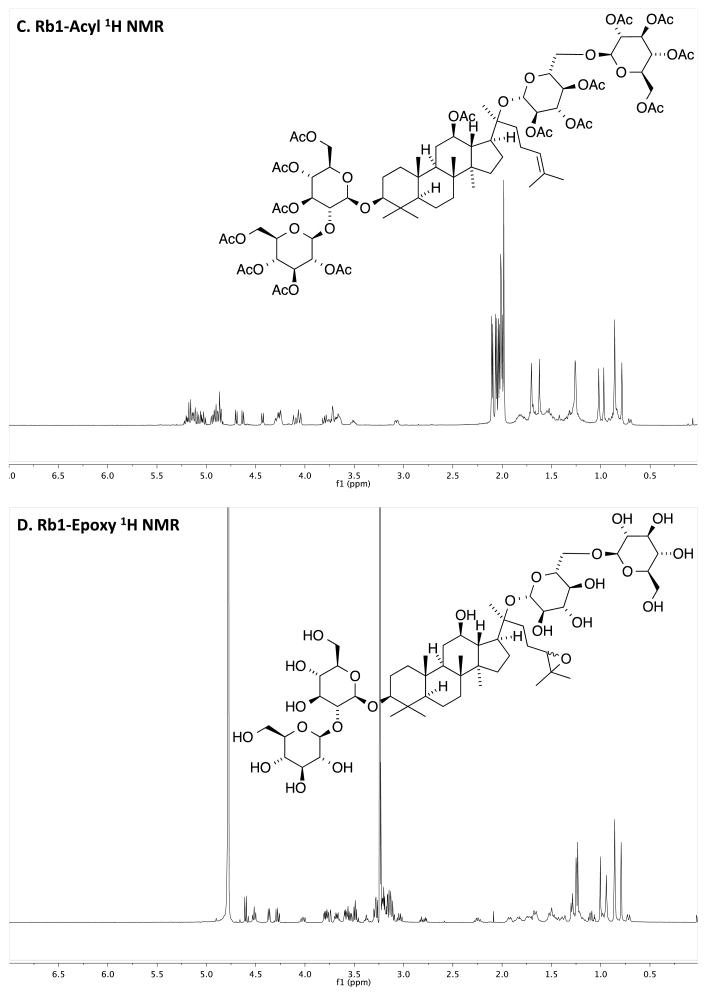
Fig. (2).
NMR Spectra of Rb1 and chemically modified Rb1. A) Rb1 without chemical modification. B) Rb1-H2. C) Rb1-Acyl. D) Rb1-Epoxy. See “Result” section for a description on changes in 1H NMR signals corresponding to hydrogenation, acylation and epoxidation of Rb1.
Rb1-Acyl
Acetic anhydride (0.60 μL, 6.76 μmol) was added to a reaction flask charged with Rb1 (5 mg) in pyridine (1.50 μL, 18.03 μmol) and the reaction was stirred at room temperature overnight. The mixture was evaporated under vacuum (0.5 mm Hg) and residual pyridine, acetic anhydride, and acetic acid were removed by co-evaporation with MeOH (3 × 1 mL) under reduced pressure to yield a colorless oil in 96% yield. The 1H NMR spectrum of the product (Rb1-Acyl) exhibited signals consistent with the formation of the desired product [22] including the olefin triplet at δ 5.1 ppm, multiple singlets in the 2.0–2.2 ppm range expected for the 7 acetyl methyl groups, and downfield shifted signals δ 3–4 ppm expected for the C-Hs with the acetoxy groups (Fig. 2A and C). The LCMS trace exhibited one major peak that was shown to be comprised of molecules with the expected m/z: MS (m/z) calcd for C84H123O38+ [M+H]+ 1739.8, found 1739.8.
Rb1-Epoxide
To a stirred solution of Rb1 (11.2 mg, 10.1 μmol) in MeOH (2 mL) was added m-CPBA (2.8 mg, 16.2 μmol) [23] and the reaction was stirred at room temperature overnight. The reaction was concentrated in vacuo and the oil was chromatographed (reversed-phase column chromatography using a C18 Sep-Pak and gradient elution from water to MeOH). Evaporation and solvent removal by lyophilization gave Rb1-Epoxide in 24% yield. The product exhibited a 1H NMR spectrum consistent with that of the data provided for known Rb1-Epox [24], however we present the 1H NMR in d-MeOH for easier interpretation. The spectrum included the loss of the diagnostic triplet at δ 5.1 for the tri-substituted olefin substrate of the reaction (Fig. 2A and D). The LCMS trace exhibited one major peak that was shown to have expected m/z of Rb1-Epoxy: MS (m/z) calcd for C54H91O24− [M-H] − 1123.6, found 1123.6.
Cell Culture
Adult retinal pigment epithelial cells (ARPE19) were obtained from the American Type Culture Center (ATCC) and grown to confluence in T75 flasks in Dulbecco’s Modified Eagle Medium (DMEM)-F12 containing 5.5 mM glucose and 10% FBS at 37°C and 5% CO2. Cell were seeded into 24-well plates at a density of 20,000 cells per well and incubated for 24 hours at 37°C before treatments.
Introduction of Rb1 and Rb1 Modified Compounds to ARPE-19 Cells in Culture
Compounds (Rb1, Rb1-H2, Rb1-Acyl, Rb1-Epoxy) were diluted into serum free medium (DMEM: F12) to a final concentration of 250 nM. Each cell sample received a total of 250 nM of its respective treatment/compound and were incubated for 72 hrs at 37°C and 5% CO2. DMSO (vehicle for Rb1) and appropriate vehicles for Rb1 modified compounds were used as controls.
Cell Viability and Cytokine Determination
Cell viability was determined by the trypan-blue dye exclusion method (Cat No. MT25900Cl; Corning via Fisher Scientific) and counting with a Neubauer hemacytometer. VEGF cytokine concentration in conditioned media was determined by Enzyme-Linked Immunosorbent Assay (ELISA) (Cat No. DVE00, R&D Systems, USA). Quantification of samples was determined by comparison to VEGF standard calibration curve (supplied with kit and performed per manufacturer’s instructions).
RESULTS
Chemical Modification of Rb1 by Hydrogenation, Acylation and Epoxidation
The hydrogenation and epoxidation transformations convert the double bond in native Rb1 ginsenoside (Fig. 1) to the corresponding saturated chain and epoxide, respectively. The hydrogenation of Rb1 ginsenoside was confirmed upon observing the loss of the alkene proton of the substrate at 5.1 ppm. The Rb1-H2 1H NMR spectrum exhibited all other signals expected for the product (Fig. 2A and B). In contrast, the peracetylation converts all of the hydroxyl groups to acetoxy groups, diminishing aqueous solubility. The presence of the acetoxy group was confirmed by the appearance and appropriate integration of singlets near 2.0 ppm (Fig. 2C). In the epoxidation reaction, the alkene proton of Rb1 ginsenoside disappeared but other signals appeared as expected (Fig 2D). Rb1-Epoxy is anticipated to exist as a mixture of diastereomers, though neither spectroscopy nor spectrometry provided evidence for these stereoisomers.
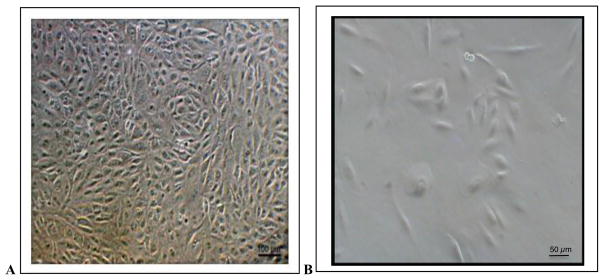
Morphology of ARPE19 Cells
Cells were grown to confluence at P9 in T75 flasks (Fig. 3A). Cell seeded into 1 ml well and incubated for 24 hr exhibit morphology consistent with pre-confluent ARPE 19 cells (Fig. 3B). Cell treated with Rb1 and Rb1 derivatives exhibited similar morphology suggesting that these compound did not induce major upregulation of protein expression and cell differentiation.
Fig. (3).
Photomicrographs of ARPE-19 cells in culture. A) confluent cells a Passage 9 after 5 days in culture in a P75 flask, 100× magnification B) Cells at Passage 10 were seeded in a 1 ml well at a seeding density of 20,000 cells per well and incubated for 24 hr in 37C, 5% CO, 200× magnification.
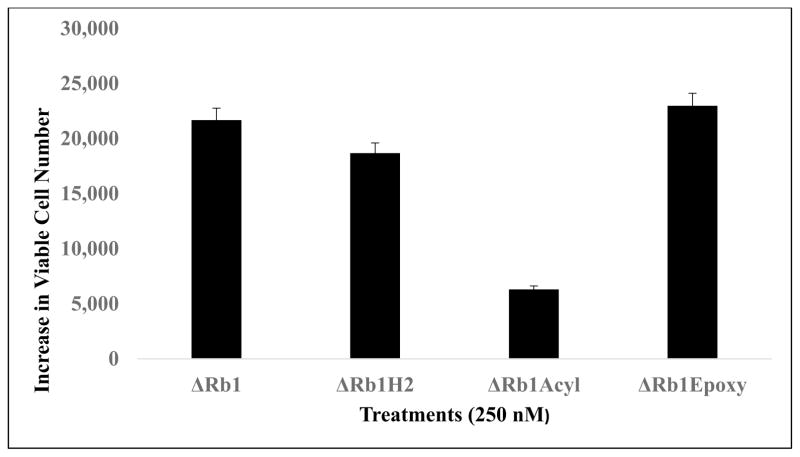
Increase in Viable Cells After 72h Treatment with Rb1 and Rb1-Modified Compounds (Concentration = 250nM)
After treatment for 72 hrs, there was an increase of 33,000 cells between vehicle and Rb1 groups (Fig. 4). Likewise, there were also increases in viable cell numbers between vehicle controls vs. treatments by Rb1 modified compounds: increase by 18,000 cells with Rb1-H2 treatment, 6,000 cells with Rb1-Acyl treatment and 22,000 cells with Rb1-Epoxy (n = 3 for all data points; ±SEM, see Fig. 3). One-way analysis of variance shows a significant treatment effects (F=50; r 30.001).
Fig. (4).
Increase in cell proliferation after with Rb1 and Rb1-modified compounds. Data in this figure show the increase in viable cell number between vehicle control (0-Rb1) group and group of cell with Rb1 (or Rb1 modified compounds) treatment at 250 nM for 72 hr. (n = 3 for all data points; ±SEM).
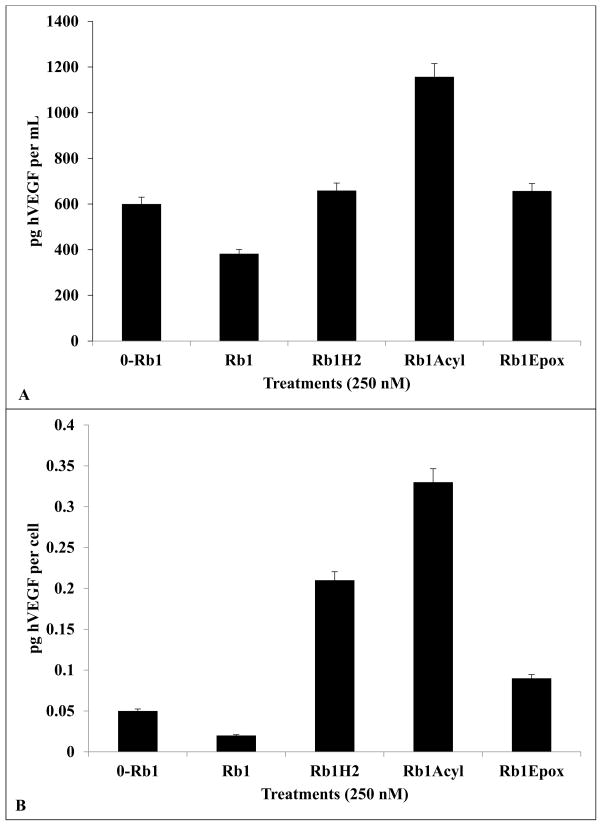
Decrease in VEGF Release by Rb1 Treatment and Increase VEGF Release by Rb1-H2, Rb1-Acyl and Rb1-Epoxy After 72hrs with 250nM
After 72 hrs of treatment, VEGF secretion from cells treated with Rb1 decreased from 0.05 pg/cell (without Rb1) to 0.02 pg/cell (with 250 nM Rb1; Fig. 5). Meanwhile, treatment by Rb1-H2 resulted in an increase of VEGF to 0.21 pg/cell (Fig. 5). Treatment with Rb1-Acyl resulted in the highest level of increase VEGF (to 0.33 pg/cell) while treatment with Rb1-Epoxy resulted in a much lower level of VEGF increase (to 0.05 pg/ml). Vehicle added to the conditioned media did not induce any changes in VEGF from control (0mM Rb1). (n = 3 for all data points; ±SEM). One-way analysis of variance shows a significant treatment effects (F=30; r 30.001).
Fig. (5).
hVEGF secretion by ARPE 19 cells is inhibited by Rb1 and enhanced by Rb1H2, Epoxide, or Rb1 Acylated Treatments. A) After 72 hrs, hVEGF secretion from cells treated with Rb1 at 250 nM decreased to 382 pg/mL (compared to 600 pg/mL in vehicle control of 0-Rb1) whereas cells treated with Rb1-H2 at 250 nM increased to 659 pg/mL. Similarly, cell treated with Rb1-Acyl increased to 1157 pg/mL and Rb1-Epoxy, increased to 657 pg/mL. B) Compared to 0.05 pg/cell/mL in vehicle control of 0-Rb1, hVEGF decreased to 0.02 pg/cell in respond to Rb1. In contrast, Rb1-H2 treatment increased hVEGF secretion to 0.21 pg/cell/mL, Rb1-H2, to 0.33 pg/cell by Rb1-Acyl, and to 0.09 pg/cell by Rb1-Epoxy treatment (n = 3 for all data points; ±SEM).
DISCUSSION
Previous reports have shown that Rb1 possesses potent anti-angiogenic activity in both in-vivo and in-vitro studies [14, 3]. Results from the present study are consistent with those from our previous report that Rb1 induces ARPE-19 cell proliferation and inhibits VEGF secretion [14]. However, it is not known if chemical modifications of Rb1 could impact the efficacy of this cellular activity. Such a study on the structure versus function relationship (SAR) is important because Rb1 affects a significant change in VEGF (a known proangiogenic cytokine) secretion by RPE cells which are located near choroidal capillaries and other ocular vasculatures in the eye, and are a major producer of VEGF which contributes to the development of AMD and diabetic retinopathy.
Results from the present study strongly suggest that chemical modifications of Rb1 led to significant changes in its ability to induce cell proliferation and VEGF secretion. Chemically modified Rb1s produced upon hydrogenation, acylation and epoxidation all induce cell proliferation. In comparison to unmodified Rb1, epoxidation resulted in a slight increase in the efficacy of Rb1 to induce cell proliferation whereas hydrogenation resulted in a somewhat lower level of increase in cell proliferation (Fig. 4). In contrast, the addition of acyl group significantly reduced Rb1’s ability to induce an increase in cell proliferation. Although it is tempting to speculate that a change in hydrophobicity by the addition of the acetyl group and the epoxide may correlate to the decrease and increase in this cell (proliferation) activity, additional experiments are needed to substantiate this suggestion. Further experiments can be carried out to study if changes in Rb1 efficacy on cell proliferation are due to a change in its compatibility with hydrophobic cell membrane and/or intracellular signaling including gene activation [25, 26]
Results from the present study also show that chemical modification of Rb1 significantly affects ARPE19 secretion of VEGF. Consistent with our prior report, the unmodified Rb1 inhibited VEGF secretion (see Fig. 5) [5]. In contrast, chemical modification of Rb1 by hydrogenation, acylation and epoxidation resulted in an increase of VEGF release by ARPE19. The addition of acetyl groups resulted in the highest level of change – about 7-fold increase in VEGF release in comparison to control without Rb1. This is followed by hydrogenation, which induced a 4-fold increase and by epoxidation, with a 2-fold increase in VEGF release (compared to unmodified Rb1 which induced a 50% reduction in VEGF release; Fig. 5). It is not clear at this time how such structural changes render such large differences in this biological activity. Further experiments will be needed to investigate underlying mechanisms.
There are few pharmacokinetic and pharmacodynamic studies on the effects of Rb1 in humans, and therefore the toxic levels of ginsenosides are currently unknown [27]. Betts et al. [14] used concentrations of 0.25, 2.5, 25, and 250 nM Rb1 in an earlier experiment and observed a significant decrease in VEGF with an increase in cell proliferation, and therefore we chose to use 250 nM for all of our modified compounds in our experiment. Based on the information presented in several studies [16, 17], retinal angiogenesis is not a fully understood process in which retinal vascular endothelial cells proliferate and migrate through the damaged membrane. Based on the results of this and similar studies, Rb1 and its modified derivative Rb1H2 may be considered a viable homeopathic or pharmacological treatment for ocular disease like AMD and diabetic retinopathy which involves the process of choroidal neovascularization mediated by VEGF. This is the first study that compares the effects of Rb1 and a hydrogenated form of Rb1, as well as acylation and epoxidation. These modified compounds, compared to current therapy, could have a significant impact in the diabetic retinopathy and AMD markets.
CONCLUSION
Chemical modifications of ginsenoside Rb1 significantly change its biological activities in terms of cell viability and cytokine secretion by ARPE 19 cells. Additional experiments will provide results to illustrate mechanistic insight on how these structure changes lead to alternation of biological activities.
Acknowledgments
This project was supported by a grant from the National Institute on Minority Health and Health Disparities (G12MD007591) from the National Institutes of Health. MS was supported by a grant from the NIGMS (NIH UTSA RISE GM060655). Authors thank Phillip Rodriguez for literature review and support, Jessica Buikema for technical assistance, and Dr. Oleg Larionov and Mr. David E. Stephens for assistance with mass spectrometry.
LIST OF ABBREVIATIONS
- AMD
Age-related Macular Degeneration
- RPE
Retinal Pigment Epithelium
- VEGF
Vascular Endothelial Growth Factor
- NMR
Nuclear Magnetic Resonance
- Rb1
Ginsenoside
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Fulder S. The Book of Ginseng and other Chinese Herbs for Vitality. Rochester: Healing Arts Press; 1993. [Google Scholar]
- 2.Nocerino E, Amato M, Izzo AA. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 2000;71(Suppl 1):S1–5. doi: 10.1016/s0367-326x(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 3.Pardianto G. Understanding diabetic retinopathy. Mimbar Ilmiah Oftalmologi Indonesia. 2005;2:65–6. [Google Scholar]
- 4.Einwallner E, Ahlers C, Golbaz I, et al. Neovascular age related macular degeneration under anti-angiogenic therapy: Subretinal fluid is a relevant prognostic parameter. Ophthalmologie. 2010;107(2):158–64. doi: 10.1007/s00347-009-1948-7. [DOI] [PubMed] [Google Scholar]
- 5.Evans JR. Risk Factors for age-related macular degeneration. Prog Retinal Eye Res. 2001;20(2):227–53. doi: 10.1016/s1350-9462(00)00023-9. [DOI] [PubMed] [Google Scholar]
- 6.Kim IW, Hong HD, Choi SY, Hwang DH, Her Y, Kim SK. Characterizing a full spectrum of physico-chemical properties of ginsenosides Rb1 and Rg1 to be proposed as standard reference. J Ginseng Res. 2011;35:487–96. doi: 10.5142/jgr.2011.35.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geraldes P, Yamamoto H, Matsumoto J, et al. Activation of PKC-3 and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2012;15(11):1298–306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toke B. Experimental approaches to diabetic retinopathy. In: Hammes P, Porta M, editors. Front Diabetes. Vol. 20. Basel: Karger; 2010. pp. 1–19. [Google Scholar]
- 9.Bangaru LK, Sultana M. In silico homology modeling of contactin 5 protein which is responsible for diabetic retinopathy. Int J Novel Trends Pharmaceutical Sci. 2012;2:145–9. [Google Scholar]
- 10.Vidro E, Yendluri B, Thai T, Tsin A. NBHA modulates acrolein-induced pro-angiogenic cytokine release by ARPE-19 cells: Involvement of TGFb and SMAD3. Curr Eye Res. 2011;36(4):370–8. doi: 10.3109/02713683.2010.549601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossi E, Koerner F. Retinopathy of prematurity. Intensive Care Med. 1995;21(3):241–6. doi: 10.1007/BF01701481. [DOI] [PubMed] [Google Scholar]
- 12.Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev. 1997;13(1):37–50. doi: 10.1002/(sici)1099-0895(199703)13:1<37::aid-dmr174>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J Pediatr Ophthalmol Strabismus. 1999;3(1):26–32. doi: 10.1016/s1091-8531(99)70091-1. [DOI] [PubMed] [Google Scholar]
- 14.Betts B. Ginsenoside-Rb1 induces ARPE-19 proliferation and reduces VEGF release. ISRN Ophthalmol. 2011;2011:184295. doi: 10.5402/2011/184295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: A possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76(4):397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 16.Amin R, Puklin JE, Frank RN. Growth factor localization in choroidal neovascular membranes of age related macular degeneration. Invest Ophthalmol Vis Sci. 1994;35(8):3178–88. [PubMed] [Google Scholar]
- 17.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285(5425):245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi T, Hata Y, Yoshikawa H, Nakagawa K, Sueishi K, Inomata H. Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1997;235(3):159–67. doi: 10.1007/BF00941723. [DOI] [PubMed] [Google Scholar]
- 19.Qian T, Cai Z. Biotransformation of ginsenosides Rb1, Rg3 and Rh2 in rat gastrointestinal tracts. Chin Med. 2010;5:19. doi: 10.1186/1749-8546-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felpin F, Fouquet E. A Useful, reliable and safer protocol for hydrogenation and the hydrogenolysis of O-Benzyl groups: The in situ preparation of an active Pd0/C catalyst with well-defined properties. Chem Eur J. 2010;16:12440–5. doi: 10.1002/chem.201001377. [DOI] [PubMed] [Google Scholar]
- 21.Sakanaka M. Vascular regeneration promoters. 02/067950A1. International Patent No WO. 2002
- 22.Kanaoka M, Kato H, Shimada F, Yano S. Studies on the enzyme immunoassay of bio-active constituents in oriental medicinal drugs. VI. Enzyme immunoassay of ginsenoside Rb1 from Panax ginseng. Chem Pharm Bull (Tokyo) 1992;40:314–7. doi: 10.1248/cpb.40.314. [DOI] [PubMed] [Google Scholar]
- 23.Goeddel D, Shi Y. Science of synthesis-methods of molecular transformations: Synthesis from alkenes with organic oxidants. Georg Thieme Verlag KG; Stuttgart: 2008. pp. 277–320. [Google Scholar]
- 24.Wang JR, Yau LF, Tong TT, et al. Characterization of oxygenated metabolites of ginsenoside Rb1 in plasma and urine of rat. J Agric Food Chem. 2015;63:2689–700. doi: 10.1021/acs.jafc.5b00710. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya TK, Jackson P, Patel MK, Thomas JR. Epidermal cell proliferation in calorie-restricted aging rats. Curr Aging Sci. 2012;5:96–104. doi: 10.2174/1874609811205020096. [DOI] [PubMed] [Google Scholar]
- 26.Luo L, Luo JZ, Jackson I. Tripeptide Amide L-pyroglutamyl-Histidyl-L-Prolineamide (L-PHP-Thyrotropin-Releasing Hormone, TRH) Promotes Insulin-Producing Cell Proliferation. Curr Aging Sci. 2013;6:8–13. doi: 10.2174/1874609811306010002. [DOI] [PubMed] [Google Scholar]
- 27.Du GJ, Dai Q, Williams S, Wang CZ, Yan CS. Synthesis of protopanaxadiol derivatives and evaluation of their anticancer activities. Anticancer Drugs. 2011;22(1):35–45. doi: 10.1097/CAD.0b013e32833fde29. [DOI] [PMC free article] [PubMed] [Google Scholar]