Abstract
Rapid, sensitive, and selective pathogen detection is of paramount importance in infectious disease diagnosis and treatment monitoring. Currently available diagnostic assays based on polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA) are time-consuming, complex, and relatively expensive, thus limiting their utility in resource-limited settings. Loop-mediated isothermal amplification (LAMP) technique has been used extensively in the development of rapid and sensitive diagnostic assays for pathogen detection and nucleic acid analysis and hold great promise for revolutionizing point-of-care molecular diagnostics. Here, we review novel LAMP-based lab-on-a-chip (LOC) diagnostic assays developed for pathogen detection over the past several years. We review various LOC platforms based on their design strategies for pathogen detection and discuss LAMP-based platforms still in development and already in the commercial pipeline. This review is intended as a guide to the use of LAMP techniques in LOC platforms for molecular diagnostics and genomic amplifications.
Keywords: nucleic acid tests, molecular diagnostics, lab on a chip, microfluidics, LAMP, pathogen detection
Graphical Abstract
1. INTRODUCTION
The detection and identification of nucleic acids (NA) using point-of-care (POC) micro total analysis systems (μTAS) is a rapidly growing field with numerous clinical and industrial applications.1 μTAS-based platforms enhance nucleic acid amplification by increasing the ratio of surface area to volume, improving the transfer of heat in portable lab-on-a-chip (LOC) devices for diagnostic applications.2 Nucleic acid–based POC diagnostic platforms require cell lysis, NA extraction, and purification prior to amplification,3 all of which is of paramount importance in detecting small quantities of NA in fingerprick volumes of biological samples. Though polymerase chain reaction (PCR) is the most common method for DNA amplification, it involves multiple thermo-cycling steps. Isothermal amplification of NA eliminates the thermo-cycling steps, reducing cost and increasing assay quality.4
In addition to loop-mediated isothermal amplification (LAMP), other isothermal amplification techniques, including rolling circle amplification (RCA),5 strand displacement amplification (SDA),6 signal-mediated amplification of RNA technology (SMART),7 nucleic acid sequence-based amplification (NASBA),8 single primer-triggered isothermal amplification,9 helicase-dependent amplification (HDA),10 and cross-priming amplification (CPA)11,12 are used to isothermally amplify NA. However, LAMP13 has been shown to be faster and more stable, sensitive, and specific for NA detection. Because of the nature of the reaction, LAMP-based methods produce >50-fold more amplicon than PCR-based techniques.14 LAMP also has the ability to amplify medium- to long-range template strands of nucleic acids (>130 bp and <300 bp), which makes it suitable for amplification of various DNA of pathogens.13a Moreover, it can amplify the target NA in complex substrates even in the presence of substances that usually inhibit PCR reactions, such as blood components of hemoglobin, IgG, IgM,15 and some food ingredients.16 LAMP-based methods have higher specificity because they use four to six different primers that bind to specific sites on the template strand, whereas PCR uses only two primers.12b LAMP amplifies DNA at temperatures between 60 and 66 °C by using the Bst polymerase enzyme and high strand displacement activity.13a,17 However, RNA targets require additional reverse transcriptase enzyme to transcript RNA into cDNA prior to the amplification step.
Over the past decade, significant improvements have been made in monitoring LAMP amplicon detection at the end-point of LAMP reaction and in real-time. In particular, colorimetric, electrophoresis, immunoassay, and electrochemical modalities are leveraged for end-point detection. Optical, electrical, electrochemical, and pH-sensing mechanisms are used for real-time monitoring of LAMP amplicon. LAMP amplicon detection relies on production of magnesium pyrophosphate. Higher concentration of pyrophosphate ions reacts with the magnesium buffer, which yields white precipitate and increases the turbidity. This precipitate can be visualized by a naked eye or can be monitored in a real-time manner using optical instruments.18 Another detection mechanism is electrophoresis, which has been widely used for amplicon detection. Electrophoresis separates amplicon based on their sizes. LAMP amplicon can also be detected utilizing lateral flow assay by immobilization of a specific antibody to biotin at the test line followed by capturing biotin-labeled amplicon hybridized with fluorescein isothiocyanate (FITC)-labeled DNA probes.18 Electrochemical detection has been widely used for LAMP amplicon as well. Electrochemical detection mechanism relies on intercalating redox molecule with amplicon, which can detect at the end point or real-time manner.19 pH sensing modality has also been used for LAMP amplicon detection by leveraging the field effect transistor to monitor pH variation during LAMP reaction.20
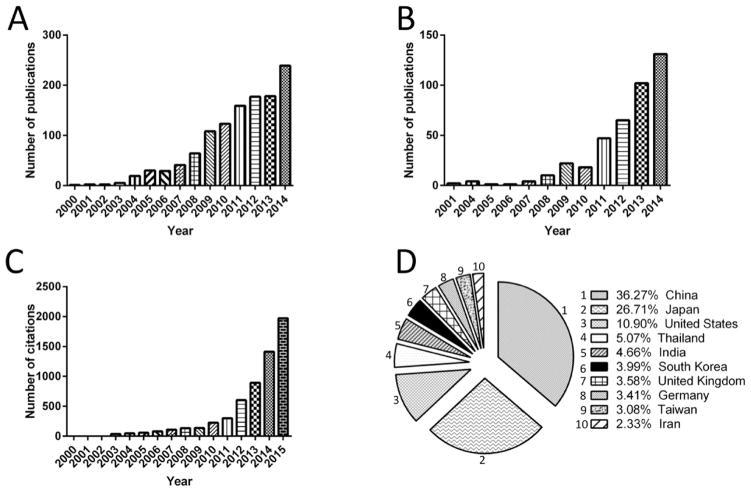
Since 2001 there has been exponential growth in the number of publications and citations related to the applications of LAMP in medicine, biology, environmental monitoring, and food industries (Figure 1A–C). In 2014 alone, 131 papers and 1414 citations were recorded. Scientists in China, Japan, and the United States have made the most contributions to the scientific and commercial advancements of LAMP-based LOC devices (Figure 1D). Here, we specifically and thoroughly review LAMP-based microchip technologies developed for NA detection, including colorimetric, electrochemical, optical, electrophoresis, and pH-based sensing modalities. We also discuss the advantages and disadvantages of various LAMP-based LOC design concepts developed for NA and pathogen detection.
Figure 1.
Search results for number of publications and citations related to LAMP-based technologies. (A) Number of publications with keywords of “loop-mediated isothermal amplification”. (B) Number of publications with keywords of “loop-mediated isothermal amplification” and “lab on a chip”. (C) Number of citations with keywords of “loop-mediated isothermal amplification” and “lab on a chip”. These statistics indicate the exponential growth in the interest in developing LAMP-based techniques. (D) Major contributing countries that published in science and commercial advancement based on LAMP LOCs. All statistical data were collected from SCOPUS.
2. END-POINT DETECTION
2.1. Colorimetric Detection
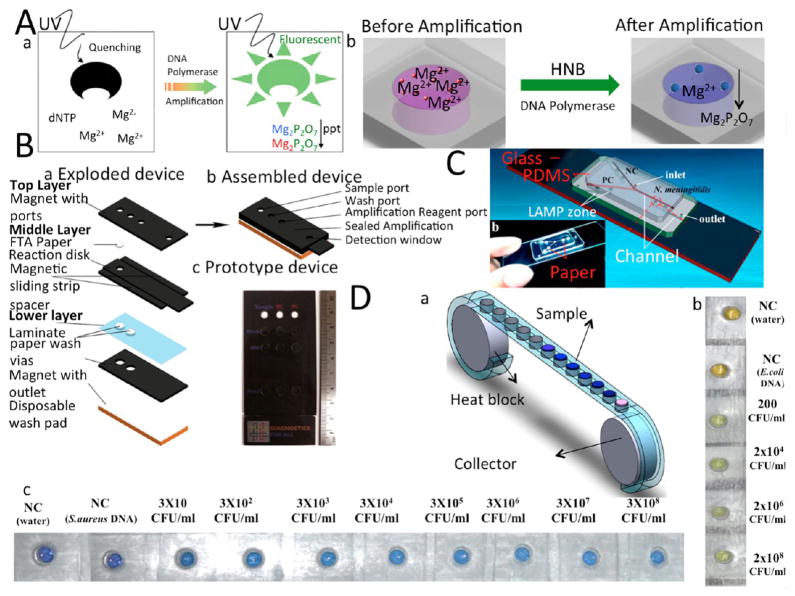
The main concept behind colorimetric detection is the production of magnesium pyrophosphate (Mg2P2O7) as a side product of the reaction of Deoxynucleotide triphosphate (dNTP) and magnesium sulfate (MgSO4). Magnesium pyrophosphate is an insoluble compound and will precipitate out of the background solution, increasing its turbidity.21 Though this technique requires no instruments, it is dependent on human interpretation of color. Various colorimetric dyes can be used to detect the presence of amplicon, but such dyes should not inhibit the amplification reaction, and the color change should be easily distinguished by the naked eye. Dyes used for LAMP amplicon detection, including fluorescent probes and pH-sensitive dyes, are listed in Table 1.22 Calcein and hydroxynaphthol blue (HNB) are the main dyes that can be added to preamplification samples without inhibiting the LAMP reaction.23 The colorimetric detection mechanism are described in Figure 2A. Other dyes such as propodium iodine, SYBR GREEN I, and Picogreen are used after amplification, as they inhibit LAMP and terminate amplification. Figure 2 shows various LAMP-based microchip platforms with colorimetric detection mechanisms.
Table 1.
Various Dyes Used for LAMP Detection
| dye name | color before amplification | color in presence of amplicon | inhibit LAMP | refs |
|---|---|---|---|---|
| hydroxynaphtol blue (HNB) | violet | blue | no | 23 |
| calcein | yellow | green | no | 21 |
| SYBR GREEN I | dark orange | green | yes | 101 |
| PicoGreen | light orange | green | yes | 102 |
| propodium iodine | orange | white/silver | yes | 103 |
| SYTO-81, SYTO-9 | orange | green | no | 104 |
| YO-PRO-1 | green | green | no | 105 |
| GeneFinder | orange | green | yes | 106 |
| EvaGreen | orange | green | no | 107 |
| ethidium bromide | no color (under UV) | yellow/red (under UV) | no | 105,107 |
| AuNP | red | purple | no | 108,109 |
| gold nano rod (GNR) | red | purple | no | 110 |
| fluorescein isothiocyanate (FITC) | yellow-orange | green | no | 111 |
| leuco triphenylmethane | no color | violet | no | 112 |
| SYBR Premix Ex TaqTM II | red | green | yes | 106 |
Figure 2.
LAMP-based LOC devices with colorimetric detection modalities. (A) Schematic of color change during amplification using (a) Calcein and (b) HNB dye. (B) Paper microchip for LAMP amplification and detection. (a) Exploded and (b) assembled schematic of paper microchip, which consists of three different layers to wash the lysate, purify the DNA sample, amplify the DNA template, and detect the amplification product using SYBR Green I. (c) image of the prototype. (C) Paper-plastic hybrid assay for detecting H1N1 virus. (a) 3D schematic of the chip, which consists of PDMS chip, glass, and paper chromatography paper. (b) The actual image of a fabricated microchip. Chromatography paper is placed at the amplification zones to preload primers. (D) LAMP-based cassette device (a) Cassette device with an array of chambers on a flexible ribbon for high-throughput bacterial detection. (b) S. aureus pathogen was detected using Calcein with the LOD of 200 CFU/mL. (c) E. coli bacteria were detected using HNB dye with LOD of 30 CFU/mL in 60 min amplification time. Reproduced with the permission from refs 21 and 24–26. Copyright 2008 Nature, 2014 American Chemical Society, 2015 American Chemical Society, and 2014 Royal Society of Chemistry.
Connelly et al.24 developed a cellulose paper microchip for sample preparation and LAMP amplification. Three layers of magnetic slips provide a robust platform for cell lysis as well as DNA purification and amplification, while cellulose paper is a suitable matrix for DNA adsorption and isolation. SYBR GREEN I was used to detect the amplified product with limit of detection (LOD) of 5 cells in 80 μL of human plasma blood sample (Figure 2B). In another effort, a hybridized paper/plastic microfluidic chip was developed for the detection of Neisseria meningitidis using calcein that showed a LOD of 3 DNA copies in a 26 μL sample of cerebrospinal fluid (Figure 2C).25 The LAMP amplification was implemented in cerebrospinal fluid, since it did not inhibit the reaction. In another study, a cassette device consisting of two aluminum reels and a plastic ribbon with an array of chambers was developed to detect E. coli (30 CFU/mL) and S. aureus (200 CFU/mL) utilizing HNB and calcein dyes, respectively.26 These pathogenic bacteria species and a LAMP reagent mixture were placed in the chambers, and then the array was covered by simple plastic tape. Cellular lysis was implemented through thermal shock at 65 °C for E. coli and 90 °C for S. aureus for 2 min. The color of HNB changed from purple to blue, and the color of calcein changed from yellow to green (Figure 2D).26 A similar approach was also used to develop a plastic pouch microchip to detect Herpes simplex virus (HSV-1) and HSV-2, with LOD of 6.08 copies/μL and 0.598 copies/μL, respectively, in 25 μL spiked PBS samples.27 The simplicity of the design, ease of fabrication, and use of a polyethylene sheet make these chips highly desirable for POC diagnosis in resource-limited settings. Wu et al.28 developed a glass microfluidic chip for NA detection using SYBR GREEN I dye. The chip featured a flow control system to avoid LAMP inhibition via an interaction with solid-phase extraction (SPE) reagents. Chemical lysis took place off-chip, and DNA extraction was achieved using pillar structures coated with chaotropic salt guanidine hydrochloride. Highly sensitive DNA extraction and amplification were completed within 2 h by passivation of the LAMP chamber and extraction region. In another effort, an easy to operate, octopus-like microfluidic chip was developed for selectively detecting different subtypes of the human influenza A virus.29 The LOD of the system was 10 fg/μL using 0.4 μL of DNA sample within an hour. Though colorimetric detection is less expensive than other methods (it does not require readers or signal processing), its main drawback is that it is mostly qualitative.
2.2. Electrochemical Detection
Electrochemical sensing has shown a strong promise for the development of rapid, sensitive, selective, miniaturized, and inexpensive POC diagnostic platforms.30 Electrochemical sensing has been utilized for detection of single nucleic polymorphisms,31 nucleic acids,30d,32 and amplification products.33 LAMP-based electrochemical sensing relies upon the oxidation–reduction of electroanalyte as monitored by linear sweep voltammetry (LSV),34 square wave voltammetry (SWV),33b,35 differential pulse voltammetry (DPV),36 or conductivity.37 Electrochemical detection is achieved either by redox labeling of immobilized LAMP amplicon on the surface of the working electrode or by detection of redox molecules in the presence or absence of amplification product. Sun et al.36 detected LAMP amplicons on a carbon ionic liquid electrode (CILE) using DPV. The electrode surface was modified with V2O5 nanobelt, multiwalled carbon nanotubes (MWCNT), and chitosan for electrode surface modification. The electrode was then set as a substrate for immobilization of a single-stranded DNA probe38 for Yersinia enterocolitica. The assay had a LOD of 1.76 aM and used methylene blue (MB) as an electrochemical redox for labeling the LAMP amplicon. Various electrochemical redox molecules commonly used for LAMP amplicon detection and their potential peaks with respect to Ag/Agcl (Ep vs Ag/AgCl) are listed in Table 2. Some of them, such as Hoechst 23458, inhibit the amplification reaction and cannot be rendered for real-time LAMP amplicon monitoring.39 MB or ruthenium hexamine, are among redox molecules that can be used for real-time monitoring as it does not interfere with LAMP amplification process.
Table 2.
Various Redox Used for LAMP Detection
| redox name | electrochemical technique | electrode | EP Vs Ag/Agcl | refs |
|---|---|---|---|---|
| methylene blue (MB) | DPV | carbon | −0.2 | 35 |
| Hoechst 33258 | LSV | carbon | 0.52 | 113 |
| ruthenium hexamine | SWV | carbon | −0.275 | 33b |
| [(Bpy)2Dppz2+(Os)] Dppz | SWV | carbon | 0.4 | 39 |
| daunomycin | DPV | carbon | 0.42 | 114 |
| 2′-deoxyguanosine 5′-triphosphated (GTP) | DPV | carbon | 0.81 | 115 |
| Ru(Phen)32+ | NA | gold | Na | 116 |
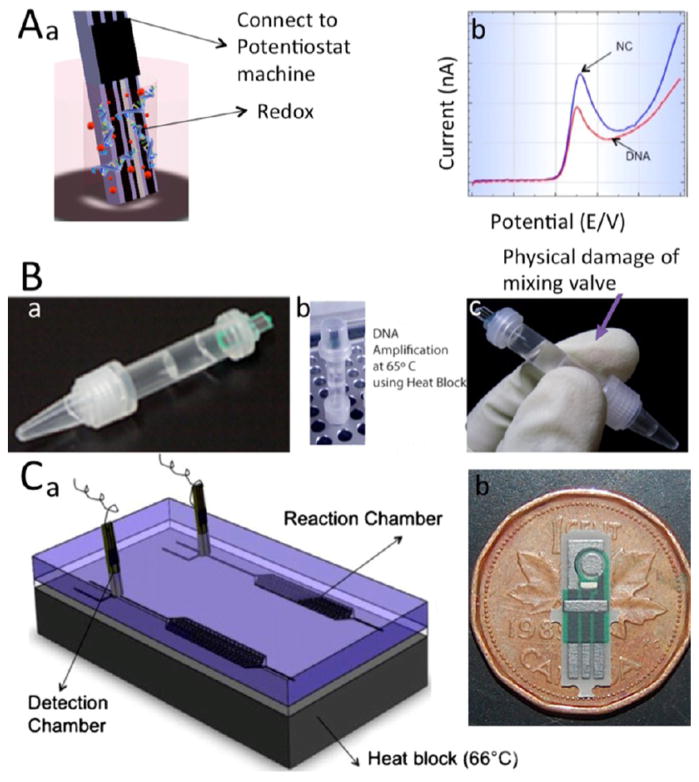
Generally, LAMP amplicon can be detected either at the termination of the amplification reaction or monitored in real-time. Electrochemical detection mechanism of LAMP amplicon at the end point was shown in Figure 3A. Ahmed et al.34 developed a LAMP-based portable device for maze CBH 351 GMO DNA detection and quantification in a tubelike chamber with separation valve between the detection and quantification chamber. LAMP reagents with target DNA were amplified; then by slight shaking, the separating wall was damaged, mixing redox with amplicon for further electrochemical detection. The LAMP amplicons were detected using screen-printed electrodes (SPE)40 in an electrochemical chamber with LOD of 200 fg/μL (Figure 3B).34 Safavieh et al.41 developed an LSV-based microfluidic device for the detection and quantification of E. coli. As shown in Figure 3C, this microfluidic device contained two parallel chips, including a reaction chamber, an active valve, and an electrochemical detection chamber. Carbon SPEs were fabricated vertically in the detection chamber. The mixture of E. coli and LAMP reagents was loaded onto the chip, and the bacteria were lysed through a 66 °C thermal shock process and detected with a LOD of 24 CFU/ml.41 Figure 3 demonstrates LOC platforms for the detection of LAMP amplicon using endpoint electrochemical sensing. Others used a combination of LAMP and bacteriophages for viability screening and confirmation through a dual response biosensor.42 The bacteriophage was immobilized on a cysteamine-modified gold electrode by cross-linking with 1,4-phenylene diisothiocyanate. The impedance biosensor showed reproducible and sensitive detection within a range of 1 × 103 to 1 × 109 CFU/ml. The impedimetric response of live and dead E. coli was tested after incubation of 1 × 108 CFU/ml live and dead cells on electrodes for 15 min. After washing with PBS, faradaic impedance was measured for 1 h to determine bacterial viability. Increasing the LAMP amplification time to 40 min enhanced the chip sensitivity by at least 1 order of magnitude (1 × 102 CFU/mL).
Figure 3.
LAMP-based microchip devices with electrochemical sensing modalities (end-point detection). (A) 3D schematic of electrochemical sensing of amplified NAs (a) Schematic of binding redox and LAMP amplicon at the end of LAMP reaction, which results in the LSV peak reduction. (b) LSV of Hoechst redox. (B) Microchip for electrochemical detection of maize CBH 351 GMO DNA. (a) Image of DNA stick, which consists of amplification and detection sections that are separated from each other by a valve. (b) The amplification reaction was first performed on a hot plate. (c) by damaging the interface valve with gentle mixing, redox and amplification products were mixed and further electrochemical detection was possible. (C) Microfluidic electrochemical assay for detecting E. coli.. (a) Schematic of microfluidic chip for negative control and sample detection. Each microfluidic chip consists of PDMS chip, glass substrate, and aluminum heater. The detection chamber has a disposable carbon screen-printed electrode. (b) Image of disposable screen-printed electrode. Reproduced with the permission from refs 34 and 41. Copyright 2009 Royal Society of Chemistry and 2013 American Chemical Society. respectively.
2.3. Electrophoresis
Electrophoresis is one of the most common techniques for separation of NA (DNA or RNA) and proteins based on their molecular size and charge.13a,21,43 Gel electrophoresis (GE) has been used for LAMP amplicon detection with 2% agarose gel as a matrix. To visualize amplicon on the gel, ethidium bromide was used as an intercalating fluorescent dye.44 The length of amplicon can be measured using a specific molecular size standard, reducing the risk of nonspecific detection. GE is usually used as a postprocessing method for amplicon detection and has not been used for on-chip detection analysis. Capillary electrophoresis (CE) has the advantage of on-chip multiplex detection and relatively low power consumption.45 Although CE requires complex equipment and is time-consuming, it can provide endpoint amplicon detection by using fluorescent dyes such as ethidium bromide.46 Several microchip platforms have been developed for LAMP amplicon detection using electrophoresis. Hataoka et al.47 developed an electrophoresis-based poly-(methyl methacrylate) (PMMA) chip to detect gene fragments of LAMP amplicon with a LOD of 23 fg/μL and 15 min amplification time. In another study, a microreactor chip was developed for single molecule detection on polyacrylamide gel (PAA).48 Because of its porous structure, various ions diffuse into the PAA gel matrix and initiated amplification. The chip consisted of a microheater and a temperature sensor, both of which were fabricated at the bottom of each chamber. The amplification product was then visualized by fluorescent imaging analysis at the end of the reaction.
Isotachophoresis (ITP) is another electrophoresis technique to separate NA from complex samples based on their ionic mobility. By utilizing ITP, leading electrolyte (LE) and trailing electrolyte (TE) buffers,49 a chip with minimal sample pipetting was developed. E. coli bacterial DNA were separated and purified from milk samples and a LOD of 103 CFU/ml was achieved.
2.4. Immunoassays
Immunoassays, including lateral flow test (LFT) and enzyme-linked immunosorbent assay (ELISA), have also been used for developing LAMP-based diagnostic technologies by immobilizing biotin-labeled LAMP amplicon and labeled DNA probes.50 Lateral flow assays detect biotin-labeled LAMP amplicon hybridized with a FITC-labeled DNA probe conjugated with gold-based anti-FITC antibody. Non-hybridized FITC bind to gold-labeled anti-FITC antibody and move forward to the test line.50 The bioanalyte detection time, excluding DNA extraction, in the so far developed LFT-based assays were less than 40 min. The sensitivity of the LFT-based assays for LAMP amplicon detection was 2.4 fg/μL for Roundup Ready soybean (RRS),51 3 fg/μL for Mycobacterium tuberculosis genomic DNA,52 and 0.039 fg/μL for extracted Staphylococcus pseudintermedius DNA.53 LFT provides robust specificity due to the fact that the probe is complementary to a specific amplicon.
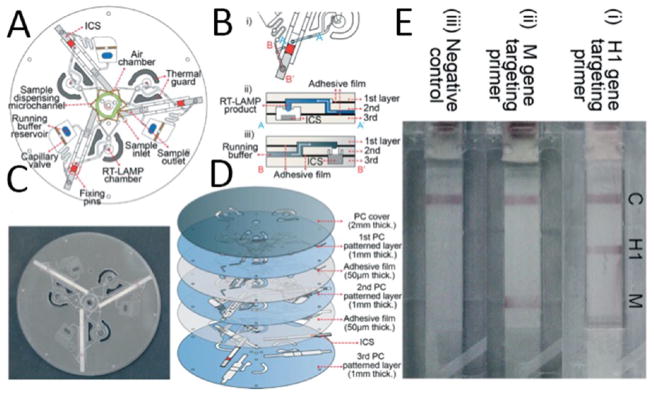
A centrifuge-based microchip integrating three separate LFTs and LAMP on a compact disk (CD)-based microfluidic device was developed for detecting H1N1 virus through the detection of H1 and M genes.54 Each microfluidic device consisted of an inlet for injecting LAMP reagents, a chamber for amplification, and a zigzag-shaped dispensing microchannel which was set to aliquot LAMP solution equally in three chambers. A 2 μL sample was injected into the inlet and filled the zigzag channel by capillary action. Using an air pump, the sample was forced into the reaction chamber and a siphon channel was connected to the chamber to prevent overflow of the LAMP sample into the LFT. The flow process is shown in Figure 4. With the use of different centrifugal speeds and various amplification times, LAMP amplicons reached the LFT and different target genes in H1N1 were detected with LOD of 10 copies per sample (7 μL). LFT-LAMP has great potential in the development of POC diagnostic platforms by integrating the sensitivity and specificity of LAMP with the simplicity of lateral flow immunoassay which does not require any trained personnel. Integration of this method with self-contained microfluidic platforms could also eliminate the potential of cross-contamination and can provide high-throughput analysis.
Figure 4.
Multiplex RT-LAMP integrated with LFT to control the flow (A) 2D schematic of the CD microfluidic device. (B) (i) An illustration of how the microchannels were connected to the LFT, (ii) cross-sectional schematic for the connection between microchannel, RT-LAMP chamber, and the LFT, and (iii) schematic for the connection between the running buffer reservoir and the LFT. (C) Image of the RT-LAMP-LFT CD microfluidic. (D) Exploded 3D schematic of the RT-LAMP-LFT microchip. Different layers cover the LFT, hold the sample, and control the flow. (E) Actual image of the assay results for detecting (i) H1 gene, (ii) M gene, and (iii) negative control. Reproduced with permission from ref 54. Copyright 2015 Royal Society of Chemistry.
Combining LAMP and ELISA also yields a strong technique for developing high-throughput diagnostic assays with immense sensitivity. The developed LAMP-ELISA-based assays for detecting Mycobacterium tuberculosis and Salmonella serogroup D demonstrated LODs of 100 copies in 100 μL sample (1 copy/μL)55 and 10 CFU/ml,56 respectively. The sensitivity of LAMP-ELISA-based assay for detecting Salmonella was 100 times more sensitive than PCR- and turbidity-based LAMP assays.56 The principle behind the LAMP-ELISA method is direct incorporation of labeled nucleotide into the amplicon during the amplification process, hybridization to a specific probe, and detection of captured antigen by an immunoassay.56 The main drawbacks are that the assay can take up to several hours, and the fact that it is a multistep process, which makes the creation of a POC diagnostic device unpractical. However, the method does not require an expensive reader. In another work, ELISA-LAMP was integrated with phage to detect organophosphorous pesticides. Phage g3p-displayed short peptide libraries were shown to be efficient tools for selecting the mimotope peptides. The unique feature of phage-born peptide yields binding with a specific target on a phage particle containing amplified NA.57 This strategy makes it possible to detect small molecules that do not have any DNA strands within the range 2–128 ng/mL.
3. REAL-TIME DETECTION
3.1. Optical Detection
Optical-based LAMP detection methods utilize the production of magnesium pyrophosphate Mg2P2O7 as the byproduct of the LAMP polymerase reaction. Magnesium pyrophosphate increases the sample’s turbidity, which can be detected with a turbidimeter in real time,58 optical fiber,59 surface plasmon resonance (SPR),60 spectrophotometer,61 or fluorescent imaging by a charged coupled device (CCD) camera in microchips.62 DNA copies can be quantified by plotting turbidity against amplification time. Fang et al.59 developed an eight-channel microchip made out of poly-dimethylsiloxane (PDMS) and glass for detection and quantification of pseudorabies virus DNA. The chip utilized a digital fiber optic sensor to emit LED light at 640 nm and a phototransistor to measure turbidity. Using only a 0.4 μL sample, a LOD of a 10 fg DNA sample was achieved after an hour amplification process. In a similar method, but more applicable for POC diagnostics, a valve-less microfluidic device was developed to detect multiple genes, including stx2 and eaeA of E. coli and mecA and vicK genes of S. aureus.63 The polyester film microfluidic device was fabricated using hot embossing. In this method, SYTO-81 dye was mixed with reagents before being applied to the microchambers. All primers and LAMP reagents were dehydrated in each chamber. A LOD of 13 copies per sample (1 μL) was achieved by employing LED light at the bottom of each chamber (Figure 5A). The amplification time was an hour at a temperature of 63 °C. The main drawbacks are heterogeneity of the particle size, uneven spatial distribution, and redissolution of magnesium pyrophosphate particles.18,60
Figure 5.
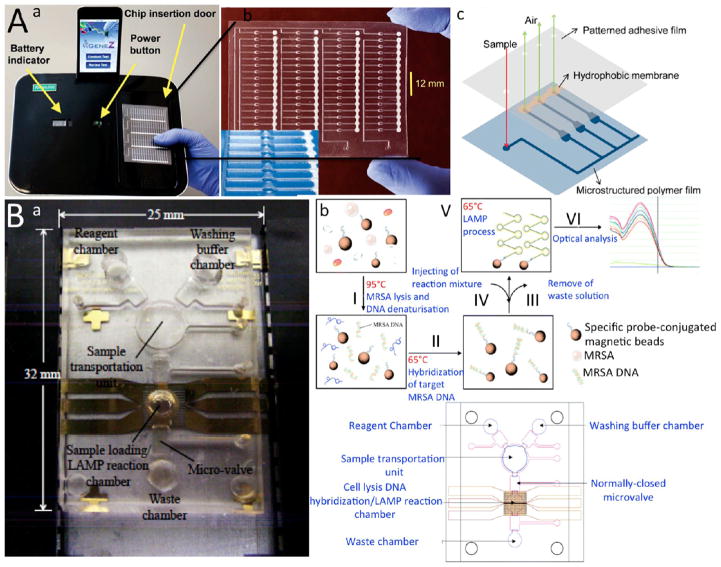
LAMP-based microfluidics integrated with optical sensing apparatus. (A) Microchip integrated with fiber optics. (a) Image of Gene-Z device integrated with an iPod dock, a rechargeable port, and a disposable microchip. (b) An actual image of the microfluidic device with four parallel arrays of microchannels and close-up of the reaction wells. (c) 3D schematic of the microchannels demonstrating the working principle behind the Gene-Z technology. After sample loading through the sample inlet the reaction wells are filled within several seconds. Air inside the reaction wells is purged from the vents located downstream of each channel to distribute samples in all chambers equally. (B) A LAMP-based microchip for bacteria detection integrated with a spectrophotometer. (a) Actual image of the integrated microfluidic device connected to an air vent, a heat block, and a magnet. (b) Schematic of the principle behind the technology for MRSA detection. MRSA bacteria are injected into the chip with a specific probe conjugated with magnetic beads and lysed in lysis chamber through heating at 95 °C. After releasing MRSA DNA, the molecular probe is hybridized with DNA at 63 °C. The target DNA is separated from background using magnetic separation. LAMP reagents are added to the solution and amplified targets are optically detected with a spectrophotometer. (c) 2D schematic of the microchip and its components. Reproduced with the permission from refs 61 and 63. Copyright 2011 and 2012 Royal Society of Chemistry, respectively.
A LAMP-based microfluidic device was developed to detect methicillin-resistant Staphylococcus aureus (MRSA) bacteria in clinical samples using a spectrophotometer.61 The chip consists of lysing, washing, and reaction chambers for sample treatment and amplification. Oligonucleotide probe conjugated with magnetic beads was mixed with MRSA bacteria (Figure 5B). The bacteria was lysed at 95 °C, and the probe conjugated with the magnetic beads bound to the released DNA. Target DNA was then purified through magnetic separation in the hybridization chamber. LAMP reagents were applied to the chamber, and amplification product was measured via a spectrophotometer. The entire sample processing and amplification procedure was carried out automatically, and a LOD of 10 fg/μL was achieved (Figure 6A). FLUO SENS DO fluorescence detection module was integrated in a microfluidic device for HIV viral load testing using saliva samples.64 The cassette microfluidic device consisted of a chamber for isolation and purification of RNA and removing critical inhibitors using a Flinders Technology Associates (FTA) Whatman membrane. The amplified target amplicons were then detected using SYTO-9 green dye and a portable ESE fluorescence detector. A flexible, polyimide-based heater attached to the bottom of the microfluidic chamber provided temperature regulation for amplification. This assay showed a LOD of 10 copies of HIV in saliva samples (20 μL) with an assay time of 27 min.
Figure 6.
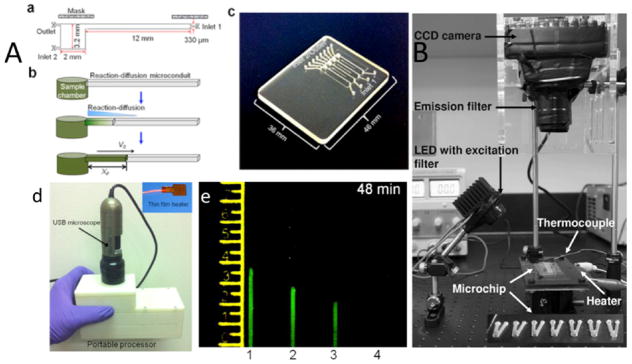
LAMP-based microchips integrated with CCD imaging. (A) The nuclemeter. (a) The nuclemeter has a reaction chamber and a microconduit where reaction-diffusion takes place. (b) NA template is amplified and diffused through the channel. (c) Actual image of a fabricated nuclemeter microchip with four separate microchannels for detecting HIV RNA. (d) Actual image of the portable nuclemeter with its components, includinga flexible thin heater and USB-size microscope. (e) Quantification of HIV-1 in samples based on the diffusion length of amplified amplicons in each channels. (B) Microfluidic device platform for detecting various bacterial DNA using a CCD camera, emission filter, LED with excitation filter, and a microchip. Reproduced with permission from refs 62 and 67. Copyright 2011 Springer and 2014 Nature, respectively.
CCD-based fluorescent imaging was used for real-time monitoring of LAMP amplification to detect waterborne pathogens. SYTO-81 was used as a florescent dye, and real-time imaging was implemented for Campylobacter jejuni 0414 gene detection. The microfluidic chip had 8 times higher signal-to-noise ratio (SNR) and half of threshold time (Tt) in comparison with a commercially available real-time PCR instrument. A single copy of a gene was detected within only 19 min of threshold time.62 The chip was later modified to detect multiple food- and water-borne pathogens of S. enterica, C. parvum, C. jejuni, L. pneumophila, E. coli O157:H7, V. cholerae (Figure 6B).62 An optical photomultiplier (PMT)-based microchip was developed for multiplexing Streptococcus agalactiae, Koi herpesvirus, Irido virus, and Aeromonas hydrophila.65 DNA from cell lysate was isolated using magnetic beads coated with target-specific probes. The extracted DNA was amplified and optically detected within 65 min. The sensitivity analysis for this microchip technology showed a LOD of 20 copies in 25 μL sample.65 Liu et al.66 developed a sample-to-answer portable microfluidic chip for determining the genotype of a malaria mosquito. The device was integrated with a CCD camera from a cell phone and a blue LED, which enhanced the image analysis of the detected target malaria genome. The chip consisted of three separate chambers to identify Anopheles gambiae, Anopheles arabiensis, and the negative control sample. A piece of the malaria mosquito’s leg was first placed on the FTA Whatman paper and crushed until it formed a small disc-shaped sample. Then the FTA paper was placed into each chamber, and the lysing reagent (AL lysing solution) was added to the sample and washed to remove debris and inhibitors. LAMP reagents along with SYTO-9 fluorescent dye were added to each chamber. LED light excited the intercalating dye which made it easier to visualize by naked eye.66 A combination of real-time RT-LAMP and a hand-held USB-based fluorescent microscope on a microfluidic chip was used for HIV viral load testing.67 As shown in Figure 6, the chip included a chamber and a diffusion-reaction micro conduit. The sample laden with target DNA was introduced into the chamber and LAMP amplification was initiated thermally. The microconduit was filled with 4% (w/v) hydroxypropyl-methyl-cellulose (HPMC) to reduce the diffusion speed and confine the reaction front. As the amplification reaction progresses, the amplicon diffused through the microconduit. The diffusion length of the amplicon is proportional to the amplification progress. The sensitivity of this method was 50 copies in 15 μL of sample.
SPR chips have also been used for LAMP amplicon detection.60,68 A SPR-LAMP-based microchip with a PMMA reactor and polycarbonate prism coated with 50 nm Au was developed for Hepatitis B virus (HBV) detection.60 HBV DNA was used as a template and mixed with 10 μL of LAMP reagents, which yielded a LOD of 2 fg/mL.60 In another SPR-LAMP-based microchip, single-layer graphene was constructed on the surface of the Au SPR chip for detecting tuberculosis bacillus (TB) DNA.69 Though label-free optical detection modalities are powerful in developing real-time monitoring of LAMP reactions, such methods usually require bulky and expensive readers and have shown poor sensitivity.
3.2. Real-Time Electrochemical Detection
Electrochemical modality has also been used for real-time monitoring of LAMP reaction (Figure 7A). Hsieh et al.70 developed a microfluidic device for real-time monitoring of DNA amplicon using electrochemical sensing modality. Au working electrode and Pt counter and reference electrodes were fabricated at the bottom of the chip (Figure 7B). MB was used as a redox molecule, as it does not inhibit LAMP, and SWV scanning was implemented to monitor intercalating of MB with LAMP amplicon during amplification (Figure 7C). This assay detected the target amplicon in less than 50 min with a LOD of 16 copies/μL. Similarly, an octopus-like microfluidic system with an indium tin oxide (ITO) electrode fabricated on a glass substrate was developed for multiplexing bacteria DNA.71 The sensitivity of this method for detecting Mycobacterium tuberculosis (MTB), Haemophilus influenzae (HIN), and Klebsiella pneumoniae (KPN) was 28, 17, and 16 copies/μL, respectively. The assay time for these target bacteria was between 30 min and 1 h. Safavieh et al.39 also developed a cassette-type device for real-time electrochemical detection of E. coli as a Gram-negative model and S. aureus as a Gram-positive model. The device featured two aluminum reels and a plastic ribbon that formed an array of reaction chambers. Carbon SPEs were fabricated at the bottom of each chamber. One of the aluminum reels acted as a ribbon collector and was connected to the heater to maintain a constant 65 °C temperature for amplification. The other reel acted as a sample provider. When samples were added to the chamber, the array of chambers was covered with heat tape to prevent sample evaporation. Then the sample rolled around the collector reel to initiate amplification. Simultaneously, each electrode was connected to a potentiostat for signal processing. Since 65 °C was not sufficient to lyse S. aureus, the samples were first covered with tape and heated on-chip at 90 °C for 2 min. Then the tape was removed and LAMP reagents were added to each sample for further electrochemical monitoring, and the temperature was set at 65 °C.39 The sensitivity of 200 CFU/mL of S.aureus was achieved. Overall, electrochemical-based methods for the detection of LAMP-amplified agents are rapid, low-cost, and sensitive. However, various factors such as electrode material, pH, and ionic strength of the sample can potentially affect the electrochemical signal.72
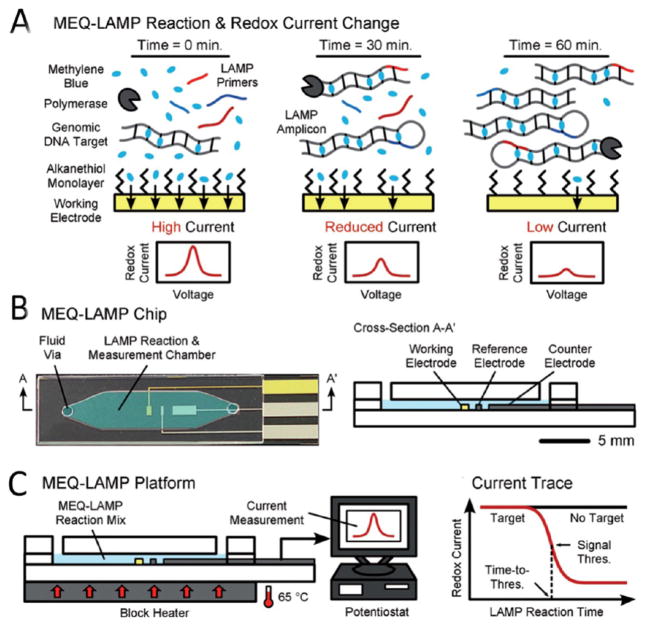
Figure 7.
LAMP-based microfluidic device with real-time electrochemical sensing modalities. (A) Schematic of the principle behind real-time electrochemical detection using MB active redox compound. Prior to amplification, MB is free in the solution and generates high redox current due to rapid diffusion on the surface of gold working electrodes. During LAMP reaction, MB intercalates with newly formed double stranded product, which leads to a decrease in the current peak. (B) Schematic of the microfluidic electrochemical quantification (MEQ)-LAMP chip and its cross-section (C) Microchip is placed on a heat block to provide the required temperature (65 °C) for amplification. The amplified target is detected through SWV using a potentiostat. Adapted with permission from ref 70a. Copyright 2012 Wiley.
3.3. pH-Sensing Platforms
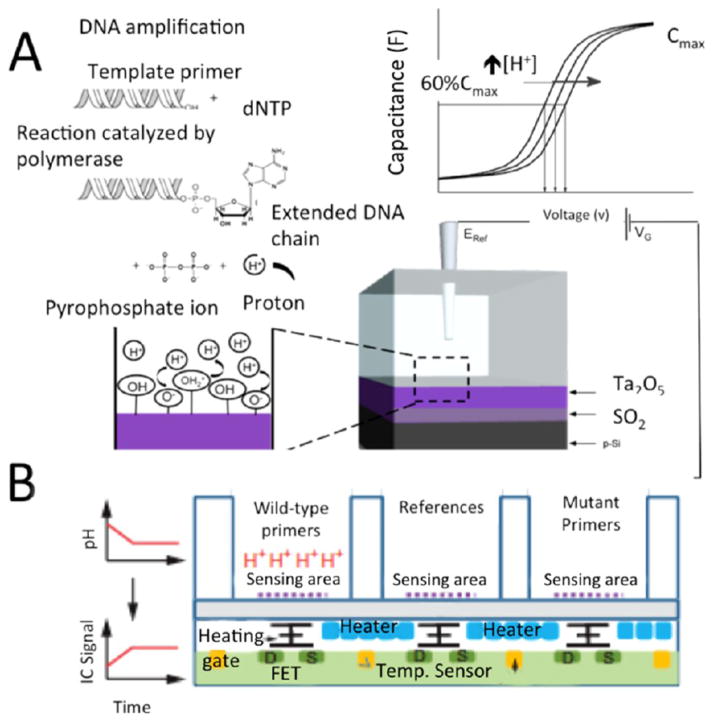
The pH of the solution changes during the LAMP polymerization reaction due to ion release, which can be monitored by electrochemical or electrical sensing.73,76 A simple pH meter can be used for real-time monitoring of LAMP amplification process.74 Tomazou et al.20,75 developed an ion-sensitive field-effect transistor (ISFET), which converts a chemical signal into an electrical signal. The ISFET was integrated with a temperature control sensor. As the amplification reaction proceeded, a significant amount of proton ion (H+) was released into the chamber. The increase of the charge at the sensing surface caused an increase in the charge distribution between the source and drain gates of the ISFET, which increased the integrated circuit (IC) output signal voltage yielding a LOD of 10 copies of genome DNA in 2 μL of sample. In another approach, a FET-based sensor made of tantalum oxide (Ta2O5) combined with a AgCl reference electrode was also developed for real-time detection of DNA amplification by detecting generated protons (H+) during the LAMP process through on-chip capacitance measurement.76 The measured voltages were calculated at 60% of the maximum obtained capacitance in a C–V curve. Label-free LAMP amplicons were quantified in the range of 1 × 108 to 1 × 1011 DNA copies/ml as shown in Figure 8.
Figure 8.
LAMP-based microchip with pH sensing modality. (A) The principle behind the ion sensing platform is illustrated. Schematic of how C–V curve changes throughout the LAMP amplification process due to the production of H+. The elongation process yields accumulation of protons. Thus, the pH shift in during the LAMP process is proportional to the number of nucleotides. The hydrogen accumulation can be measured by impedance spectroscopy. (B) Schematic of pH sensing with its ISFET platform. Cross section of array of an ISFET-based system with its gate, source (S), drain (D), temperature sensors (yellow), and controlled heater (blue), all are embedded at the underneath of a silicon nitride-sensing surface. Reproduced with the permission from refs 20 and 76. Copyright 2013 Nature and 2014 Elsevier, respectively.
Most of the ion-sensitive FET-based devices rely on the establishment of fluid gate potential, which requires complex microfabrication of reference electrodes and limits parallel detection. To overcome this problem, a novel method for the fabrication of a solid-state platinum quasi-reference electrode (QRE) coupled with pH-insensitive field-effect transistor (FET) was developed for real-time pH monitoring in LAMP reactions.77 Polyvinyl chloride (PVC) was used to cover ISFET, which made it insensitive to pH change. The pH of the solution containing the LAMP reagents was optimized using HCl-Tris buffer, and the reaction was monitored for pH change. A LOD of 1 × 102 to 1 × 103 CFU/mL was achieved with a detection time of less than 30 min.
4. DESIGN CRITERIA
4.1. Capillary Tube
Glass capillary are nonpermeable and chemically neutral and have been used in developing LAMP-based detection assays. Several capillary-LAMP-based assays were developed for detecting Mycobacterium tuberculosis with sensitivities down to 1pg/mL and assay time less than 15 min.78,79 The sample handling in such capillary-based assays is simple. The capillary-based microchip developed by Liu et al. utilized magnetic beads for sample processing and DNA purification.79 Different sample preparation, detection, and amplification zones were categorized in capillary glass. The magnetic area of the capillary glass was sandwiched between two electrical magnets. The detection zone was an LED array with emission and excitation filters. After DNA purification, the sample droplet was transported into the reaction zone for DNA amplification. The amplified targets were then quantified through fluorescence detection of the sample using a blue-light LED. A LOD of 10 bacteria (in 10 μL sample) was achieved within 50 min, including sample analysis.79 Using an FTA membrane, an integrated glass capillary device was fabricated to detect single-nucleotide polymorphisms (SNPs) in CYP2C19 from untreated blood in 150 min. A small flake of FTA membrane was picked up by syringe needle and placed inside the capillary. Several reagents for purification and washing, including purification reagent, Tris-EDTA (TE) buffer, LAMP reagents, and water, were injected from the other side of the capillary at various positions, while 0.2 μL of blood sample was injected from the opposite side and permeated the FTA paper. The purification solution and TE buffer washed the extracted DNA on the FTA paper, which was then immersed in LAMP solution for further amplification.80 Later on, this concept was modified for high-throughput sample purification using micropipette tips.81 FTA paper was inserted at the top part of the micropipette tip. As the sample contacted the FTA, RNA/DNA was extracted and trapped in the FTA card. Washing buffer was introduced to purify the extracted DNA, following by applying LAMP reagent. The pipet tip was sealed by lighter flame, and the amplified target was detected using fluorescent dye (Calcein).
4.2. High-Throughput and Multiplex Analysis
Despite the complexity of LAMP amplicon structure, several LAMP-based assays have been developed for simultaneous detection of pathogens in biological samples.82–86 To allow multiplexing using LAMP technology, Liang et al.83 designed a series of target-specific barcodes and tagged the LAMP amplicon with FIP primer. The barcodes were decoded utilizing pyro-sequencing on nicked amplification product. To enable the nicking reaction near the barcode regions, the recognition sequence of the nicking endonuclease was attached on the FIP primer. After nicking, pyro-sequencing was initiated at the 3′ end using dNTP complementary to the non-nicked strand to simultaneously detect four pathogens of HBV, hepatitis C virus (HCV), human immunodeficiency virus (HIV), and Treponema pallidum. In other work, a multiplex endonuclease restriction real-time LAMP assay was developed to detect multiple genome targets in one LAMP sample.84 FIP and BIP primers, which contain 5′-end short sequences, were recognized by restriction endonuclease enzyme and new FIP and BIP were modified at the 5′ end with fluorophore as well as a dark quencher. Later, restriction endonuclease digested the newly synthesized double-stranded terminal sequences, releasing the quenching and gaining the florescent signal. The positive signal was obtained within 12 min.
Various fluidic components such as reaction chambers, microvalves, micropumps, and microdispensers can be integrated in LOC-based NA detection assays to provide micro/nano droplet-size samples for multiplexing and high-throughput analysis.85 Zhou et al.86 developed a centrifuge-based microfluidic platform to detect 10 different bacteria, including Nocardia seriolae Pseudomonas putida, Streptococcus iniae, Vibrio alginolyticus, Vibrio anguillarum, Vibrio fluvialis, Vibrio harveyi, Vibrio parahemolyticus, Vibrio rotiferianus, and Vibrio vulnificus using LAMP technology. Real-time and endpoint fluorescent intensity measurement was implemented to achieve a LOD of 0.4 pg in a 1.414 μL sample (Figure 9A). In another study, an octopus-like microfluidic device was created for multiplexed real-time electrochemical analysis of three different bacteria: Mycobacterium tuberculosis (MTB), Haemophilus influenzae (HIN), and Klebsiella pneumoniae (KPN) with LOD of 28, 17, and 16 copies/μL, respectively (Figure 9B).71 The assay time in this method was less than 45 min. Cassette format devices with the ability to simultaneously analyze 3626 and 1239 samples for detecting biotargets, including E. coli, were also developed using end-point colorimetric and real-time electrochemical detection modalities with a sensitivity down to 30 CFU/mL within 29 min.
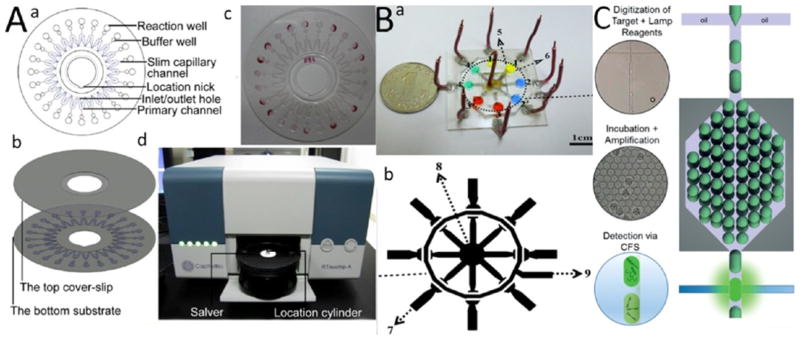
Figure 9.
Multiplexing and high-throughput LAMP LOC devices. (A) CapitalBio RTisochip. (a) Microfluidic disc platform. Each disk consists of 24 reaction wells, which are attached to the buffer well. The buffer wells are connected with crooked primary channels with a slim capillary channel. The capillary channel is then cut off by thermal shock during the amplification process, which isolates the reaction well to protect the sample from potential contamination. Each reaction well has a volume of 1.414 μL and all the primers are first added into the wells and dehydrated. (b) Schematic of top and bottom coverslips. (c) Actual image of a fabricated CD-based microchip. (d) Image of the CapitalBio RTisochip platform. (B) A LAMP-based microchip for multiplexing biotargets using real-time electrochemical sensing modality. (a) Actual image of a fabricated microchip. (b) 2D schematic of the device and its three sections for detecting three different DNA pathogens. (C) A droplet-LAMP-based microfluidic for high-throughput biotarget detection. Amplified droplets are detected using Calcein fluorescent signal (CFS) analysis with confocal microscopy. Reproduced with the permission from refs 71, 86, and 90. Copyright 2014 Elsevier, 2014 Elsevier, and 2015 Royal Society of Chemistry, respectively.
SlipChip is a novel microfluidic platform with the ability of high-throughput analysis of nanoliter sized biological samples. The chip consists of two plates that are in contact with each other. The bottom plate has an array of chambers that are filled with reagents and ducts. The top plate acts as a lead for the reservoirs and a series of wells. The sample is first introduced into the wells on the top layer through microchannels, which is then mixed with the reagents in the bottom plate wells after slipping the top plate. This platform allows multiplexing and eliminates the need for valves and on-chip pumps for sample handling. It also reduces the amount of sample required to pico/nanoliter volumes.87 In a similar approach, a SlipChip-based microchip was developed for quantification of HIV RNA using RT-LAMP and Poisson distribution.88 RNA molecules were first compartmentalized, then cDNA was produced through reverse transcription of RNA, which was amplified using LAMP for detection. This method increased the efficiency of the assay more than 10-fold (from 2% to ~23%) compared to one step RT-LAMP.
A LAMP-based digital microfluidic device was also developed for detecting amplified NAs.89 The chip structure was completely valve-free and contained four separate panels, which contained 1200 microchambers with 6 nL volume each. The chip consisted of four channels, enabling detection of four separate samples. The device was mainly fabricated from PDMS and was vacuum packaged. The pressure difference of the air dissolved in PDMS provides the driving force for the liquid and oil to be sucked into the channels and microwells. The fluorescent intensities of an array of various concentrations of DNA were detected using the Poisson analytical model. Droplet-based microfluidics is also a promising method in developing high-throughput LAMP-based detection assays for analyzing water-soluble samples in oil droplets. A continuous-flow digital LAMP-based microchip was developed using droplet-based microfluidics and microdispensers. Each droplet contains LAMP reagent as well as NA target. The droplets flow into the serpentine chamber for incubation. The structure of the serpentine chamber allows the droplets to flow side by side, while narrowing the channel enhances the droplets’ flow to the detection region in which fluorescent detection takes place via confocal fluorescent spectroscopy (Figure 9C).90 Using this system, a throughput of 1 million droplets in 110 min were obtained with LOD of 600 copies/μL.
4.3. Electricity-Free Cartridges
Electricity-free cartridges can be used in developing POC LAMP-based microchips for providing the temperature required for target amplification. One of the main methods to maintain consistent temperature for LAMP amplification is to create an exothermal reaction.91 Calcium oxide (CaO2) reacts exothermically with water and generates 63.7 kJ/mol.91,92 A high-capacity reaction chamber with a consistent temperature at a melting point of 65 °C were developed by employing an engineered fat-based compound or parafin. Liu et al.93 developed an electricity-free detection platform based on water-Mg/Fe metal alloy reaction, in which the flow rate was controlled using a filter paper. The amplicon was detected using a colorimetric fluorescent detection method with LOD of 10 copies in 25 μL sample. In a similar approach, an electricity-free cartridge was integrated with LFT for HIV detection with a sensitivity of 6 copies/μL.94 It was also shown that even a pocket hand warmer could be used for LAMP amplification.95 Zhang et al.96 used a pocket warmer and capillary glass for LAMP-based multiplexing target NAs. Various target DNAs and negative controls were injected at different positions of the channel, and the two sides of the channel were sealed with epoxy glue. The chip was then sandwiched between two hand-warmers for sample heating. This method had a LOD of 2 copies of standard plasmid per sample volume ranging from 100 nl to 2.5 μL. Figure 10 shows various electricity-free cartridges.
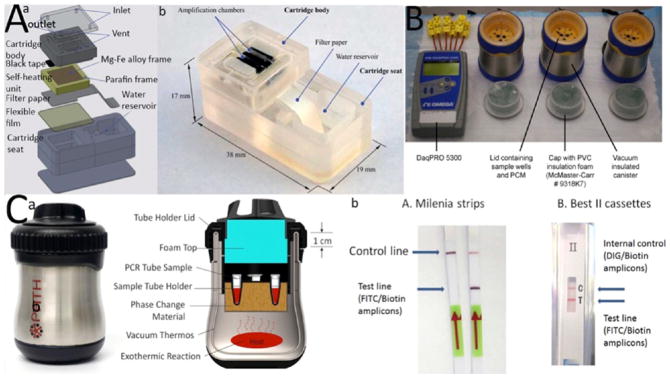
Figure 10.
Electricity-free cartridges for on-chip LAMP amplification. (A) (a) Exploded 3D schematic of the cartridge is demonstrated. (b) Actual image of a fabricated cartridge. (B) NINA electricity free cartridge that uses CaO for heat generation. (C) (a) NINA-PATH cartridge. (b) The cartridge was integrated with lateral flow test for detecting HIV amplicons. Reproduced with the permission from refs 91, 93, and 94. Copyright 2012 Public Library of Science, 2011 Royal Society of Chemistry, and 2014 Public Library of Science, respectively.
5. CONCLUSION AND FUTURE PERSPECTIVES
Isothermal amplification techniques are promising alternatives to the traditional gold standard, i.e., PCR. Among isothermal amplification methods, LAMP seems to be a promising and robust amplification method which has been used more than any other isothermal amplification technique for detecting pathogens such as viruses and bacteria in biological samples.97 The traditional analytical steps such as sample purification, NA extraction and detection can be integrated on a compact microfluidic-based platform to develop LAMP-based POC diagnostic assays.4b Optical- and electrochemical-based detection modalities are the most frequently used mechanisms in developing LAMP-based microchip platforms. However, novel detection techniques such as those based on giant magneto-resistance (GMR)98 and bioluminescence16a have also been developed for the detection and monitoring of LAMP-amplified biotargets. LAMP-LFT-based POC assays seem to have significant potential for commercialization due to its low cost and ease of use by nonskilled personnel. In addition, electricity-free cartridges based on exothermic chemical reactions can help amplify target genes in geographically remote areas and provide an accurate test without access to laboratories. An important consideration for the current LAMP-based POC platforms is the final cost of the product for applications in resource-limited settings. Paper-based microfluidics is an attractive tool for developing low-cost, mass-producible molecular diagnostic assays that can be used to design POC LAMP-based tests.24 LAMP-based products in the market or in the development pipeline are listed in Table 3 and categorized based on their detection mechanism, type of readers used in the system, cost per test and reader, and throughput.
Table 3.
LAMP-Based Products in the Market or Development Pipeline
| company name | product | reader’s cost ($) | cost per test ($) | detection mechanism | throughput analysis | ref |
|---|---|---|---|---|---|---|
| Eiken Chemicals | LA-320C | discontinued | discontinued | turbidimeter | 32 | 117 |
| Eiken Chemicals | LA-500 | 25000 | 3.3 | turbidimeter | 16 | 118 |
| CapitalBio | RTisochip-A | 15000 | 1.25 | fluorescent | 24 | 86 |
| Gene-Z | Gene-Z | 1000 | 2–10 for 24 tests | LED-optical fiber | 15 | 63 |
| Opti Gene | Genie II | 13000 | 4 | fluorescent | 16 | 119 |
| Opti Gene | Genie III | 17995 | 1.85 | fluorescent | 8 | 120 |
| QIAGEN | ESEQuant Tube Scanner | 16000 | 10 | fluorescent | 12 | 121 |
| Meridian Bioscience, Inc. | Meridian illumipro-10 | 7500 | 28–30 | fluorescent | 10 | 122 |
| Diagenetix, Inc. | Smart DART v.30 | 2490 | 10–15 | turbidity | 8 | 123 |
| Lucigen, Inc. | ClairLight | TBD | 1.5 | TBD | 1 | 99 |
| diagnostic for all | under development | 1.83 | fluorescent | 1 | 24 |
LAMP-based microchip strategies have the potential to be utilized in developing functional portable devices if upstream and downstream procedures are carefully optimized based on multidisciplinary approaches. However, as of today, LAMP-based methods still require vigilant optimization of loop primers for reproducible and sensitive target detection. Initial primer optimization could be shortened by rapid in silico or simulation approaches. In silico DNA amplification already exists for PCR-based methods. On the other hand, unlike PCR, LAMP-based in vitro DNA amplification relies on Bst Polymerase, which is produced, supplied, and licensed solely by New England Biolabs of the United States. Unless new universal enzymes for LAMP strategy are developed in the near future, this proprietary component may be a limiting factor for widespread use of LAMP-based microchip approaches. In addition, the primary components of this technique require the maintenance of the cold-chain, because the Bst Polymerase and dNTP cannot tolerate warm or even room temperatures. For example, Lucigen developed OmniAmp reverse transcriptase enzyme, which could be lyophilized with a storage life of over 6 months99 and can be used for the POC applications in resource-limited settings.100 Biotechnologists may work on recombinant microorganisms and improved nucleic acid synthesizers to develop more stable LAMP enzymes and other LAMP chemical components. New chemical processes and novel strategies may also be generated with nonenzymatic loop-mediated isothermal nucleic acid amplifications to be more practical and useful for resource-limited settings. An ideal point-of-care LAMP-based assay requires the integration of up- and downstream sample processes such as sample preparation and NA extraction and amplification with a sensitive and rapid sensing modality on a compact microchip for high throughput and multiplex detection of target pathogens in complex biological samples. Smart integration is the key to advances in LAMP-based self-contained devices.
Acknowledgments
The authors acknowledge the support received from the National Institute of Allergy and Infectious Disease (NIAID), National Institute of Health (NIH) through F32AI102590 and 1R01AI118502; Brigham and Women’s Hospital (BWH), Harvard Medical School (HMS), through the Bright Futures Prize and Fund to Sustain Research Excellence; Department of Medicine, Harvard Medical School, through the Innovation Evergreen Fund; Center for Nanotechnology at the King Abdolaziz University, Saudi Arabia; and the National Engineering Research Council of Canada (NSERC) through NSERC-CREATE fellowship, NSERC Postdoctoral fellowship and Programme de Bourse pour de courts séjours d’études universitaires á l’extérieur du Québec (PBCSE). The authors also extend their gratitude to Collin Preston for manuscript proofreading.
Footnotes
Author Contributions
All authors have made contributions by writing the manuscript.
The authors declare no competing financial interest.
References
- 1.(a) Arora A, Simone G, Salieb-Beugelaar GB, Kim JT, Manz A. Latest Developments in Micro Total Analysis Systems. Anal Chem. 2010;82:4830–4847. doi: 10.1021/ac100969k. [DOI] [PubMed] [Google Scholar]; (b) Kovarik ML, Ornoff DM, Melvin AT, Dobes NC, Wang Y, Dickinson AJ, Gach PC, Shah PK, Allbritton NL. Micro Total Analysis Systems: Fundamental Advances and Applications in the Laboratory, Clinic, and Field. Anal Chem. 2013;85(2):451–472. doi: 10.1021/ac3031543. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gauglitz G. Point-of-Care Platforms. Annu Rev Anal Chem. 2014;7:297–315. doi: 10.1146/annurev-anchem-071213-020332. [DOI] [PubMed] [Google Scholar]; (d) Auroux PA, Iossifidis D, Reyes DR, Manz A. Micro total analysis systems. 2. Analytical standard operations and applications. Anal Chem. 2002;74(12):2637–52. doi: 10.1021/ac020239t. [DOI] [PubMed] [Google Scholar]; (e) Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annu Rev Biomed Eng. 2008;10:107–44. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]; (f) Lui C, Cady NC, Batt CA. Nucleic Acid-based Detection of Bacterial Pathogens Using Integrated Microfluidic Platform Systems. Sensors. 2009;9(5):3713–3744. doi: 10.3390/s90503713. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 2.Gervais L, De Rooij N, Delamarche E. Microfluidic Chips for Point-of-Care Immunodiagnostics. Adv Mater. 2011;23(24):H151–H176. doi: 10.1002/adma.201100464. [DOI] [PubMed] [Google Scholar]
- 3.Easley CJ, Karlinsey JM, Bienvenue JM, Legendre LA, Roper MG, Feldman SH, Hughes MA, Hewlett EL, Merkel TJ, Ferrance JP, Landers JP. A fully integrated microfluidic genetic analysis system with sample-in–answer-out capability. Proc Natl Acad Sci U S A. 2006;103(51):19272–19277. doi: 10.1073/pnas.0604663103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Asiello PJ, Baeumner AJ. Miniaturized isothermal nucleic acid amplification, a review. Lab Chip. 2011;11:1420–1430. doi: 10.1039/c0lc00666a. [DOI] [PubMed] [Google Scholar]; (b) Chang CM, Chang WH, Wang CH, Wang JH, Mai JD, Lee GB. Nucleic acid amplification using microfluidic systems. Lab Chip. 2013;13:1225. doi: 10.1039/c3lc41097h. [DOI] [PubMed] [Google Scholar]; (c) Yan L, Zhou J, Zheng Y, Gamson AS, Roembke BT, Nakayama S, Sintim HO. Isothermal amplified detection of DNA and RNA. Mol BioSyst. 2014;10:970. doi: 10.1039/c3mb70304e. [DOI] [PubMed] [Google Scholar]
- 5.Monsur Ali M, Li F, Zhang Z, Zhang K, Kang D-K, Ankrum JA, Le XC, Zhao W. Rolling circle amplification: a versatile tool for chemical biology, materials science and medicine. Chem Soc Rev. 2014;43:3324–3341. doi: 10.1039/c3cs60439j. [DOI] [PubMed] [Google Scholar]
- 6.Shi C, Liu Q, Ma C, Zhong W. Exponential Strand-Displacement Amplification for Detection of MicroRNAs. Anal Chem. 2014;86(1):336–339. doi: 10.1021/ac4038043. [DOI] [PubMed] [Google Scholar]
- 7.Hall MJ, Wharam SD, Weston A, Cardy DL, Wilson WH. Use of signal-mediated amplification of RNA technology (SMART) to detect marine cyanophage DNA. BioTechniques. 2002;32(3):608–611. doi: 10.2144/02323rr02. [DOI] [PubMed] [Google Scholar]
- 8.Compton J. Nucleic acid sequence-based amplification. Nature. 1991;350(6313):91–92. doi: 10.1038/350091a0. [DOI] [PubMed] [Google Scholar]
- 9.Ma C, Han D, Deng M, Wang J, Shi C. Single primer-triggered isothermal amplification for double-stranded DNA detection. Chem Commun. 2015;51:553–556. doi: 10.1039/c4cc07845d. [DOI] [PubMed] [Google Scholar]
- 10.Vincent M, Xu Y, Kong H. Helicase-dependent isothermal DNA amplification. EMBO Rep. 2004;5(8):795–800. doi: 10.1038/sj.embor.7400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu G, Hu L, Zhong H, Wang H, Yusa S-i, Weiss TC, Romaniuk PJ, Pickerill S, You Q. Cross priming amplification: mechanism and optimization for isothermal DNA amplification. Sci Rep. 2012;2:246. doi: 10.1038/srep00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Chang CM, Chang WH, Wang CH, Wang JH, Mai JD, Lee GB. Nucleic acid amplification using microfluidic systems. Lab Chip. 2013;13:1225–1242. doi: 10.1039/c3lc41097h. [DOI] [PubMed] [Google Scholar]; (b) Craw P, Balachandran W. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: a critical review. Lab Chip. 2012;12:2469–2486. doi: 10.1039/c2lc40100b. [DOI] [PubMed] [Google Scholar]
- 13.(a) Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Notomi T, Mori Y, Tomita N, Kanda H. Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol. 2015;53(1):1–5. doi: 10.1007/s12275-015-4656-9. [DOI] [PubMed] [Google Scholar]; (c) Mori Y, Notomi T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother. 2009;15(2):62–69. doi: 10.1007/s10156-009-0669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seyrig G, Ahmad F, Stedtfeld RD, Tourlousse DM, Hashsham SA. Environmental Microbiology: Current Technology and Water Applications. Caister Academic; Cambridge, U.K: 2011. Simple, Powerful, and Smart: Using LAMP for Low-cost Screening of Multiple Waterborne Pathogens; pp. 103–125. [Google Scholar]
- 15.Abdul-Ghani R, Al-Mekhlafi AM, Karanis P. Loop-mediated isothermal amplification (LAMP) for malarial parasites of humans: Would it come to clinical reality as a point-of-care test? Acta Trop. 2012;122:233–240. doi: 10.1016/j.actatropica.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 16.(a) Kiddle G, Hardinge P, Buttigieg N, Gandelman O, Pereira C, McElgunn CJ, Rizzoli M, Jackson R, Appleton N, Moore C, Tisi LC, Murray JA. GMO detection using a bioluminescent real time reporter (BART) of loop mediated isothermal amplification (LAMP) suitable for field use. BMC Biotechnol. 2012;12:15. doi: 10.1186/1472-6750-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang L, Shi L, Alam MJ, Geng Y, Li L. Specific and rapid detection of foodborne Salmonella by loop-mediated isothermal amplification method. Food Res Int. 2008;41(1):69–74. [Google Scholar]; (c) Kaneko H, Kawana T, Fukushima E, Suzutani T. Tolerace of loop-mediated isothermal amplification to a culture medium and biological substances. J Biochem Biophys Methods. 2007;70(3):499–501. doi: 10.1016/j.jbbm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 17.(a) Tanner NA, Evans TC. Current Protocol in Molecular Biology. John Wiley & Sons; New York: 2014. Loop-Mediated Isothermal Amplification for Detection of Nucleic Acids; pp. 15.14.1–15.14.14. [DOI] [PubMed] [Google Scholar]; (b) Parida M, Sannarangaiah S, Dash PK, Rao PVL, Morita K. Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol. 2008;18(6):407–421. doi: 10.1002/rmv.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Lowe SB, Gooding JJ. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP) Biosens Bioelectron. 2014;61:491–499. doi: 10.1016/j.bios.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed MU, Hossain MM, Tamiya E. Electrochemical biosensors for medical and food applications. Electroanalysis. 2008;20(6):616–626. [Google Scholar]
- 20.Toumazou C, Shepherd LM, Reed SC, Chen GI, Patel A, Garner DM, Wang C-JA, Ou C-P, Amin-Desai K, Athanasiou P, Bai H, Brizido IMQ, Caldwell B, Coomber-Alford D, Georgiou P, Jordan KS, Joyce JC, La Mura M, Morley D, Sathyavruthan S, Temelso S, Thomas RE, Zhang L. Simultaneous DNA amplification and detection using a pH-sensing semiconductor system. Nat Methods. 2013;10:641–646. doi: 10.1038/nmeth.2520. [DOI] [PubMed] [Google Scholar]
- 21.Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc. 2008;3:877–882. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- 22.Tanner NA, Zhang Y, Evans TC. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes. Biotechniques. 2015;58(2):59–68. doi: 10.2144/000114253. [DOI] [PubMed] [Google Scholar]
- 23.Goto M, Honda E, Ogura A, Nomoto A, Hanaki KI. Colorimetric detection of loopmediated isothermal ampli!cation reaction by using hydroxy naphthol blue. BioTechniques. 2009;46:167–172. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- 24.Connelly JT, Rolland JP, Whitesides GM. Paper Machine” for Molecular Diagnostics. Anal Chem. 2015;87(15):7595–7601. doi: 10.1021/acs.analchem.5b00411. [DOI] [PubMed] [Google Scholar]
- 25.Dou M, Dominguez DC, Li X, Sanchez J, Scott G. A Versatile PDMS/Paper Hybrid Microfluidic Platform for Sensitive Infectious Disease Diagnosis. Anal Chem. 2014;86(15):7978–7986. doi: 10.1021/ac5021694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safavieh M, Ahmed MU, Sokullu E, Ng A, Braescu L, Zourob M. A simple cassette as point-of-care diagnostic device for naked-eye colorimetric bacteria detection. Analyst. 2014;139(2):482–487. doi: 10.1039/c3an01859h. [DOI] [PubMed] [Google Scholar]
- 27.Nahar S, Ahmed MU, Safavieh M, Rochette A, Toro C, Zourob M. A Flexible and Low-cost Polypropylene Pouch for Naked-eye Detection of Herpes Simplex Viruses. Analyst. 2015;140:931–937. doi: 10.1039/c4an01701c. [DOI] [PubMed] [Google Scholar]
- 28.Wu Q, Jin W, Zhou C, Han S, Yang W, Zhu Q, Jin Q, Mu Y. Integrated Glass Microdevice for Nucleic Acid Purification, Loop-Mediated Isothermal Amplification, and Online Detection. Anal Chem. 2011;83:3336–3342. doi: 10.1021/ac103129e. [DOI] [PubMed] [Google Scholar]
- 29.Fang X, Chen H, Yu S, Jiang X, Kong J. Predicting viruses accurately by a multiplex microfluidic loop-mediated isothermal amplification chip. Anal Chem. 2011;83(3):690–695. doi: 10.1021/ac102858j. [DOI] [PubMed] [Google Scholar]
- 30.(a) Odenthal KJ, Gooding JJ. An introduction to electrochemical DNA biosensors. Analyst. 2007;132:603–610. doi: 10.1039/b701816a. [DOI] [PubMed] [Google Scholar]; (b) Wang J. Electroanalytical Techniques in Clinical Chemistry and Laboratory Medicine. Wiley-VCH; New York, NY: 1988. [Google Scholar]; (c) Wang J. Analytical Electrochemistry. WILEY-VCH; New York, NY: 2006. [Google Scholar]; (d) Paleček E, Bartošík M. Electrochemistry of Nucleic Acids Chem Rev. 2012;112(6):3427–3481. doi: 10.1021/cr200303p. [DOI] [PubMed] [Google Scholar]; (e) Ronkainen NJ, Halsall HB, Heineman WR. Electrochemical biosensors. Chem Soc Rev. 2010;39:1747–1763. doi: 10.1039/b714449k. [DOI] [PubMed] [Google Scholar]
- 31.(a) Yang AHJ, Hsieh K, Patterson AS, Ferguson BS, Eisenstein M, Plaxco KW, Soh HT. Accurate Zygote-Specific Discrimination of Single-Nucleotide Polymorphisms Using Microfluidic Electrochemical DNA Melting Curves†. Angew Chem. 2014;126(12):3227–3231. doi: 10.1002/anie.201310059. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nakamura N, Ito K, Takahashi M, Hashimoto K, Kawamoto M, Yamanaka M, Taniguchi A, Kamatani N, Gemma N. Detection of Six Single-Nucleotide Polymorphisms Associated with Rheumatoid Arthritis by a Loop-Mediated Isothermal Amplification Method and an Electrochemical DNA Chip. Anal Chem. 2007;79(24):9484–9493. doi: 10.1021/ac0715468. [DOI] [PubMed] [Google Scholar]
- 32.(a) Hamidi-Asl E, Palchetti I, Hasheminejad E, Mascini M. A review on the electrochemical biosensors for determination of microRNAs. Talanta. 2013;115:74–83. doi: 10.1016/j.talanta.2013.03.061. [DOI] [PubMed] [Google Scholar]; (b) Drummond TG, Hill MG, Barton JK. Electrochemical DNA sensors. Nat Biotechnol. 2003;21:1192–1199. doi: 10.1038/nbt873. [DOI] [PubMed] [Google Scholar]
- 33.(a) Ahmed MU, Hasan Q, Hossain MM, Saito M, Tamiya E. Meat species identification based on the loop mediated isothermal amplification and electrochemical DNA sensor. Food Control. 2010;21(5):599–605. [Google Scholar]; (b) Ahmed MU, Nahar S, Safavieh M, Zourob M. Real-time electrochemical detection of pathogen DNA using electrostatic interaction of a redox probe. Analyst. 2013;138(3):907–915. doi: 10.1039/c2an36153a. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed MU, Saito M, Hossain MM, Rao SR, Furui S, Hino A, Takamura Y, Takagi M, Tamiya E. Electrochemical genosensor for the rapid detection of GMO using loop-mediated isothermal amplification. Analyst. 2009;134:966–972. doi: 10.1039/b812569d. [DOI] [PubMed] [Google Scholar]
- 35.Nagatani N, Saito KYM, Koketsu R, Sasaki T, Ikut K, Miyahara T, Tamiya E. Semi-real time electrochemical monitoring for influenza virus RNA by reverse transcription loop-mediated isothermal amplification using a USB powered portable potentiostat. Analyst. 2011;136:5143–5150. doi: 10.1039/c1an15638a. [DOI] [PubMed] [Google Scholar]
- 36.Sun W, Qin P, Gao H, Li G, Jiao K. Electrochemical DNA biosensor based on chitosan/nano-V2O5/MWCNTs composite film modified carbon ionic liquid electrode and its application to the LAMP product of Yersinia enterocolitica gene sequence. Biosens Bioelectron. 2010;25(16):1264–1270. doi: 10.1016/j.bios.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 37.(a) Zhang X, Liu W, Lu X, Gooding JJ, Li Q, Qu K. Monitoring the progression of loop-mediated isothermal amplification using conductivity. Anal Biochem. 2014;466:16–18. doi: 10.1016/j.ab.2014.08.002. [DOI] [PubMed] [Google Scholar]; (b) Zhang X, Li Q, Jin X, Jiang C, Lu Y, Tavallaie R, Gooding JJ. Quantitative determination of target gene with electrical sensor. Sci Rep. 2015;5(12539):12539. doi: 10.1038/srep12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards E, Li S, Chen N, Battig MR, Wang Y. Polymerization of Affinity Ligands on a Surface for Enhanced Ligand Display and Cell Binding. Biomacromolecules. 2014;15(12):4561–4569. doi: 10.1021/bm501347s. [DOI] [PubMed] [Google Scholar]
- 39.(a) Safavieh M, Ahmed MU, NGA, Zourob M. High-throughput real-time electrochemical monitoring of LAMP for pathogenic bacteria detection. Biosens Bioelectron. 2014;58:101–106. doi: 10.1016/j.bios.2014.02.002. [DOI] [PubMed] [Google Scholar]; (b) Safavieh M, Ahmed MU, Zourob M. High throughput low cost electrochemical device for S. aureus bacteria detection. IEEE Sensors. 2013:1–4. [Google Scholar]
- 40.Ahmed MU, Hossain MM, Safavieh M, Wong YL, Abd Rahman I, Zourob M, Tamiya E. Toward the development of smart and low cost point of care biosensors using screen printed electrodes. Crit Rev Biotechnol. 2015:1–11. doi: 10.3109/07388551.2014.992387. [DOI] [PubMed] [Google Scholar]
- 41.Safavieh M, Ahmed MU, Tolba M, Zourob M. Microfluidic Electrochemical Assay for Rapid Detection and Quantification of Escherichia Coli. Biosens Bioelectron. 2012;31(1):523–528. doi: 10.1016/j.bios.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 42.Tlili C, Sokullu E, Safavieh M, Tolba M, Ahmed MU, Zourob M. Bacteria Screening, Viability, And Confirmation Assays Using Bacteriophage-Impedimetric/Loop-Mediated Isothermal Amplification Dual-Response Biosensors. Anal Chem. 2013;85(10):4893–4901. doi: 10.1021/ac302699x. [DOI] [PubMed] [Google Scholar]
- 43.(a) Lam L, Sakakihara S, Ishizuka K, Takeuchi S, Arata HF, Fujita H, Noji H. Loop-mediated isothermal amplification of a single DNA molecule in polyacrylamide gel-based microchamber. Biomed Microdevices. 2008;10(4):539–546. doi: 10.1007/s10544-008-9163-x. [DOI] [PubMed] [Google Scholar]; (b) Johansson BG. Agarose Gel Electrophoresis. Scand J Clin Lab Invest. 1972;29(124):7–19. doi: 10.3109/00365517209102747. [DOI] [PubMed] [Google Scholar]; (c) Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244(16):4406–4412. [PubMed] [Google Scholar]
- 44.Sigmon J, Larcom L. The effect of ethidium bromide on mobility of DNA fragments in agarose gel electrophoresis. Electrophoresis. 1996;17(10):1524–1527. doi: 10.1002/elps.1150171003. [DOI] [PubMed] [Google Scholar]
- 45.Grossman PD, Colburn JC. Capillary Electrophoresis: Theory and Practice. Academic Press; San Diego, CA: 2012. [Google Scholar]
- 46.Almassian DR, Cockrell LM, Nelson WM. Portable nucleic acid thermocyclers. Chem Soc Rev. 2013;42:8769. doi: 10.1039/c3cs60144g. [DOI] [PubMed] [Google Scholar]
- 47.Hataoka Y, Zhang L, Mori Y, Tomita N, Notomi T, Baba Y. Analysis of specific gene by integration of isothermal amplification and electrophoresis on poly (methyl methacrylate) microchips. Anal Chem. 2004;76(13):3689–3693. doi: 10.1021/ac035032u. [DOI] [PubMed] [Google Scholar]
- 48.Lam L, Sakakihara S, Ishizuka K, Takeuchi S, Arata HF, Fujita H, Noji H. Loop-mediated isothermal amplification of a single DNA molecule in polyacrylamide gel-based microchamber. Biomed Microdevices. 2008;10(4):539–546. doi: 10.1007/s10544-008-9163-x. [DOI] [PubMed] [Google Scholar]
- 49.Borysiak MD, Kimura KW, Posner JD. NAIL: Nucleic Acid detection using Isotachophoresis and Loop-mediated isothermal amplification. Lab Chip. 2015;15:1697–1707. doi: 10.1039/c4lc01479k. [DOI] [PubMed] [Google Scholar]
- 50.(a) Jaroenram W, Kiatpathomchai W, Flegel TW. Rapid and sensitive detection of white spot syndrome virus by loop-mediated isothermal amplification combined with a lateral flow dipstick. Mol Cell Probes. 2009;23(2):65–70. doi: 10.1016/j.mcp.2008.12.003. [DOI] [PubMed] [Google Scholar]; (b) Arunrut N, Prombun P, Saksmerprome V, Flegel TW, Kiatpathomchai W. Rapid and sensitive detection of infectious hypodermal and hematopoietic necrosis virus by loop-mediated isothermal amplification combined with a lateral flow dipstick. J Virol Methods. 2011;171(1):21–25. doi: 10.1016/j.jviromet.2010.09.022. [DOI] [PubMed] [Google Scholar]; (c) Patel JS, Brennan MS, Khan A, Ali GS. Implementation of loop-mediated isothermal amplification methods in lateral flow devices for the detection of Rhizoctonia solani. Can J Plant Pathol. 2015;37(1):118–129. [Google Scholar]; (d) Kaewphinit T, Arunrut N, Kiatpathomchai W, Santiwatanakul S, Jaratsing P, Chansiri K. Detection of Mycobacterium tuberculosis by using loop-mediated isothermal amplification combined with a lateral flow dipstick in clinical samples. BioMed Res Int. 2013;2013:926230. doi: 10.1155/2013/926230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Teng D, Guan Q, Tian F, Wang J. Detection of Roundup Ready soybean by loop-mediated isothermal amplification combined with a lateral-flow dipstick. Food Control. 2013;29:213–220. [Google Scholar]
- 52.Roskos K, Hickerson AI, Lu HW, Ferguson TM, Shinde DN, Klaue Y, Niemz A. Simple System for Isothermal DNA Amplification Coupled to Lateral Flow Detection. PLoS One. 2013;8:e69355. doi: 10.1371/journal.pone.0069355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diribe O, North S, Sawyer J, Roberts L, Fitzpatrick N, La Ragione R. Design and application of a loop-mediated isothermal amplification assay for the rapid detection of Staphylococcus pseudintermedius. J Vet Diagn Invest. 2014;26(1):42–48. doi: 10.1177/1040638713516758. [DOI] [PubMed] [Google Scholar]
- 54.Jung JH, Park BH, Oh SJ, Choi G, Seo TS. Integration of reverse transcriptase loop-mediated isothermal amplification with an immunochromatographic strip on a centrifugal microdevice for influenza A virus identification. Lab Chip. 2015;15:718–725. doi: 10.1039/c4lc01033g. [DOI] [PubMed] [Google Scholar]
- 55.Lee MF, Chen YH, Peng CF. Evaluation of reverse transcription loop-mediated isothermal amplification in conjunction with ELISA–hybridization assay for molecular detection of Myco-bacterium tuberculosis. J Microbiol Methods. 2009;76(2):174–180. doi: 10.1016/j.mimet.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Ravan H, Yazdanparast R. Development and evaluation of a loop-mediated isothermal amplification method in conjunction with an enzyme-linked immunosorbent assay for specific detection of Salmonella serogroup D. Anal Chim Acta. 2012;733:64–70. doi: 10.1016/j.aca.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 57.Hua X, Yin W, Shi H, Li M, Wang Y, Wang H, Ye Y, Kim HJ, Gee SJ, Wang M, Liu F, Hammock BD. Development of Phage Immuno-Loop-Mediated Isothermal Amplification Assays for Organophosphorus Pesticides in Agro-products. Anal Chem. 2014;86(16):8441–8447. doi: 10.1021/ac5020657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee SY, Huang JG, Chuang TL, Sheu JC, Chuang YK, Holl M, Meldrum DR, Lee CN, Lin CW. Compact optical diagnostic device for isothermal nucleic acids amplification. Sens Actuators, B. 2008;133(2):493–501. doi: 10.1016/j.snb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang X, Liu Y, Kong J, Jiang X. Loop-mediated isothermal amplification integrated on microfluidic chips for point-of-care quantitative detection of pathogens. Anal Chem. 2010;82(7):3002–3006. doi: 10.1021/ac1000652. [DOI] [PubMed] [Google Scholar]
- 60.Chuang TL, Wei SC, Lee SY, Lin CW. A polycarbonate based surface plasmon resonance sensing cartridge for high sensitivity HBV loop-mediated isothermal amplification. Biosens Bioelectron. 2012;32(1):89–95. doi: 10.1016/j.bios.2011.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang CH, Lien KY, Wu JJ, Lee GB. A magnetic bead-based assay for the rapid detection of methicillin-resistant Staphylococcus aureus by using a microfluidic system with integrated loop-mediated isothermal amplification. Lab Chip. 2011;11:1521–1531. doi: 10.1039/c0lc00430h. [DOI] [PubMed] [Google Scholar]
- 62.Ahmad F, Seyrig G, Tourlousse DM, Stedtfeld RD, Tiedje JM, Hashsham SA. A CCD-based fluorescence imaging system for real-time loop-mediated isothermal amplification-based rapid and sensitive detection of waterborne pathogens on microchips. Biomed Microdevices. 2011;13(5):929–937. doi: 10.1007/s10544-011-9562-2. [DOI] [PubMed] [Google Scholar]
- 63.Stedtfeld RD, Tourlousse DM, Seyrig G, Stedtfeld TM, Kronlein M, Price S, Ahmad F, Gulari E, Tiedje JM, Hashsham SA. Gene-Z: a device for point of care genetic testing using a smartphone. Lab Chip. 2012;12:1454–1462. doi: 10.1039/c2lc21226a. [DOI] [PubMed] [Google Scholar]
- 64.Liu C, Geva E, Mauk M, Qiu X, Abrams WR, Malamud D, Curtis K, Owen SM, Bau HH. An isothermal amplification reactor with an integrated isolation membrane for point-of-care detection of infectious diseases. Analyst. 2011;136:2069–2076. doi: 10.1039/c1an00007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang WH, Yang SY, Wang CH, Tsai MA, Wang PC, Chen TY, Chen SC, Lee GB. Rapid isolation and detection of aquaculture pathogens in an integrated microfluidic system using loop-mediated isothermal amplification. Sens Actuators, B. 2013;180:96–106. [Google Scholar]
- 66.Liu C, Mauk MG, Hart R, Bonizzoni M, Yan G, Bau HH. A low-cost microfluidic chip for rapid genotyping of malaria-transmitting mosquitoes. PLoS One. 2012;7(8):e42222. doi: 10.1371/journal.pone.0042222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu C, Sadik MM, Mauk MG, Edelstein PH, Bushman FD, Gross R, Bau HH. Nuclemeter: A Reaction-Diffusion Based Method for Quantifying Nucleic Acids Undergoing Enzymatic Amplification. Sci Rep. 2014;4:7335. doi: 10.1038/srep07335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang K, Wu S, Kwok H, Ho H, Kong S. Using Loop-Mediated Isothermal DNA Amplification (LAMP) and Spectral Surface Plasmon Resonance (SPR) to Detect Methicillin-resistance S. aureus (MRSA). Proceedings of the 2012 International Conference on Biomedical Engineering and Biotechnology; Macau, Macao. May 28–30: 2012; Piscataway, NJ: IEEE; 2012. pp. 647–650. [Google Scholar]
- 69.Chiu N-F, Huang T-Y, Kuo C-C, Lee W-C, Hsieh M-H, Lai H-C. Single-layer graphene based SPR biochips for tuberculosis bacillus detection. Proc SPIE. 2012;8427(8427):84273M. [Google Scholar]
- 70.(a) Hsieh K, Patterson AS, Ferguson BS, Plaxco KW, Soh HT. Rapid, Sensitive, and Quantitative Detection of Pathogenic DNA at the Point of Care through Microfluidic Electrochemical Quantitative Loop-Mediated Isothermal Amplification. Angew Chem. 2012;124(20):4980–4984. doi: 10.1002/anie.201109115. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Patterson AS, Hsieh K, Soh HT, Plaxco KW. Electrochemical real-time nucleic acid amplification: towards point-of-care quantification of pathogens. Trends Biotechnol. 2013;31(12):704–712. doi: 10.1016/j.tibtech.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Luo J, Fang X, Ye D, Li H, Chen H, Zhang S, Kong J. A real-time microfluidic multiplex electrochemical loop-mediated isothermal amplification chip for differentiating bacteria. Biosens Bioelectron. 2014;60:84–91. doi: 10.1016/j.bios.2014.03.073. [DOI] [PubMed] [Google Scholar]
- 72.(a) Grieshaber D, MacKenzie R, Voros J, Reimhult E. Electrochemical Biosensors - Sensor Principles and Architectures. Sensors. 2008;8:1400–1458. doi: 10.3390/s80314000. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Váradi MBJ, Pungor E. Comparison of Electrochemical Detectors with Non-Electrochemical Detetors in Chromatophraphy. Pure Appl Chem. 1979;51:1175–1182. [Google Scholar]
- 73.Zhang F, Wu J, Wang R, Wang L, Ying Y. Portable pH-inspired electrochemical detection of DNA amplification. Chem Commun. 2014;50:8416–8419. doi: 10.1039/c4cc03011g. [DOI] [PubMed] [Google Scholar]
- 74.Xie S, Yuan Y, Song Y, Zhuo Y, Li T, Chai Y, Yuan R. Using the ubiquitous pH meter combined with a loop mediated isothermal amplification method for facile and sensitive detection of Nosema bombycis genomic DNA PTP1. Chem Commun. 2014;50:15932–15935. doi: 10.1039/c4cc06449f. [DOI] [PubMed] [Google Scholar]
- 75.Kalofonou M, Toumazou C. Semiconductor technology for early detection of DNA methylation for cancer: From concept to practice. Sens Actuators, B. 2013;178:572–580. [Google Scholar]
- 76.Veigas B, Branquinho R, Pinto JV, Wojcik PJ, Martins R, Fortunato E, Baptista PV. Ion sensing (EIS) real-time quantitative monitorization of isothermal DNA amplification. Biosens Bioelectron. 2014;52:50–55. doi: 10.1016/j.bios.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 77.Salm E, Zhong Y, Reddy B, Duarte-Guevara C, Swaminathan V, Liu YS, Bashir R. Electrical Detection of Nucleic Acid Amplification Using an On-Chip Quasi-Reference Electrode and a PVC REFET. Anal Chem. 2014;86(14):6968–6975. doi: 10.1021/ac500897t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rafati A, Gill P. Microfluidic method for rapid turbidimetric detection of the DNA of Mycobacterium tuberculosis using loop-mediated isothermal amplification in capillary tubes. Microchim Acta. 2015;182(3):523–530. [Google Scholar]
- 79.Liu D, Liang G, Zhang Q, Chen B. Detection of Mycobacterium tuberculosis Using a Capillary-Array Microsystem with Integrated DNA Extraction, Loop-Mediated Isothermal Amplification, and Fluorescence Detection. Anal Chem. 2013;85(9):4698–4704. doi: 10.1021/ac400412m. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L, Zhang Y, Wang C, Feng Q, Fan F, Zhang G, Kang X, Qin X, Sun J, Li Y, Jiang X. Integrated Microcapillary for Sample-to-Answer Nucleic Acid Pretreatment, Amplification, and Detection. Anal Chem. 2014;86(20):10461–10466. doi: 10.1021/ac503072a. [DOI] [PubMed] [Google Scholar]
- 81.Lu W, Wang J, Wu Q, Sun J, Chen Y, Zhang L, Zheng C, Gao W, Liu Y, Jiang X. High-throughput sample-to-answer detection of DNA/RNA in crude samples within functionalized micro-pipette tips. Biosens Bioelectron. 2016;75:28–33. doi: 10.1016/j.bios.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 82.(a) Iseki H, Alhassan A, Ohta N, Thekisoe OMM, Yokoyama N, Inoue N, Nambota A, Yasuda J, Igarashi I. Development of a multiplex loop-mediated isothermal amplification (mLAMP) method for the simultaneous detection of bovine Babesia parasites. J Microbiol Methods. 2007;71:281–287. doi: 10.1016/j.mimet.2007.09.019. [DOI] [PubMed] [Google Scholar]; (b) Nurul Najian AB, Engku Nur Syafirah EAR, Ismail N, Mohamed M, Yean CY. Development of multiplex loop mediated isothermal amplification (m-LAMP) label-based gold nanoparticles lateral flow dipstick biosensor for detection of pathogenic Leptospira. Anal Chim Acta. 2015;903:142. doi: 10.1016/j.aca.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Liang C, Chu Y, Cheng S, Wu H, Kajiyama T, Kambara H, Zhou G. Multiplex Loop-Mediated Isothermal Amplification Detection by Sequence-Based Barcodes Coupled with Nicking Endonuclease-Mediated Pyrosequencing. Anal Chem. 2012;84(8):3758–3763. doi: 10.1021/ac3003825. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y, Wang Y, Lan R, Xu H, Ma A, Li D, Dai H, Yuan X, Xu J, Ye C. Multiple Endonuclease Restriction Real-Time Loop-Mediated Isothermal Amplification: A Novel Analytically Rapid, Sensitive, Multiplex Loop-Mediated Isothermal Amplification Detection Technique. J Mol Diagn. 2015;17(4):392–401. doi: 10.1016/j.jmoldx.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 85.(a) Safavieh M, Nahar S, Zourob M, Ahmed MU. Microfluidic biosensors for high-throughput screening of pathogens in food. In: Bhunia AK, Kim MS, Taitt CR, editors. High Throughput Screening for Food Safety Assessment: Biosensor Technologies, Hyperspectral Imaging and Practical Applications. Woodhead Publishing; Cambridge, U.K: 2015. pp. 327–357. [Google Scholar]; (b) Gehring AG, Tu SI. High-Throughput Biosensors for Multiplexed Food-Borne Pathogen Detection. Annu Rev Anal Chem. 2011;4:151–172. doi: 10.1146/annurev-anchem-061010-114010. [DOI] [PubMed] [Google Scholar]; (c) Sharma H, Mutharasan R. Review of biosensors for foodborne pathogens and toxins. Sens Actuators, B. 2013;183:535–549. [Google Scholar]
- 86.Zhou QJ, Wang L, Chen J, Wang RN, Shi YH, Li CH, Zhang DM, Yan XJ, Zhang YJ. Development and evaluation of a real-time fluorogenic loop-mediated isothermal amplification assay integrated on a microfluidic disc chip (on-chip LAMP) for rapid and simultaneous detection of ten pathogenic bacteria in aquatic animals. J Microbiol Methods. 2014;104:26–35. doi: 10.1016/j.mimet.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Du W, Li L, Nichols KP, Ismagilov RF. SlipChip. Lab Chip. 2009;9:2286–2292. doi: 10.1039/b908978k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun B, Shen F, McCalla SE, Kreutz JE, Karymov MA, Ismagilov RF. Mechanistic Evaluation of the Pros and Cons of Digital RT-LAMP for HIV-1 Viral Load Quantification on a Microfluidic Device and Improved Efficiency via a Two-Step Digital Protocol. Anal Chem. 2013;85(3):1540–1546. doi: 10.1021/ac3037206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu Q, Gao Y, Yu B, Ren H, Qiu L, Han S, Jin W, Jin Q, Mu Y. Self-priming compartmentalization digital LAMP for point-of-care. Lab Chip. 2012;12:4755–4763. doi: 10.1039/c2lc40774d. [DOI] [PubMed] [Google Scholar]
- 90.Rane TD, Chen L, Zec HC, Wang TH. Microfluidic continuous flow digital loop-mediated isothermal amplification (LAMP) Lab Chip. 2015;15:776–782. doi: 10.1039/c4lc01158a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Curtis KA, Rudolph DL, Nejad I, Singleton J, Beddoe A, Weigl B, LaBarre P, Owen SM. Isothermal Amplification Using a Chemical Heating Device for Point-of-Care Detection of HIV-1. PLoS One. 2012;7(2):e31432. doi: 10.1371/journal.pone.0031432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.(a) Weigl B, Domingo G, LaBarre P, Gerlach J. Towards non-and minimally instrumented, microfluidics-based diagnostic devices. Lab Chip. 2008;8:1999–2014. doi: 10.1039/b811314a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) LaBarre P, Boyle D, Hawkins K, Weigl B. Instrument-free nucleic acid amplification assays for global health settings. Proc SPIE. 2011:8029. doi: 10.1117/12.882868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu C, Mauk MG, Hart R, Qiu X, Bau HH. A self-heating cartridge for molecular diagnostics. Lab Chip. 2011;11:2686–2692. doi: 10.1039/c1lc20345b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singleton J, Osborn JL, Lillis L, Hawkins K, Guelig D, Price W, Johns R, Ebels K, Boyle D, Weigl B, LaBarre P. Electricity-Free Amplification and Detection for Molecular Point-of-Care Diagnosis of HIV-1. PLoS One. 2014;9(11):e113693. doi: 10.1371/journal.pone.0113693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.(a) Hatano B, Maki T, Obara T, Fukumoto H, Hagisawa K, Matsushita Y, Okutani A, Bazartseren B, Inoue S, Sata T, Katano H. LAMP using a disposable pocket warmer for anthrax detection, a highly mobile and reliable method for anti-bioterrorism. J Infect Dis. 2010;63:36–40. [PubMed] [Google Scholar]; (b) Hatano B, Fujii T, Imakiire T, Kikuchi Y, Takahashi R, Kanai M, Tamura K, Izumi T, Takahashi Y, Iwamoto Y, Mimura S, Goto M, Mukai Y, Takita K, Takeo H, Kitamura R, Shimizu E, Fukushima K, Hakozaki Y, Uehata A, Sakai M, Ohshima S, Fukumoto H, Shirotani T, Oba K, Hasegawa H, Sata T, Katano H, Obara T, Maki T, Suzuki G, Yamamoto T, Hagisawa K, Matsushita Y. Mobile and accurate detection system for infection by the 2009 pandemic influenza A H1N1 virus with a pocket-warmer reverse-transcriptase loop-mediated isothermal amplification. J Med Virol. 2011;83(4):568–573. doi: 10.1002/jmv.22031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y, Zhang L, Sun J, Liu Y, Ma X, Cui S, Ma L, Xi JJ, Jiang X. Point-of-Care Multiplexed Assays of Nucleic Acids Using Microcapillary-based Loop-Mediated Isothermal Amplification. Anal Chem. 2014;86(14):7057–7062. doi: 10.1021/ac5014332. [DOI] [PubMed] [Google Scholar]
- 97.Foudeh AM, Fatanat Didar T, Veres T, Tabrizian M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip. 2012;12(18):3249–3266. doi: 10.1039/c2lc40630f. [DOI] [PubMed] [Google Scholar]
- 98.Zhi X, Deng M, Yang H, Gao G, Wang K, Fu H, Zhang Y, Chen D, Cui D. A novel HBV genotypes detecting system combined with microfluidic chip, loop-mediated isothermal amplification and GMR sensors. Biosens Bioelectron. 2014;54:372–377. doi: 10.1016/j.bios.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 99.Lucigen OmniAmp RNA & DNA LAMP Kit. http://www.lucigen.com/OmniAmp-RNA-and-DNA-LAMP-Kit/-subcat-tabs3.
- 100.(a) Chander Y, Koelbl J, Puckett J, Moser MJ, Klingele AJ, Liles MR, Carrias A, Mead DA, Schoenfeld TW. A novel thermostable polymerase for RNA and DNA loop-mediated isothermal amplification (LAMP) Front Microbiol. 2014;5(395) doi: 10.3389/fmicb.2014.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Schoenfield T. Metagenomic discovery and engineering of thermostable viral DNA polymerase for detection and analysis of RNA and DNA targets. Point of Care Diagnostics and Global Health World Congress; SELECTBio; San Diego, CA. 2015. [Google Scholar]
- 101.Parida MM, Santhosh SR, Dash PK, Lakshmana Rao PV. Rapid and Real-time Assays for Detection and Quantification of Chikungunya Virus. Future Virol. 2008;3(2):179–192. [Google Scholar]
- 102.Carnevale ML, Roche PJR, Najih M, Paliouras M, Beitel LK. Trifiro MAA rapid diagnostic method for E. coli serogroups responsible for gastro-intestinal diseases using loop-mediated isothermal amplification. Anal Methods. 2015;7:287–295. [Google Scholar]
- 103.Hill J, Beriwal IS, Chandra s, Paul VK, Kapil A, Singh T, Wadowsky RM, Singh V, Goyal A, Jahnukainen T, Johnson JR, Tarr PI, Vats A. Loop-Mediated Isothermal Amplification Assay for Rapid Detection of Common Strains of Escherichia coli. J Clin Microbiol. 2008;46(8):2800–2804. doi: 10.1128/JCM.00152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Njiru ZK, Mikosza ASJ, Armstrong T, Enyaru JC, Ndung’u JM, Thompson ARC. Loop-Mediated Isothermal Amplification (LAMP) Method for Rapid Detection of Trypanosoma brucei rhodesiense. PLoS Neglected Trop Dis. 2008;2(2):e147. doi: 10.1371/journal.pntd.0000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hara-Kudo Y, Yoshino M, Kojima T, Ikedo M. Loop-mediated isothermal amplification for the rapid detection of Salmonella. FEMS Microbiol Lett. 2005;253:155–161. doi: 10.1016/j.femsle.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 106.Almasi MA, Hosseini Dehabadi SM, Moradi A, Eftekhari Z, Aghapour Ojaghkandi M, Aghaei S. Development Application of Loop-Mediated Isothermal Amplification Assay for Rapid Detection of Fusarium Oxysporum fSp. lycopersici. J Plant Pathol Microb. 2013;4(5):1–7. [Google Scholar]
- 107.Qiao Y-M, Guo Y-C, Zhang X-E, Zhou Y-F, Zhang Z-P, Wei H-P, Yang R-F, Wang D-B. Loop-mediated isothermal amplification for rapid detection of Bacillus anthracis spores. Biotechnol Lett. 2007;29:1939–1946. doi: 10.1007/s10529-007-9472-9. [DOI] [PubMed] [Google Scholar]
- 108.Wong JKF, Yip SP, Lee TMH. Ultrasensitive and Closed-Tube Colorimetric Loop-Mediated Isothermal Amplification Assay Using Carboxyl-Modified Gold Nanoparticles. Small. 2014;10(8):1495–1499. doi: 10.1002/smll.201302348. [DOI] [PubMed] [Google Scholar]
- 109.Zhou C, Mu Y, Yang M-c, Wu Q-q, Xu W, Zhang Y, Jin W, Song Q, Wu Z-y, Jin Q-h. Gold nanoparticles based colorimetric detection of target DNA after loop-mediated isothermal amplification. Chem Res Chin Univ. 2013;29(3):424–428. [Google Scholar]
- 110.Nikbakht H, Gill P, Tabarraei A, Niazi A. Nanomolecular detection of human influenza virus type A using reverse transcription loop-mediated isothermal amplification assisted with rod-shaped gold nanoparticles. RSC Adv. 2014;4:13575–13580. [Google Scholar]
- 111.Maruyama F, Kenzaka T, Yamaguchi N, Tani K, Nasu M. Detection of Bacteria Carrying the stx2 Gene by In Situ Loop-Mediated Isothermal Amplification. Appl Environ Microbiol. 2003;69(8):5023–5028. doi: 10.1128/AEM.69.8.5023-5028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miyamoto S, Sano S, Takahashi K, Jikihara T. Method for colorimetric detection of double-stranded nucleic acid using leuco triphenylmethane dyes. Anal Biochem. 2015;473:28–33. doi: 10.1016/j.ab.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 113.Kobayashi M, Kusakawa T, Saito M, Kaji S, Oomura M, Iwabuchi S, Morita Y, Hasan Q, Tamiya E. Electrochemical DNA quantification based on aggregation induced by Hoechst 33258. Electrochem Commun. 2004;6(4):337–343. [Google Scholar]
- 114.Wang J, Ozsoz M, Cai X, Rivas G, Shiraishi H, Grant DH, Chicharro M, Fernandes J, Paleček E. Interactions of antitumor drug daunomycin with DNA in solution and at the surface. Bioelectrochem Bioenerg. 1998;45(1):33–40. [Google Scholar]
- 115.Zhang X, Qu K, Li Q, Cui Z, Zhao J, Sun X. Recording the Reaction Process of Loop-Mediated Isothermal Amplification (LAMP) by Monitoring the Voltammetric Response of 22-Deoxyguanosine 52-Triphosphate. Electroanalysis. 2011;23(10):2438–2445. [Google Scholar]
- 116.Xie S, Chai Y, Yuan Y, Bai L, Yuan R. Development of an electrochemical method for Ochratoxin A detection based on aptamer and loop-mediated isothermal amplification. Biosens Bioelectron. 2014;55:324–329. doi: 10.1016/j.bios.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 117.Chemicals E. SAS Loopamp Realtime Turbidimeter LA-320C. http://www.uib.no/med/avd/ii/nyhetsbrev/2010/15/Turbidimeter.pdf.
- 118.Eiken Chemicals, LA-500. http://loopamp.eiken.co.jp/e/products/la500/img/02.pdf.
- 119.Optigene Genie II. http://www.optigene.co.uk/instruments/instrument-genie-ii/
- 120.Optigene Genie III. http://www.optigene.co.uk/instruments/instrument-genie-iii/
- 121.QIAGEN. ESEQuant Tube Scanner. http://www.qiagen.com/resources/download.aspx?id=3bf98195-8fe6-4b11-aadf-b2499a920461&lang=en.
- 122.meridianbioscience. Meridian illumipro-10. http://www.meridianbioscience.com/Content/Assets/Files/2.1C.difficileProducts/illumigeneC.difficile/illumigenetechnologypage/illumigene_product_folder.pdf.
- 123.(a) SMART. Smart-DART 8-Well V.3.0. http://diagenetix.com/smart-dart-platform/; (b) Keremane ML, Ramadugu C, Rodriguez E, Kubota R, Shibata S, Hall DG, Roose ML, Jenkins D, Lee RF. A rapid field detection system for citrus huanglongbing associated ‘Candidatus Liberibacter asiaticus’ from the psyllid vector, Diaphorina citri Kuwayama and its implications in disease management. Crop Prot. 2015;68:41–48. [Google Scholar]