Abstract
Pulmonary barrier function plays a pivotal role in protection from inhaled particles. However, some nano-scaled particles, such as carbon nanotubes (CNT), have demonstrated the ability to penetrate this barrier in animal models, resulting in an unusual, rapid interstitial fibrosis. To delineate the underlying mechanism and specific bio-effect of inhaled nanoparticles in respiratory toxicity, models of lung epithelial barriers are required that allow accurate representation of in vivo systems; however, there is currently a lack of consistent methods to do so. Thus, this work demonstrates a well-characterized in vitro model of pulmonary barrier function using Calu-3 cells, and provides the experimental conditions required for achieving tight junction complexes in cell culture, with trans-epithelial electrical resistance measurement used as a biosensor for proper barrier formation and integrity. The effects of cell number and serum constituents have been examined and we found that changes in each of these parameters can greatly affect barrier formation. Our data demonstrate that use of 5.0 × 104 Calu-3 cells/well in the Transwell cell culture system, with 10% serum concentrations in culture media is optimal for assessing epithelial barrier function. In addition, we have utilized CNT exposure to analyze the dose-, time-, and nanoparticle property-dependent alterations of epithelial barrier permeability as a means to validate this model. Such high throughput in vitro cell models of the epithelium could be used to predict the interaction of other nanoparticles with lung epithelial barriers to mimic respiratory behavior in vivo, thus providing essential tools and bio-sensing techniques that can be uniformly employed.
Keywords: Nanoparticles, Lung, In vitro model, Pulmonary barrier, Toxicology
1. Introduction
The rapidly growing nanotechnology industry has revolutionized various industrial fields. Engineered nanomaterials (ENM) are of significant relevance owing to their unique physical, electrical and chemical properties, which have been exploited for widespread applications in electronics, aerospace, medicinal drug delivery, engineering, and cosmetics. However, their mass production, and exposures associated with workplace handling, raises serious health concerns, especially in the context of lung hazards, since ENMs are readily aerosolized.
The biological effects of ENMs, including carbon nanotubes (CNTs), remain poorly understood and are an object of debate regardless of several attempts to fully characterize them [7,11,12,20,23]. Despite this, it is commonly accepted that inhalation of ENMs, the primary route of exposure for CNTs, can cause major airway and lung disorders [4,7,12]. Recent research has divulged potential harmful effects of CNTs in the lungs including oxidative stress, inflammatory cytokine production, fibrosis, granuloma formation, and lung cancer promotion [8,16,20,31], while other studies have found similar effects as a result of various alternative ENMs such as cerium dioxide [14,15] and titanium dioxide [3,25].
Airway epithelial cells, present as one of the first lines of defense for inhaled particulate matter, represent a major determinant in the interaction of a foreign body with other major body compartments. In addition, epithelial cells are involved with the formation and maintenance of tight junctions between neighboring epithelial cells, only permitting polarized secretory functions (such as ion transport) and routine cellular trafficking, while preventing access to xenobiotics and pathogens [1,28,32,34]. Moreover, these tight junctions assist in keeping cytokines, toxins, and pathogens from infiltrating the epithelial layer. Indeed, these dynamic protein structures govern the paracellular permeability of the epithelium, permitting only that which is necessary under normal circumstances [13,26]. Consequently, pulmonary barrier function plays a pivotal role in controlling penetration of inhaled nanoparticles into the interstitium, which can lead to rapid interstitial fibrosis [19,35]. For example, dispersed single- or multi-walled CNTs rapidly enter the alveolar interstitial space and induce a progressive interstitial fibrotic response with minimal lung inflammation [19,23,35]. However, due to a lack of studies evaluating the effects of CNT exposure on lung epithelial barriers, a key determinant of pulmonary toxicity due to ENMs, the pathogenic mechanisms underlying these effects have not been fully elucidated. This is due, in part, to a lack of effective, consistent methods to reliably predict in vivo outcomes in an in vitro setting.
Further compounding this issue is the notion that unique physicochemical characteristics of ENMs, such as particle size, shape, and surface modification, may contribute to progressive, toxic responses, including heightened lung epithelial barrier permeability. To date, it remains inadequately understood how each of these fundamental features distinctly affects the airway epithelium. In addition, due to the escalating costs and limitations of evaluating individual cell type-specific outcomes when animals are exposed to ENMs in vivo, more in vitro approaches are being explored as alternatives.
Epithelial cells in culture form tight junction complexes, thereby making in vitro models of lung epithelium a favorable choice to mimic and predict the respiratory behavior in vivo upon exposure to ENMs such as CNT materials [30]. Several studies have sought to explore the effects of various ENMs on epithelial function in vitro using the Calu-3 small airway epithelial cell line [21,28,38]; however, inconsistent methods to do so limit comparisons and conclusions. Hence, our current study utilized the Calu-3 cell line that demonstrates the characteristics of differentiated, functional human epithelia [9,33] with the primary objective to provide the technical details and characterization of an in vitro pulmonary barrier model to consistently screen for the pathogenicity of various nanoparticles on lung epithelium. Trans-epithelial electrical resistance (TEER) is a commonly used endpoint to assess integrity and permeability of epithelial monolayers, as it is an instantaneous measure of ion flux [32] and an indirect measurement of the formation of tight junctions [36]. Thus, we have employed this parameter for use as a biosensor for epithelial monolayer formation and integrity. In addition, the peculiar effects of cell number and serum constituents have been examined in an effort to reliably study the effect of exposed particles on lung epithelial barrier permeability in vitro. Providing the results of our various testing strategies to optimize these conditions will not only provide consistent methodologies that can be implemented by others, but will also help to elucidate potential differences in past studies that have used alternate culture conditions with similar model systems, particularly Calu-3 cells.
Additionally, as a means to substantiate this model, we have utilized the optimized conditions outlined in this study with an exposure to CNTs. We hypothesized that the physicochemical properties of CNTs play a key role in determining the effects that these particles have on epithelial barrier integrity, thereby affecting penetration of CNTs into the interstitium. Such high-throughput in vitro cell models of the airway epithelium could be advantageous in predicting the interaction of other nanoparticles with lung epithelial barriers to mimic the respiratory behavior in vivo.
2. Materials and methods
2.1. Reagents
Eagle’s minimum essential medium (EMEM) was purchased from American Type Culture Collection (ATCC, Manassas, VA). Phosphate buffer saline (PBS), trypsin, fetal bovine serum (FBS), and penicillin/streptomycin antibiotic solution were purchased from Sigma–Aldrich (St. Louis, MO).
2.2. Cell monolayer assessment
Human lung epithelial cells (Calu-3, ATCC, Manassas, VA) were seeded onto Transwell® cell culture support dishes (Corning Life Sciences, Tewksbury, MA) at various densities, 1.0 × 104 cells/well, 2.0 × 104 cells/well, and 5.0 × 104 cells/well, with the optimal density determined to be 5.0 × 104 cells/well. Inserts had a diameter of 6.5 mm, with a growth area of 0.33 cm2 and 3.0 μm pores. Cells were cultured in EMEM media supplemented with L-glutamine (1%), penicillin-streptomycin (1%), and fetal bovine serum (FBS; 10%) at 37 °C in humidified air with 5% carbon dioxide. After 24 h, cells were washed and medium was changed to EMEM media with various FBS concentrations, 2%, 5%, 10%, and 15%, in an effort to determine whether FBS concentration alters monolayer formation.
Monolayer formation was evaluated by measuring electrical resistance of the cultured Calu-3 cells and subsequently confirmed using bright field microscopy (Ziess Axiovert 100 TV inverted microscope, Carl Zeiss Microscopy, LLC, Thornwood, NY), CytoViva hyperspectral microscopy (CytoViva, Auburn, AL), and analysis of the tight junction protein ZO-1 via confocal microscopy. Resistance was measured using an Epithelial Voltohmmeter (EVOM2) with a STX2 electrode (World Precision Instruments, Sarasota, FL) every 24 h after washing. Trans-epithelial electrical resistance (TEER) readings were determined by subtracting the resistance (in ohms) of the blank insert from the recorded resistance of the monolayer, and subsequently multiplying the resulting value by the effective membrane surface area of the insert to yield ohms·cm2.
2.3. Immunofluorescence of tight junctions of the Calu-3 cell monolayer
Cells were fixed in 3.7% formaldehyde for 10 min at room temperature and then permeabilized and blocked in a solution containing 0.5% saponin, 1% bovine serum, and 1.5% goat serum for 30 min. After primary antibody incubation with ZO-1 antibody (Invitrogen, Carlsbad, California) at 1:50 dilution for 1 h, cells were washed and incubated with Alexa Fluor 488-conjugated secondary antibody (Invitrogen, Carlsbad, California) for 30 min. The transwell cell culture support membrane was cut and mounted on glass microscope slides using Prolong gold anti-fade reagent containing DAPI (Invitrogen, Carlsbad, California). Images were acquired by confocal laser scanning microscopy using a Zeiss LSM 510 (Carl Zeiss Microscopy, LLC, Thornwood, NY).
2.4. Preparation of carbon nanotubes
Single-walled carbon nanotubes (SWCNT; Carbon Nanotechnologies, Inc., Houston, TX) were produced by the high pressure CO disproportionation (HiPco) technique, employing CO in a continuous-flow gas phase as the carbon feedstock and Fe (CO)5 as the iron-containing catalyst precursor. These SWCNT were then purified by acid treatment to remove metal contaminants. Elemental analysis of the supplied SWCNT by nitric acid dissolution and inductively coupled plasma–atomic emission spectrometry (ICP-AES, NMAM #7300) indicated that the SWCNT contained 99% elemental carbon and 0.23% iron. The specific surface area was measured at −196 °C by the nitrogen absorption–desorption technique (Brunauer Emmet Teller method, BET) using a SA3100 Surface Area and Pore Size Analyzer (Beckman Coulter, Fullerton, CA). The surface area of dry SWCNT was 400–1000 m2/g, and the length and width of individual (dry) SWCNT was 0.1–1 μm and 0.8–1.2 nm.
Multi-walled carbon nanotubes (MWCNT; Nanostructured & Amorphous Materials, Inc., Houston, TX) and COOH-functionalized MWCNT (f-MWCNT) were characterized fully in a previous report [29]. Briefly, f-MWCNT were prepared by the oxidation of the purified MWCNT in HNO3 (63%). The mixture was then refluxed at approximately 110 °C for 12 h. The black solution was then centrifuged to collect the f-MWCNT precipitate. The f-MWCNT was subsequently washed with deionized water to remove HNO3 until a neutral solution was obtained. The resulting f-MWCNT was then dried in a vacuum oven at room temperature for at least 24 h.
2.5. Carbon nanotube treatment
Once the transwell resistance of the monolayers reached 2000 ohms, or 660 ohms·cm2, medium was changed and allowed to equilibrate for 24 h prior to treatment. All CNTs were prepared for treatment as previously described [40]. Briefly, particles were suspended in distilled water to acquire stock solutions of 0.1 mg/mL, which were supplemented subsequently with 150 μg/mL of a natural lung surfactant, Survanta (Abbott Laboratories, Columbus, OH) to aid in their dispersion, as previously validated [39]. All particles were then dispersed using sonication and diluted in culture medium to the desired concentration. Cells were then exposed to CNTs for the indicated time periods and transwell resistance was measured. Cytoviva images were captured using an Olympus BX-51 Microscope equipped with the CytoViva Advanced Dark field Illumination System (CytoViva, Auburn, AL) and a 100 W quartz–halogen light source.
2.6. Statistical analysis
Data were pooled from three independent replicate experiments and samples were analyzed using a two-way ANOVA with Tukey’s post-test for multiple comparisons to determine significance. Statistical significance is indicated in each figure as *p < 0.05, **p < 0.01, and ***p < 0.001. All statistical analyses were performed using Graph Pad Prism Software (La Jolla, CA).
3. Results
3.1. Establishment of the epithelial monolayer
In an effort to establish reliable and realistic epithelial cell monolayers for use as an in vitro model of the lung epithelial barrier, Calu-3 cells were seeded on Transwell inserts at various cell densities and observed over time. As anticipated, cell seeding density impacted the efficient formation of monolayers, as altering the plated cell number led to significant changes in TEER measurements (Fig. 1). Although TEER measurements remained similar for all seeding densities up to approximately 5 days in culture, the higher cell density led to increased TEER measurements by as few as 6 days in culture, while 1.0 × 104 cells/well failed to form a confluent monolayer by day 14 and 2.0 × 104 cells/well demonstrated delayed and inconsistent monolayer formation (data not shown). Thus, the optimal cell seeding density was determined to be approximately 5.0 × 104 cells/well. These results suggest that even minor changes in cell culture conditions could have a very large impact on the interpretation of results when analyzing the effect of an ENM on epithelial barrier formation and integrity.
Fig. 1.
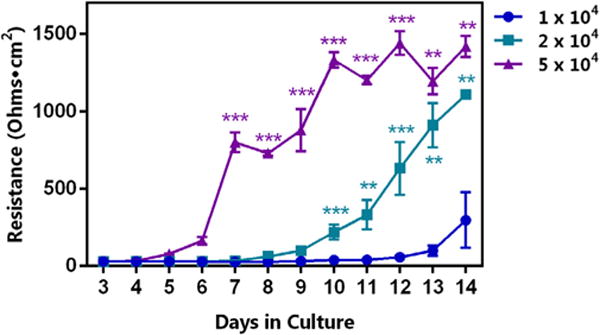
Influence of seeded cell number on Calu-3 monolayer resistance. Cells were seeded at the indicated densities per well, with growth conditions (i.e. FBS concentration, 10%) remaining constant. A significant difference in monolayer resistance, presented in ohms·cm2, due to the number of seeded cells was observed, with 5.0 × 104 cells/well achieving statistical significance compared to each of the other plating densities at 7 days and continuing until day 14. When plated at a density of 2.0 × 104 cells/well, resistances were significantly different from each of the other densities at day 10, and this difference continued for up to 14 days. Statistical significance is indicated as **p < 0.01, and ***p < 0.001.
As an additional method to assess monolayer formation, microscopic images were taken every 2 days post-seed, and monolayers were analyzed via bright field imaging, which demonstrated the presence of viable monolayers at days 2, 3, 5, and 7 (Fig. 2A), with confluence reached by day 7. Confocal microscopy was used to determine the abundance of the junctional protein ZO-1, a prominent marker for tight junction formation, which revealed a corresponding increase in the fluorescence intensity and cell periphery localization of ZO-1 as electrical resistance increased (Fig. 2B).
Fig. 2.
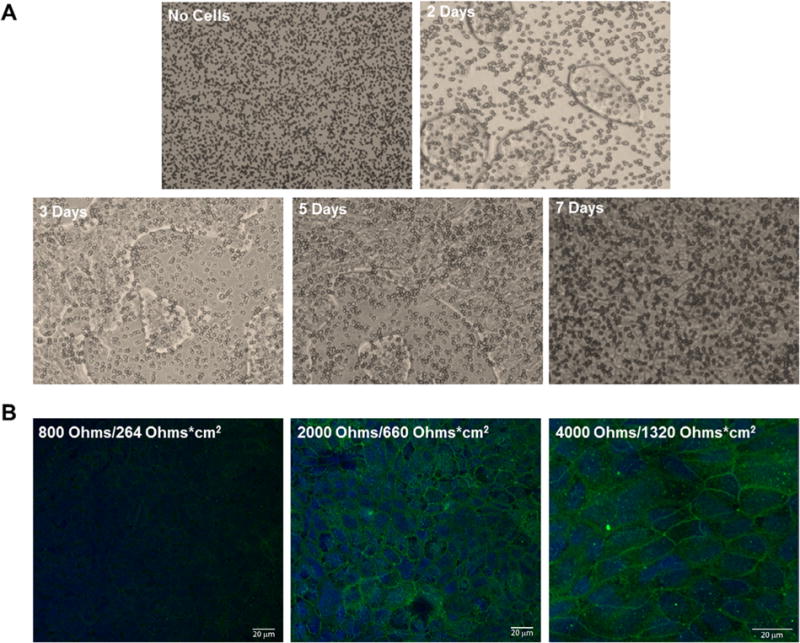
Microscopic imaging of cultured Calu-3 cells from seeding to confluent monolayer formation. (A) Calu-3 monolayer formation at 2, 3, 5, and 7 days in culture, plated in medium supplemented with 10% FBS on Transwell inserts, was imaged using an inverted microscope at 10× magnification. Transwell insert pores can be visualized in the absence of cells (top left panel), while monolayer formation can be seen over time (remaining panels) with the addition of cells. (B) Confocal microscopy revealed tight junction complexes (ZO-1, green) and nuclei (DAPI, blue) of the Calu-3 cell monolayer at the indicated electrical resistances. The observed ZO-1 fluorescent intensity increases as electrical resistance increases. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Interestingly, epithelial monolayer integrity was greatly affected by the concentration of FBS used in the culture medium, as indicated by changes in the TEER with increasing amounts of FBS (Fig. 3). Lower concentrations of FBS, 2% and 5%, resulted in TEER values that were significantly higher than when medium was supplemented with 10% and 15%, a result that was observed after as few as 5 days in culture. While 10% and 15% FBS conditions demonstrated similar trends in recorded TEER values over the course of 14 days, there were significant changes noted between the two, starting at day 10 in culture and lasting until day 13. Culture medium supplemented with 10% FBS revealed TEER values that remained relatively consistent after day 10 and lasting until day 14, while medium containing each of the other concentrations of FBS tested demonstrated larger variation in TEER values over the same time period. For this reason, in addition to the common use of 10% FBS in culture media, this concentration was determined to be optimal for use in the in vitro model described herein. Collectively, these data highlight the importance of stating all culture conditions when reporting the effects of ENMs on cells in culture.
Fig. 3.
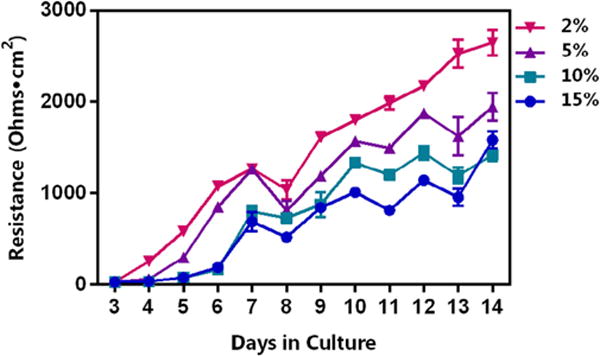
Influence of FBS concentration on Calu-3 monolayer resistance. Each well was seeded at the same Calu-3 cell density (5.0 × 104 cells/well) with increasing concentrations of FBS (2%, 5%, 10%, and 15%). Subsequently, TEER was recorded each day for up to 14 days and is presented in ohms·cm2. FBS produced a concentration-dependent effect on resistance of the monolayer. TEER values for both 2% and 5% were statistically significant from those of both 10% and 15% on nearly every day tested (with the exception of days 3 and 8). Although a similar trend was observed using 10% and 15%, a FBS concentration of 10% was determined to be the ideal concentration due to the more consistent TEER values recorded on days 10 through 14 as compared with the more variable values observed for the cells cultured in medium supplemented with 15% FBS over the same time period.
3.2. Effect of ENMs on epithelial barrier function
As previously mentioned, we sought to validate our model for the purpose of assessing the effects of ENMs on lung epithelial barrier function, comparing the in vitro treatment of Calu-3 cells with CNTs to previously published in vivo studies, which have demonstrated that both SWCNT and MWCNT can rapidly enter the interstitium once inhaled [18,35]. Calu-3 monolayers, seeded at a density of 5.0 × 104 cells/well and cultured in EMEM media containing 10% FBS, were observed until TEER readings were above 2000 ohms, or 660 ohms·cm2, at which point medium was changed and cells were allowed to equilibrate for 24 h. Subsequently, SWCNT or MWCNTs were added and TEER measurements were recorded for an additional 6 days (up to 15 total days in culture).
We first evaluated the influence of occupationally relevant doses of SWCNTs on Calu-3 monolayer formation and integrity. Interestingly, SWCNT at any of the indicated doses did not disturb the tight junctions of the Calu-3 monolayer, suggesting that penetration of SWCNT in vivo may not be due to increased epithelial barrier permeability (Fig. 4). In contrast, MWCNT at high concentrations (2 and 6 μg/cm2) significantly altered the integrity of the monolayer, as indicated by reduced TEER values after approximately 24 h of exposure (day 10 in culture; Fig. 5A), while MWCNT with COOH-functionalization did not affect the integrity at the same doses, similar to the SWCNTs (Fig. 5B and C). In addition, advanced dark field images indicated the presence of MWCNTs on the cell surface as well as those embedded within the cells (Fig. 5D). This would suggest that, unlike SWCNTs, MWCNTs observed in the interstitium in vivo [18,35] may be the result of their unique ability to penetrate the epithelial barrier and disrupt epithelial monolayer function.
Fig. 4.
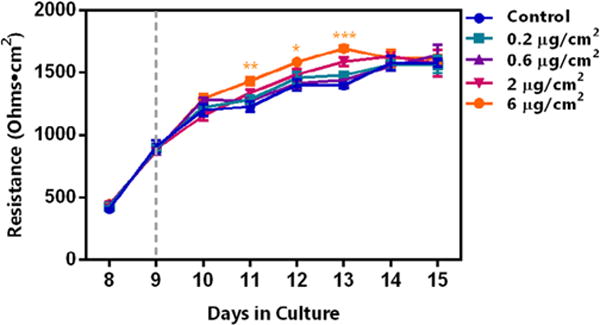
Influence of exposed SWCNT on Calu-3 monolayer permeability. Cells were seeded at a density of 5.0 × 104 cells/well, with culture media containing 10% FBS, and remained in culture until the TEER value was measured at 2000 ohms, equivalent to 660 ohms·cm2. Subsequently, the medium was changed and cultures were incubated for an additional 24 h, following which, SWCNTs were added at the indicated doses. Dashed line indicates the time of treatment initiation. TEER was recorded each day for up to 15 days (6 days post-exposure). Interestingly, the highest dose tested, 6 μg/cm2, induced a significant increase in TEER compared to the control at days 11, 12, and 13 in culture (3, 4, and 5 days post-exposure, respectively). Statistical significance is indicated as *p < 0.05, **p < 0.01, and ***p < 0.001.
Fig. 5.
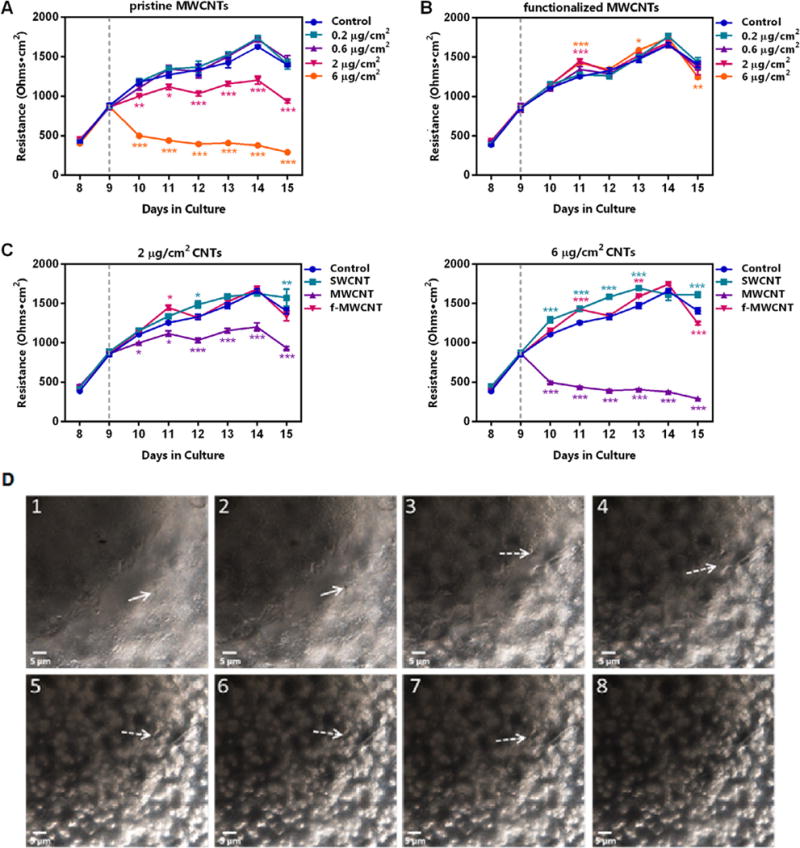
Effects of MWCNTs on Calu-3 monolayer permeability. (A) Monolayers, cultured as in Fig. 4, were exposed to pristine MWCNTs at the indicated doses (dashed lines indicate the time of treatment initiation in each panel). Only the high doses of MWCNTs, 2 μg/cm2 and 6 μg/cm2, induced a significant decrease in TEER as compared to the control, presented as ohms·cm2. (B) TEER values after treatment with f-MWCNTs (COOH-functionalized MWCNT) at the indicated doses demonstrated a similar pattern to those of the control on each day tested, with exceptions noted for the high doses on days 11 and 13, in which TEER was significantly increased, and on day 15 in which TEER was significantly decreased. (C) Cells were seeded at the same density (5.0 × 104 cells/well) and growth condition (10% FBS) and were treated with the indicated CNTs at 2 μg/cm2 (left panel) and 6 μg/cm2 (right panel). Only pristine MWCNTs, but not SWCNTs or f-MWCNTs, were able to induce a significant decrease in TEER compared to the control. Conversely, SWCNT and f-MWCNTs caused small but significant increases in TEER. (D) Interaction of dispersed MWCNT exposure at 2 μg/cm2 with the Calu-3 monolayer 1 day post-exposure as observed in a z-stack image of the cells. Note the MWCNTs (straight and dashed arrows) are on the cell surface and embedded in the cell (from 1 to 8). Statistical significance is indicated as *p < 0.05, **p < 0.01, and ***p < 0.001.
Cytotoxicity was also assessed using the same concentrations of CNTs in order to determine whether the observed alterations in barrier integrity could be due to decreased cell numbers; however, measurement of lactate dehydrogenase (LDH) activity under each of the treatment conditions remained the same at 24 h post-exposure (data not shown). Therefore, the decreased TEER measurements observed for the high doses of MWCNT cannot be attributable to cell death.
4. Discussion
This study revealed the optimal conditions required to investigate in vitro epithelial barrier formation using Calu-3 cells, to be used as a screening model for ENM exposure to mimic in vivo conditions. Details on the specific cell line, cell number, serum concentration, and duration required to achieve stable monolayer formation have been described herein to establish a consistent, reliable in vitro method for further implementation within the field. Additionally, CNT exposure, including dose range and various types of CNTs, was used to test this model for its utility in examining the potential health hazards of ENM exposure in an in vitro manner.
TEER is commonly used as an indirect parameter of tight junction formation and as a measure of epithelial monolayer integrity and permeability [32,36]. Thus, this technique offers fast and efficient analysis of the integrity of epithelial barriers, and we have employed this tool as a biosensor in our model in an effort to standardize more high-throughput in vitro screening of ENMs for potential health hazards. Our study has also utilized the Calu-3 cell line, which is commonly used for tracheobronchial epithelial cell studies given their ability to form tight junction complexes in vitro. They display several of the primary culture properties and exhibit characteristics of differentiated, functional human airway epithelia [9,33]. Previous studies have demonstrated that Calu-3 cells develop a high TEER and exhibit a similar pattern of expression of epithelial markers when compared to primary cells [2]. More importantly, Calu-3 cells are immortalized and of human origin, thereby overcoming the constraints of using primary differentiated cell cultures, including cost and limited passage number.
Our results indicate that both cell seeding density, as well as FBS concentration can influence monolayer formation, as indicated by the changes in TEER values under the various conditions. Many extracellular stimuli, including nutrients, cytokines, and immune cells, have been shown to regulate or alter the barrier function of tight junctions, while serum proteins may open the already established tight junctions or inhibit their formation, thus influencing changes in TEER [22]. Accordingly, we found that high serum concentrations (15% and 10%) altered monolayer integrity, as indicated by low TEER measurements when compared with TEER values for lower serum concentrations at the same time point. Calu-3 cells cultured with serum concentrations as low as 2% displayed considerably increased TEER values, which corresponds with increased tight junction formation. Importantly, the serum effects were found to be dose-dependent and the barrier-weakening effect was not mediated by cell density since the same cell number was utilized for all serum concentrations. Our results also agree with earlier studies showing the inhibitory effect of serum on tight junctions in the blood brain barrier [5,6]. Thus, we suggest that using a concentration of 10% FBS in culture media provides satisfactory growth conditions while minimizing the effect of serum on TEER values. It is also noteworthy that the observed TEER values for this concentration of FBS in culture medium resulted in the most consistent TEER values at days 10 through 14 in culture compared with the other concentrations tested.
The increased use and demand for ENMs in a wide range of industries has fostered the need for more in-depth analysis on the health hazards following exposure. The potential exposure to CNTs of workers in small-scale production facilities has been reported by field studies to be airborne in trace amounts up to a few thousand μg/m3 [10,17]. Consistently, a recent study determined the average inhalable elemental carbon mass concentration at 8 facilities within the United States to be approximately 10 μg/m3 [8]. Hence, correlating exposure levels in cultured cells with the aerosolized levels likely to occur in the workspace is essential, owing to the probable biological effects on respiratory barrier function and mucocilliary clearance. To achieve this goal, our study made use of the occupationally relevant concentration range of 0.2–6 μg/cm2 of CNTs [19,24,37,40]. As evident in Fig. 5, MWCNTs caused dose- and time-dependent reductions in epithelial barrier integrity using these relevant concentrations. At low doses of 0.2 and 0.6 μg/cm2, MWCNTs had no effect on TEER values; however, at higher doses of 2 and 6 μg/cm2, MWCNTs induced a significant decrease in the resistance compared to controls after only 24 h of treatment (day 10 in culture). Conversely, f-MWCNTs and SWCNTs at the same high doses did not elicit significant changes in monolayer resistance, demonstrating a particle type-dependent alteration of airway barrier function.
Consistent with our findings, a previous study by Rotoli et al. demonstrated a significant decrease in the TEER measurements of Calu-3 monolayers with relatively low doses of MWCNTs (5–10 μg/mL), and a striking TEER decrease with exposure of 100 μg/mL MWCNTs or SWCNTs [28]. Rotoli et al. also observed that the reduction in monolayer permeability was accompanied by an increase in the paracellular permeability of mannitol, and that there was no significant alteration in the cell monolayer viability. As mentioned, our data is in agreement with these results using occupationally relevant concentrations of CNTs, in the range of 0.2 – 6 μg/cm2, equivalent to approximately 0.33 μg/mL and 9.9 μg/mL, respectively. Interestingly, we observed a much more dramatic difference in TEER values between the control and MWCNTs at approximately 10 μg/mL. The study by Rotoli et al. utilizes a similar method to that reported herein, with the exception that the authors employed a cell seeding density of 7.5 × 104 cells/well. When considering the results reported in Fig. 1, it is plausible that the increased seeding density resulted in higher baseline TEER values, and thus, required larger concentrations of CNTs to induce a greater decrease in TEER. These results highlight the importance of implementing more standardized methods for the assessment of the effects of ENMs on epithelial cells, and underscore the utility of the results reported here to allow for comparisons between studies.
Previous reports have demonstrated that the physicochemical parameters of CNTs, such as surface modification, can greatly influence monolayer formation and barrier integrity. During their interaction with biological tissues, surface chemistry of ENMs plays a key role in determining potential toxic pulmonary responses, including airway perturbation. It is believed that surface functional groups can affect the stability and dispersion of the MWCNT by altering the surface charge and reactivity of the particle, thus influencing the interaction of the particles with cells. Consistently, we observed an ENM- dose dependent effect on barrier function while studying f-MWCNTs compared to non-functionalized MWCNTs. The f-MWCNTs resulted in permeability that was consistent with controls; in contrast, their pristine counterparts greatly altered epithelial integrity. This observation is consistent with the recent report by Sager et al. in which it was demonstrated that f-MWCNTs, the same CNTs that we have used in the current study, were less bioactive and showed reduced signs of inflammation in vivo as compared to non-functionalized MWCNTs [29]. Our findings also suggest that the type of CNT that the epithelium is exposed to plays a prominent role in influencing the extent of airway barrier changes. For instance, we observed that pristine MWCNT caused a large decrease in TEER as compared to controls, whereas the SWCNTs had significantly smaller effects. MWCNTs have been shown to have a lower surface/volume ratio as compared to SWCNTs, and such structural differences may contribute to the greater perturbations of barrier function induced by MWCNTs [28]. This may be related to the morphology of the MWCNTs compared to SWCNTs, as MWCNTs tend to be observed as more rigid fibers that may be capable of poking through the epithelium [20,40], while SWCNTs can often be found as more flexible, rounded particles [39,40]. In addition, length-based impairment of barrier function has been reported using SWCNTs and MWCNTs of different lengths, where longer SWCNTs and MWCNTs induced a larger reduction in TEER values, indicating that fiber length led to a greater amount of epithelial dysfunction [27].
5. Conclusions
In conclusion, this study demonstrated that the Calu-3 epithelial cell line is a highly effective in vitro epithelial barrier model that can be implemented to test the effects of ENM on the epithelium. Cell density and serum concentration are major factors affecting the formation of monolayer complexes, and should be carefully considered and reported. We have found that 5.0 × 104 cells/well in the Transwell cell culture system is optimal for assessing epithelial cell barrier function, and that 10% serum concentrations in culture media should be maintained. Furthermore, SWCNT and MWCNTs were found to induce a dose- and time-dependent change in monolayer integrity of Calu-3 cells in vitro, and the physicochemical characteristics of CNTs, such as COOH-functionalization and the type of CNT, can influence monolayer formation and permeability. Overall, this work demonstrates a well-characterized model of in vitro epithelial barrier function by providing the experimental conditions required for achieving tight junction complexes and relevant CNT exposure doses, which further aid in predicting and comparing the interaction of numerous novel nanoparticles with the biological system and mimic the respiratory behavior in vivo.
Acknowledgments
The authors would like to thank Sherri Friend for her valuable assistance with microscopy. This work was supported by the National Institute for Occupational Safety and Health, Nanotechnology Research Center Fund and by a grant from the National Institutes of Health (R01-ES022968).
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Use of brand name does not constitute product support.
Conflict of interest
The authors declare that they have no conflict of interest associated with this work.
References
- 1.Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–298. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- 2.Banga A, Witzmann FA, Petrache HI, Blazer-Yost BL. Functional effects of nanoparticle exposure on Calu-3 airway epithelial cells. Cell Physiol Biochem. 2012;29(1–2):197–212. doi: 10.1159/000337601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez E, Mangum JB, Wong BA, Asgharian B, Hext PM, Warheit DB, Everitt JI. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci. 2004;77(2):347–357. doi: 10.1093/toxsci/kfh019. [DOI] [PubMed] [Google Scholar]
- 4.Card JW, Zeldin DC, Bonner JC, Nestmann ER. Pulmonary applications and toxicity of engineered nanoparticles. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L400–411. doi: 10.1152/ajplung.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang C, Wang X, Caldwell RB. Serum opens tight junctions and reduces ZO-1 protein in retinal epithelial cells. J Neurochem. 1997;69(2):859–867. doi: 10.1046/j.1471-4159.1997.69020859.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang CW, Ye L, Defoe DM, Caldwell RB. Serum inhibits tight junction formation in cultured pigment epithelial cells. Invest Ophthalmol Vis Sci. 1997;38(6):1082–1093. [PubMed] [Google Scholar]
- 7.Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92(1):5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 8.Erdely A, Dahm M, Chen BT, Zeidler-Erdely PC, Fernback JE, Birch ME, Evans DE, Kashon ML, Deddens JA, Hulderman T, Bilgesu SA, Battelli L, Schwegler-Berry D, Leonard HD, McKinney W, Frazer DG, Antonini JM, Porter DW, Castranova V, Schubauer-Berigan MK. Carbon nanotube dosimetry: from workplace exposure assessment to inhalation toxicology. Part Fibre Toxicol. 2013;10(1):53. doi: 10.1186/1743-8977-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster KA, Avery ML, Yazdanian M, Audus KL. Characterization of the Calu-3 cell line as a tool to screen pulmonary drug delivery. Int J Pharm. 2000;208(1–2):1–11. doi: 10.1016/s0378-5173(00)00452-x. [DOI] [PubMed] [Google Scholar]
- 10.Han JH, Lee EJ, Lee JH, So KP, Lee YH, Bae GN, Lee SB, Ji JH, Cho MH, Yu IJ. Monitoring multiwalled carbon nanotube exposure in carbon nanotube research facility. Inhal Toxicol. 2008;20(8):741–749. doi: 10.1080/08958370801942238. [DOI] [PubMed] [Google Scholar]
- 11.Helland A, Wick P, Koehler A, Schmid K, Som C. Reviewing the environmental and human health knowledge base of carbon nanotubes. Environ Health Perspect. 2007;115(8):1125–1131. doi: 10.1289/ehp.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77(1):126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 13.Landau D. Epithelial paracellular proteins in health and disease. Curr Opin Nephrol Hypertens. 2006;15(4):425–429. doi: 10.1097/01.mnh.0000232883.43093.76. [DOI] [PubMed] [Google Scholar]
- 14.Ma JY, Mercer RR, Barger M, Schwegler-Berry D, Scabilloni J, Ma JK, Castranova V. Induction of pulmonary fibrosis by cerium oxide nanoparticles. Toxicol Appl Pharmacol. 2012;262(3):255–264. doi: 10.1016/j.taap.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma JY, Zhao H, Mercer RR, Barger M, Rao M, Meighan T, Schwegler-Berry D, Castranova V, Ma JK. Cerium oxide nanoparticle-induced pulmonary inflammation and alveolar macrophage functional change in rats. Nanotoxicology. 2011;5(3):312–325. doi: 10.3109/17435390.2010.519835. [DOI] [PubMed] [Google Scholar]
- 16.Manke A, Luanpitpong S, Dong C, Wang L, He X, Battelli L, Derk R, Stueckle TA, Porter DW, Sager T, Gou H, Dinu CZ, Wu N, Mercer RR, Rojanasakul Y. Effect of fiber length on carbon nanotube-induced fibrogenesis. Int J Mol Sci. 2014;15(5):7444–7461. doi: 10.3390/ijms15057444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health A. 2004;67(1):87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- 18.Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Schwegler-Berry D, Castranova V, Porter DW. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol. 2010;7:28. doi: 10.1186/1743-8977-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercer RR, Scabilloni J, Wang L, Kisin E, Murray AR, Schwegler-Berry D, Shvedova AA, Castranova V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L87–97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- 20.Mercer RR, Scabilloni JF, Hubbs AF, Battelli LA, McKinney W, Friend S, Wolfarth MG, Andrew M, Castranova V, Porter DW. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2013;10:33. doi: 10.1186/1743-8977-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mura S, Hillaireau H, Nicolas J, Le Droumaguet B, Gueutin C, Zanna S, Tsapis N, Fattal E. Influence of surface charge on the potential toxicity of PLGA nanoparticles towards Calu-3 cells. Int J Nanomed. 2011;6:2591–2605. doi: 10.2147/IJN.S24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitz T, Eisenblatter T, Psathaki K, Galla HJ. Serum-derived factors weaken the barrier properties of cultured porcine brain capillary endothelial cells in vitro. Brain Res. 2003;981(1–2):30–40. doi: 10.1016/s0006-8993(03)02834-8. [DOI] [PubMed] [Google Scholar]
- 23.Porter DW, Hubbs AF, Chen BT, McKinney W, Mercer RR, Wolfarth MG, Battelli L, Wu N, Sriram K, Leonard S, Andrew M, Willard P, Tsuruoka S, Endo M, Tsukada T, Munekane F, Frazer DG, Castranova V. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology. 2013;7:1179–1194. doi: 10.3109/17435390.2012.719649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S, Andrew M, Chen BT, Tsuruoka S, Endo M, Castranova V. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269(2–3):136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Porter DW, Wu N, Hubbs AF, Mercer RR, Funk K, Meng F, Li J, Wolfarth MG, Battelli L, Friend S, Andrew M, Hamilton R, Jr, Sriram K, Yang F, Castranova V, Holian A. Differential mouse pulmonary dose and time course responses to titanium dioxide nanospheres and nanobelts. Toxicol Sci. 2013;131(1):179–193. doi: 10.1093/toxsci/kfs261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114(5):997–1008. doi: 10.1016/j.jaci.2004.07.060. quiz 1009. [DOI] [PubMed] [Google Scholar]
- 27.Rotoli BM, Bussolati O, Barilli A, Zanello PP, Bianchi MG, Magrini A, Pietroiusti A, Bergamaschi A, Bergamaschi E. Airway barrier dysfunction induced by exposure to carbon nanotubes in vitro: which role for fiber length? Hum Exp Toxicol. 2009;28(6–7):361–368. doi: 10.1177/0960327109105159. [DOI] [PubMed] [Google Scholar]
- 28.Rotoli BM, Bussolati O, Bianchi MG, Barilli A, Balasubramanian C, Bellucci S, Bergamaschi E. Non-functionalized multi-walled carbon nanotubes alter the paracellular permeability of human airway epithelial cells. Toxicol Lett. 2008;178(2):95–102. doi: 10.1016/j.toxlet.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Sager TM, Wolfarth MW, Andrew M, Hubbs A, Friend S, Chen TH, Porter DW, Wu N, Yang F, Hamilton RF, Holian A. Effect of multi-walled carbon nanotube surface modification on bioactivity in the C57BL/6 mouse model. Nanotoxicology. 2014;8(3):317–327. doi: 10.3109/17435390.2013.779757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev. 2006;58(9–10):1030–1060. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Sargent LM, Porter DW, Staska LM, Hubbs AF, Lowry DT, Battelli L, Siegrist KJ, Kashon ML, Mercer RR, Bauer AK, Chen BT, Salisbury JL, Frazer D, McKinney W, Andrew M, Tsuruoka S, Endo M, Fluharty KL, Castranova V, Reynolds SH. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2014;11:3. doi: 10.1186/1743-8977-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286(6):C1213–1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 33.Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl-secretion. Am J Physiol. 1994;266(5 Pt 1):L493–501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- 34.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 35.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L698–708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 36.Stewart CE, Torr EE, Mohd Jamili NH, Bosquillon C, Sayers I. Evaluation of differentiated human bronchial epithelial cell culture systems for asthma research. J Allergy (Cairo) 2012:943982. doi: 10.1155/2012/943982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6(2):235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- 38.Vllasaliu D, Exposito-Harris R, Heras A, Casettari L, Garnett M, Illum L, Stolnik S. Tight junction modulation by chitosan nanoparticles: comparison with chitosan solution. Int J Pharm. 2010;400(1–2):183–193. doi: 10.1016/j.ijpharm.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Castranova V, Mishra A, Chen B, Mercer RR, Schwegler-Berry D, Rojanasakul Y. Dispersion of single-walled carbon nanotubes by a natural lung surfactant for pulmonary in vitro and in vivo toxicity studies. Part Fibre Toxicol. 2010;7:31. doi: 10.1186/1743-8977-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Stueckle TA, Mishra A, Derk R, Meighan T, Castranova V, Rojanasakul Y. Neoplastic-like transformation effect of single-walled and multi-walled carbon nanotubes compared to asbestos on human lung small airway epithelial cells. Nanotoxicology. 2014;8(5):485–507. doi: 10.3109/17435390.2013.801089. [DOI] [PMC free article] [PubMed] [Google Scholar]


