Abstract
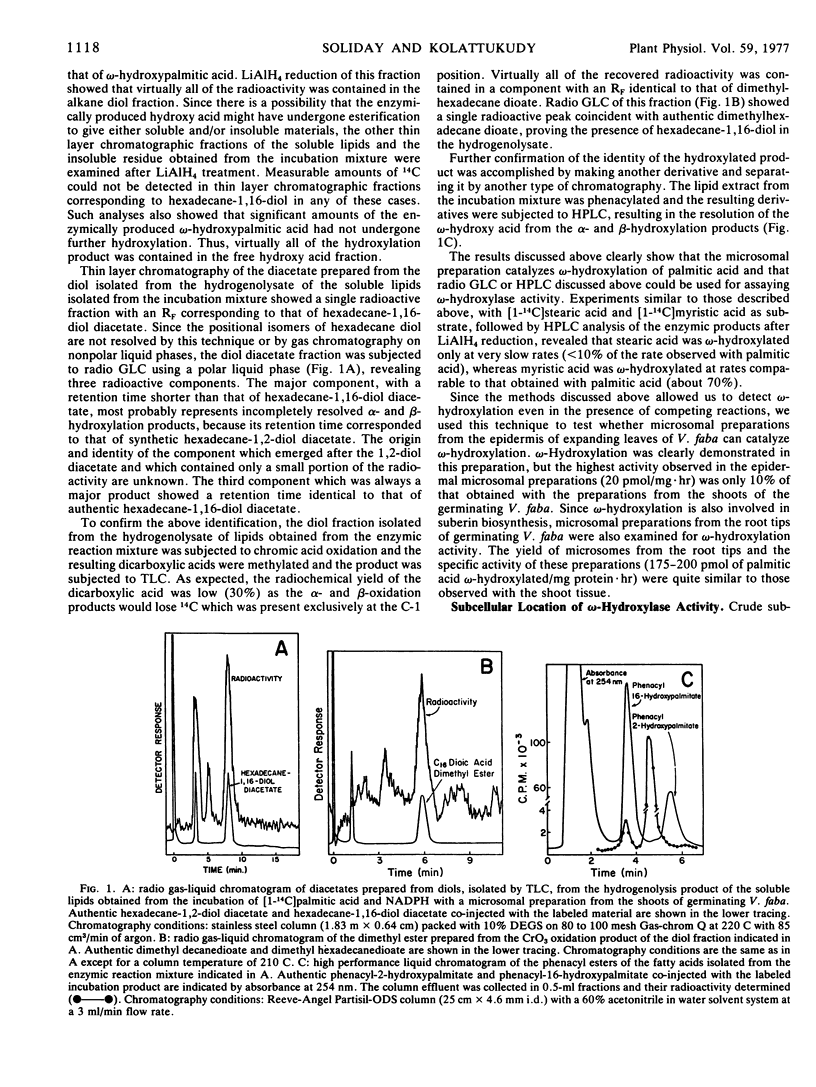
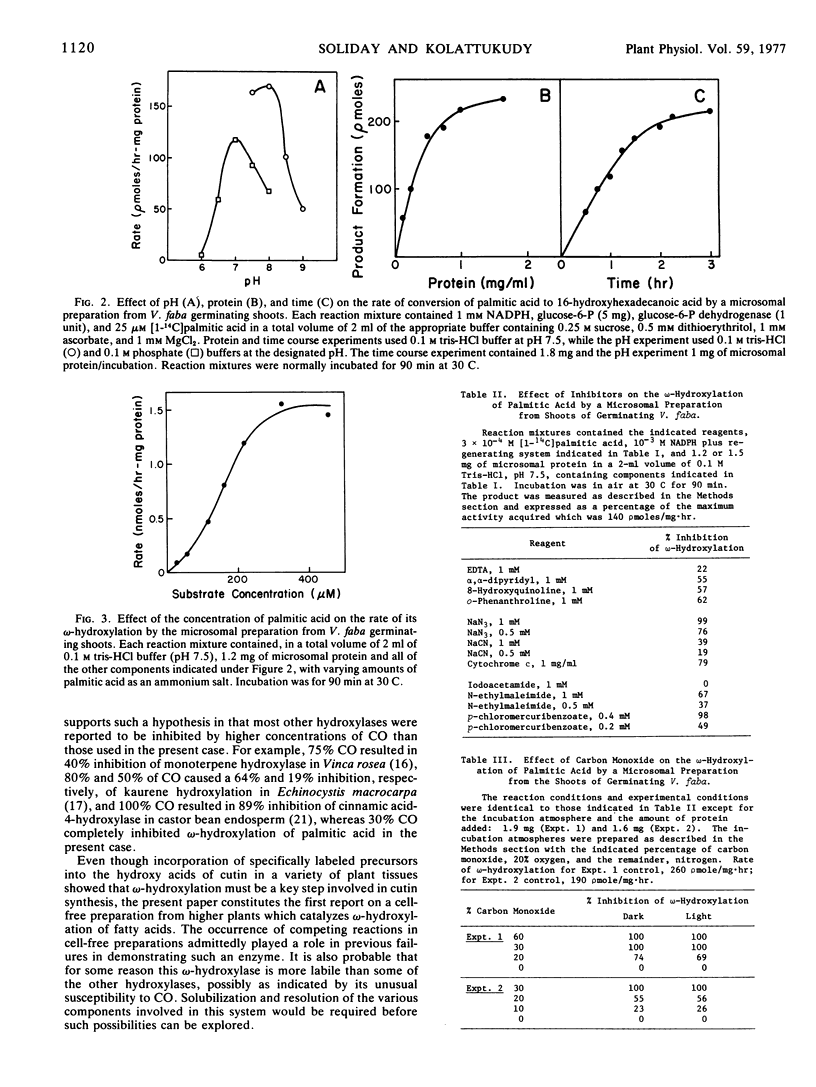
ω-Hydroxylation of fatty acids, which is a key reaction in the biosynthesis of cutin and suberin, has been demonstrated for the first time in a cell-free preparation from a higher plant. A crude microsomal fraction (105,000g pellet) from germinating embryonic shoots of Vicia faba catalyzed the conversion of palmitic acid to ω-hydroxypalmitic acid. As the crude cell-free preparation also catalyzes the formation of other hydroxy acids such as α- and β-hydroxy acids, the ω-hydroxylation product was identified by gas chromatography on a polyester column and reverse phase, high performance liquid chromatography, two techniques which were shown to resolve the positional isomers. Gas chromatographic analysis of the dicarboxylic acid obtained by CrO3 oxidation of the enzymic product also confirmed the identity of the enzymic ω-hydroxylation product. This enzymic hydroxylation required O2 and NADPH, but substitution of NADH resulted in nearly half the reaction rate obtained with NADPH. Maximal rates of ω-hydroxylation occurred at pH 8 and the rate increased in a sigmoidal manner with increasing concentrations of palmitic acid. This ω-hydroxylation was inhibited by the classical mixed function oxidase inhibitors such as metal chelators (o-phenanthroline, 8-hydroxyquinoline, and α,α-dipyridyl), NaN3 and thiol reagents (N-ethylmaleimide and p-chloromercuribenzoate). As expected of a hydroxylase, involving cytochrome P450, the present ω-hydroxylase was inhibited by CO and this enzyme system showed unusually high sensitivity to this inhibition; 10% CO caused inhibition and 30% CO completely inhibited the reaction. Another unusual feature was that the inhibition caused by any level of CO could not be reversed by light (420-460 nm).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borch R. F. Separation of long chain fatty acids as phenacyl esters by high pressure liquid chromatography. Anal Chem. 1975 Dec;47(14):2437–2439. doi: 10.1021/ac60364a037. [DOI] [PubMed] [Google Scholar]
- Croteau R., Kolattukudy P. E. Biosynthesis of hydroxyfatty acid polymers. Enzymatic epoxidation of 18-hydroxyoleic acid to 18-hydroxy-cis-9,10-epoxystearic acid by a particulate preparation from spinach (Spinacia oleracea). Arch Biochem Biophys. 1975 Sep;170(1):61–72. doi: 10.1016/0003-9861(75)90097-1. [DOI] [PubMed] [Google Scholar]
- Croteau R., Kolattukudy P. E. Biosynthesis of hydroxyfatty acid polymers. Enzymatic hydration of 18-hydroxy-cis-9,10-epoxystearic acid to threo 9,10,18-trihydroxystearic acid by a particulate preparation from apple (Malus pumila). Arch Biochem Biophys. 1975 Sep;170(1):73–81. doi: 10.1016/0003-9861(75)90098-3. [DOI] [PubMed] [Google Scholar]
- Croteau R., Kolattukudy P. E. Biosynthesis of hydroxyfatty acid polymers. Enzymatic synthesis of cutin from monomer acids by cell-free preparations from the epidermis of Vicia faba leaves. Biochemistry. 1974 Jul 16;13(15):3193–3202. doi: 10.1021/bi00712a030. [DOI] [PubMed] [Google Scholar]
- Dean B. B., Kolattukudy P. E. Biochemistry of Suberization: Incorporation of [1-C]Oleic Acid and [1-C]Acetate into the Aliphatic Components of Suberin in Potato Tuber Disks (Solanum tuberosum). Plant Physiol. 1977 Jan;59(1):48–54. doi: 10.1104/pp.59.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN P., VAGELOS P. R. The specificity of triglyceride synthesis from diglycerides in chicken adipose tissue. J Biol Chem. 1961 Oct;236:2620–2623. [PubMed] [Google Scholar]
- Gunsalus I. C., Pederson T. C., Sligar S. G. Oxygenase-catalyzed biological hydroxylations. Annu Rev Biochem. 1975;44:377–407. doi: 10.1146/annurev.bi.44.070175.002113. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Croteau R., Walton T. J. Biosynthesis of Cutin: Enzymatic Conversion of omega-Hydroxy Fatty Acids to Dicarboxylic Acids by Cell-free Extracts of Vicia Faba Epidermis. Plant Physiol. 1975 May;55(5):875–880. doi: 10.1104/pp.55.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E., Walton T. J., Kushwaha R. P. Biosynthesis of the C18 family of cutin acids: omega-hydroxyoleic acid, omega-hydroxy-9,10-epoxystearic acid, 9,10,18-trihydroxystearic acid, and their delta12-unsaturated analogs. Biochemistry. 1973 Oct 23;12(22):4488–4498. doi: 10.1021/bi00746a029. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Walton T. J. Structure and biosynthesis of the hydroxy fatty acids of cutin in Vicia faba leaves. Biochemistry. 1972 May 9;11(10):1897–1907. doi: 10.1021/bi00760a026. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meehan T. D., Coscia C. J. Hydroxylation of geraniol and nerol by a monooxygenase from Vinca rosea. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1043–1048. doi: 10.1016/0006-291x(73)90570-6. [DOI] [PubMed] [Google Scholar]
- Murphy P. J., West C. A. The role of mixed function oxidases in kaurene metabolism in Echinocystis macrocarpa Greene endosperm. Arch Biochem Biophys. 1969 Sep;133(2):395–407. doi: 10.1016/0003-9861(69)90468-8. [DOI] [PubMed] [Google Scholar]
- Potts J. R., Weklych R., Conn E. E., Rowell J. The 4-hydroxylation of cinnamic acid by sorghum microsomes and the requirement for cytochrome P-450. J Biol Chem. 1974 Aug 25;249(16):5019–5026. [PubMed] [Google Scholar]
- Russell D. W. The metabolism of aromatic compounds in higer plants. X. Properties of the cinnamic acid 4-hydroxylase of pea seedlings and some aspects of its metabolic and developmental control. J Biol Chem. 1971 Jun 25;246(12):3870–3878. [PubMed] [Google Scholar]
- Walton T. J., Kolattukudy P. E. Enzymatic conversion of 16-hydroxypalmitic acid into 10,16-dihydroxypalmitic acid in Vicia faba epidermal extracts. Biochem Biophys Res Commun. 1972 Jan 14;46(1):16–21. doi: 10.1016/0006-291x(72)90623-7. [DOI] [PubMed] [Google Scholar]


