Abstract
β-catenin is a key signalling molecule in the canonical Wnt pathway, which plays a role in cell adhesion, embryogenesis and sex determination. However, little is known about its function in teleosts. We cloned and characterized the full-length β-catenin1 gene from half-smooth tongue sole (Cynoglossus semilaevis), which was designated CS-β-catenin1. The CS-β-catenin1 cDNA consists of 2,346 nucleotides and encodes a protein with 782 amino acids. Although CS-β-catenin1 was transcribed in the gonads of both sexes, the level was significantly higher in ovaries compared to testes. Furthermore, the mRNA level of CS-β-catenin1 was significantly upregulated at 160 days and constantly increased until 2 years of age. In situ hybridization revealed that CS-β-catenin1 mRNA was mainly localized in oocyte cells, especially in stage I, II and III oocytes. When CS-β-catenin1 expression was inhibited by injection of quercetin in the ovaries, levels of CS-Figla and CS-foxl2 mRNA were significantly down-regulated, and CS-dmrt1 was up-regulated, which suggested that CS-β-catenin1 is a potential upstream gene of CS-Figla and is involved in the development of the ovaries, i.e., folliculogenesis.
Introduction
As the key molecule of cellular junctions, as well as the pivotal element of the canonical Wnt signalling pathway [1,2], β-catenin plays critical roles in cell growth and development, disease pathogenesis and cell adhesion [3,4]. Several studies have found that β-catenin has a significant link to mammalian sex determination and differentiation. In humans and mice, β-catenin is transcribed in the testis and ovaries [5–7], which suggests that it has a dual role in the gonad development of both sexes. Furthermore, the disruption of β-catenin in mouse ovaries causes severe deficiencies in gonad development, affecting the occurrence of masculinization, formation of testis-specific coelomic vessels and even the loss of female germ cells [7]. In contrast, testicular development appears to be normal regardless of mutations in β-catenin [7], which implies that β-catenin is necessary for ovary differentiation but seems to be dispensable for testicular development. Furthermore, β-catenin was found to be able to regulate the expression of many sex-related genes in mammalian ovaries. For example, β-catenin has been shown to regulate the transcription of Cyp19a1 via interactions with NR5A1 in rat ovarian granulosa cells [8] and repress Sertoli cell-specific Sox9 gene expression in mouse ovaries [9]. In addition, it was recently documented that activation of the canonical Wnt/β-catenin pathway in XY gonads of mice effectively blocked the male pathway and led to male to female sex reversal [5, 6]. In subsequent studies, the RSPO1/β-catenin signalling pathway was found to promote the meiosis of germ cells in the ovaries because the mutation resulted in impaired meiosis and down-regulated the expression of the early meiosis marker gene Stra8 [10]. Consequently, the studies described above suggest that β-catenin plays an essential role in ovary determination and differentiation in mammals. In teleosts, functional homologues of β-catenin have only been characterized in a few species, such as Danio rerio and Nile tilapia. Interestingly, in most teleosts, two subtypes of β-catenin were found, which were titled β-catenin1 and β-catenin2 [11, 12]. The two β-catenin subtypes displayed similar expression patterns in the gonads and functional similarity during development. For example, Nile tilapia β-catenin1 and β-catenin2 are both mainly expressed in the oogonia and oocytes of the ovaries, and knocking down these genes in the ovaries causes similar gonad phenotypes, including the inhibition of ovarian differentiation and masculinization [12].
The half-smooth tongue sole (Cynoglossus semilaevis) is a marine fish species with economic importance, It is generally distributed throughout the China Sea and is famous for its pleasant taste. Since its genome was deciphered, the half-smooth tongue sole has become an excellent model to study sex determination and differentiation in fish. Due to obvious growth dimorphism (females grow 2–4 times faster than males) [13], cultivating an all-female stock would be greatly beneficial for aquaculture. To achieve this goal, understanding the mechanisms of sex determination and sex differentiation is necessary [14, 15]. The half-smooth tongue sole has a ZW/ZZ sex chromosome system including male (ZZ) and female (ZW) sexes. Approximately 14% of cultivated populations comprise of neomales, which are sex-reversed from genetic females (ZW) to the phenotypic males under specific environmental conditions, such as high temperature [16, 17]. Neomales show similar growth patterns as males, which makes them disadvantageous to productivity. Therefore, elucidating the sex-reversal mechanism of neomales, and especially the identification of key genes in the reversal process, is both important for theoretical study and practical applications [18, 19]. Despite the isolation of several sex-related genes, such as dax1, foxl2, sox9a, amh, wnt4a, follistatin and cyp19a1a [20], the mechanisms of sex determination and differentiation in this species are still unclear. In the present study, we cloned and characterized a homologue of β-catenin1 in half-smooth tongue sole, then detected its pattern of tissue expression and temporal expression in gonads. We also investigated the potential pathways that are regulated by CS-β-catenin1 by treating ovaries in vivo with quercetin. Additionally, we evaluated the correlation between promoter methylation and CS-β-catenin1 expression. Our aims were to investigate the detailed functions of β-catenin1 in C. semilaevis and to further reveal its role in gonad development.
Materials and methods
Ethical statement
All experimental animal protocols were approved by Yellow Sea Fisheries Research Institute’s animal care and use committee. All tissues were removed under MS222 anesthesia, and all efforts were made to minimize fish suffering.
Experimental animals and sample collection
The half-smooth tongue sole used in this study were obtained from the Haiyang High-Tech Experiment Base (Haiyang, Shandong Province, China), and genetic and phenotypic sexuality was determined by previous method[13, 20]. Eight samples of each category; including the male, female and neomale, were randomly selected for sampling (three individuals of each gender).Thirteen tissues (heart, gill, liver, skin, kidney, blood, brain, spleen, muscle, pituitary, intestine, ovary and testis) were collected from 1-year post-hatching (yph) fish and immediately transferred into liquid nitrogen and stored at −80°C. For gonad samples at different developmental stages, three individuals at 7, 25, 48, 160 days post-hatching (dph), 8 and 10 months post-hatching (mph), 1 and 2 years post-hatching (yph) were sampled within one family. Simultaneously, parts of the gonads from 1 yph fish were fixed in 4% paraformaldehyde (pH 7.5) at 4°C for 20 h, then stored in 75% ethanol. In addition, parts of the caudal fins were collected and fixed in 100% ethanol for DNA extraction to determine the genetic sex of the fish.
Isolation of full-length CS-β-catenin1 cDNA
Total RNA was extracted from the tissues using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RACE-ready first-strand cDNA was synthesized from the total RNA using a SMART™ RACE cDNA amplification kit (Invitrogen). To obtain the full-length cDNA sequence, specific primers for the outer and nest amplifications were designed based on the partial cDNA sequence of CS-β-catenin1 from the genome sequence. The 5’-RACE and 3’-RACE reactions were performed using a SMART-RACE cDNA Amplification Kit (Clontech Inc., Mountain View, CA, USA). The specific procedures refer to Hu’s description [21].
Quantitative real-time PCR (qRT-PCR)
Total RNA from various tissues and gonads at different developmental stages was extracted using TRIzol Reagent as described previously. The cDNA was synthesized using a QuantScript RT kit (TaKaRa, Dalian, China) following the manufacturer’s protocol. Quantification was performed on a 7500 detection system (Applied Biosystems, Foster City, CA, USA) with SYBR Green Master Mix (TaKaRa BioInc). The qRT-PCR amplification was performed as previously described [22]. β-Actin was confirmed previously as a reliable gene in various tissue samples of C. semilaevis and was used as an internal control [23]. Each sample was analysed in triplicate, and three samples were handled. The relative mRNA expression of target genes was calculated with the 2−△△Ct method as described previously [24]. Data was analysed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple comparisons test using SPSS 18.0 (IBM, New York, NY, USA). Using a t-test, significance was set at P<0.05.
In situ hybridization (ISH)
To synthesize digoxigenin (DIG)-labelled RNA sense and antisense probes, we designed the primer pairs CS-β-catenin1-ISH-F and CS-β-catenin1-ISH-R (S2 Table). A 344 bp cDNA fragment from the open reading frame (ORF) region of CS-β-catenin1 was amplified using the primer pairs above then cloned into pBluescript II SK (+) and sequenced. The recombinant plasmid was linearized by EcoRI and HindIII and was used as the template for in vitro synthesis of the probes. The probes were synthesized with a DIG RNA labelling kit (Roche, Mannheim, Germany) following the manufacturer’s instructions. Gonad samples were processed in a series of ethanol gradients and embedded in paraffin wax, and 5μm sections were cut. Meanwhile, three samples were analysed with ISH which was performed using the labelled probes as previously described [25].
Expression of CS-β-catenin1, CS-Figla, CS-Wnt4a, CS-Foxl2 and CS-dmrt1 in ovaries after treatment with quercetin
Since quercetin acts as a potent inhibitor of the transcriptional activity of β-catenin [26, 27], it was selected to repress β-catenin expression. For the experiments, quercetin was diluted in 5% DMSO and injected into the gonads of 1 yph fish with female cavities at a dosage of 25 mg/kg/day. The dose was selected based on a previous study [28]. The injections were given once every 24 hours, and the fish were injected twice. The control groups had three individuals treated with an equal volume of 5% DMSO. The transcription levels of CS-β-catenin1 were detected using qRT-PCR as described above. For analysis, the mRNA of these genes was examined in 1 yph ovaries.
Analysis of CS-β-catenin1 methylation
Most gonadal DNA methylation occurs in half-smooth tongue sole at the first exon or promoter region of the gene. To determine the epigenetic regulation of β-catenin1, the data for methylation levels among females, males, and neomales were compared using information about the C. semilaevis methylome [16].
Results
Isolation and characterization of CS-β-catenin1 cDNA
A 2934 bp CS-β-catenin1 cDNA was obtained and identified after RT-PCR and RACE (GenBank accession number KX898023). The complete cDNA coding sequence contained a 204 bp 5’UTR, a 384 bp 3’UTR, and a 2346 bp ORF that encoded a 781-amino-acid protein. Structural analysis revealed that the CS-β-catenin1 protein could be divided into an N-terminal region, a central region and a C-terminal region. The central domain contained 12 armadillo (ARM) repeats and a cellulose synthase-interaction domain (Fig 1 and S1 Fig), and the ARM has been implicated in mediating protein-protein interactions.
Fig 1. Full-length cDNA sequence and the deduced amino acids of CS-β-catenin1.
Lowercase text indicates the 5’ and 3’UTR sequences of CS-β-catenin1; uppercase text indicates the coding sequence. The start codon (ATG) is in the red box. The putative ARM repeat regions and VATPaseH superfamily domain are marked with yellow arrows and black arrows, respectively. The N-terminal putative GSK-β consensus phosphorylation site is underlined, and the C-terminal transactivator region is in the box with light lines. The stop codon (TAA) is indicated by an asterisk, and the poly (A) tail is double-underlined.
Alignment and phylogenetic analysis
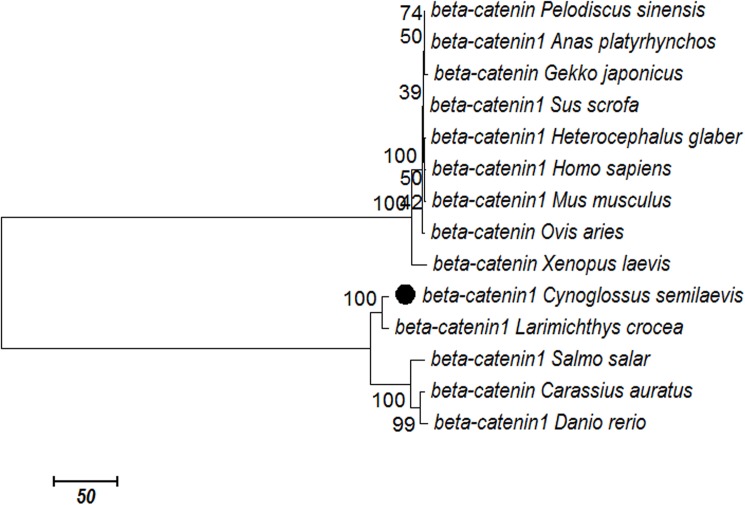
Through the BLASTx program, the CS-β-catenin1 protein showed a high degree of identity to other fish, including Larimichthys crocea (99%), Carassius auratus (96%), and Pelodiscus sinensis (96%). A phylogenetic tree was constructed using β-catenin1 from half-smooth tongue sole and 13 other species (Fig 2 and S1 Table). The sequences were clustered in two main groups, including β-catenin of the teleosts clustered in one group, and those of mammals clustered in another. Among the clusters, CS-β-catenin1 was more closely related to β-catenin in four types of fish.
Fig 2. Phylogenetic analysis of the β-catenin protein among different species.
The phylogenetic tree was constructed with the neighbour-joining algorithm in MEGA7.0. The bootstrap values were based on 10,000 resampling replicates. The relative genetic distances are indicated by the scale bar and branch lengths.
Tissue expression of CS-β-catenin1
CS-β-catenin1 expression was analysed in a wide range of tissues, e.g., the, heart, gill, brain, and liver. It is worth emphasizing that the mRNA expression of CS-β-catenin1 detected in the ovaries was the highest, whereas the lowest levels were found in muscles. The remaining tissues that were examined had very low expression (Fig 3A). Furthermore, we detected expression in the ovaries, male testis, and neomale testis of 1 yph fish. The highest levels were observed in the ovaries, followed by expression in the testis, and lowest expression was found in the neomale testis (Fig 3B).
Fig 3. Expression levels of β-catenin1 mRNA in C. semilaevis evaluated with qRT-PCR.
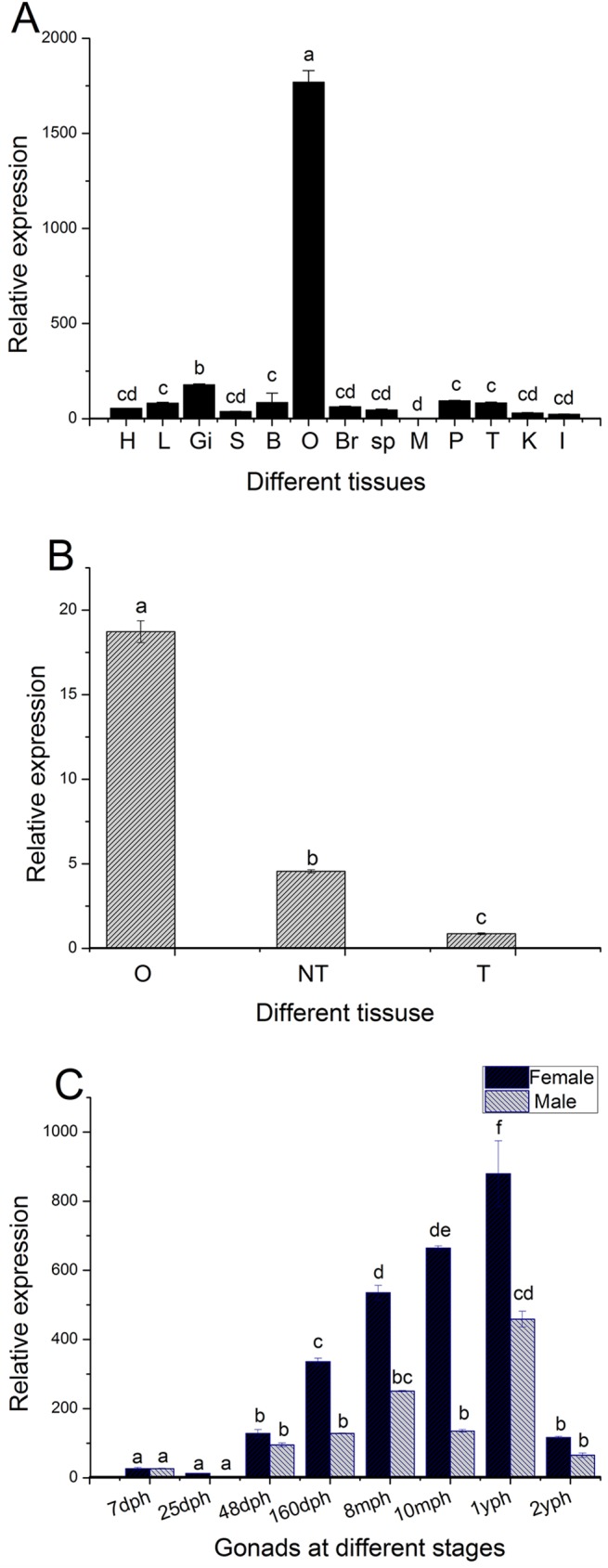
(A) Expression of CS-β-catenin1 in various tissues. H: heart, L: liver, K: kidney, I: intestine, SP: spleen, S: skin, M: muscle, B: blood, Br: brain, Gi: gill, P: pituitary, O: ovary, T: testis. (B) Expression of CS-β-catenin1 in different genotypes. O: ovary, T: testis of males, NT: testis of neomales. (C) Expression of CS-β-catenin1 in gonads at different developmental stages. β-actin was used as an internal control to normalize the expression. Three samples were performed. Data are shown as means ± SEM. The variance of this expression was represented as a ratio (the amount of CS-β-catenin1mRNA normalized to the corresponding reference genes values). Values with superscripts indicate statistical significance (P<0.05)
CS-β-catenin1 expression in developmental stages of male and female gonads
The expression profile of CS-β-catenin1 was detected by qRT-PCR, and the CS-β-catenin1 expression in the development of gonads was diverse. In the development of the female gonads, extremely low levels were observed 7 dph. Starting at 48 dph, the expression increased evidently and continued to rise until 1 yph, where peak levels were attained, and then there was a significant decline at 2 yph (Fig 3C). In the developmental stages of the testis, we detected the lowest amount of expression at 7 dph. From 48 dph to 2 yph, high expression was only observed at 8 mph and 1 yph (Fig 3C). In comparison, expression in females was higher compared to males at all tested time points except at 7 dph, when the levels were nearly equal.
Cyto-location of CS-β-catenin1
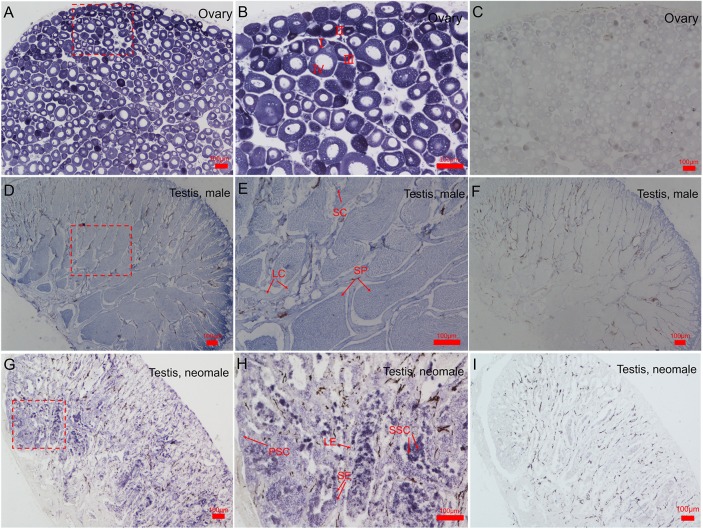
In situ hybridization (ISH) showed that much higher expression of CS-β-catenin1 occurred in the ovaries (Fig 4A and 4B) than in the testis of males and neomales (Fig 4D, 4E, 4G and 4H). In addition, ovaries contain oocytes at different developmental stages (stages I-IV). We observed the strongest hybridization signals in the stage I, II and III oocytes, and faint signals were detected in stage IV oocytes (Fig 4A and 4B). Compared to the ovary, there was relatively little expression in spermatids and sperms, and no positive signals were found in the Sertoli cells of the testis of males (Fig 4D and 4E). ISH revealed the expression of mRNA CS-β-catenin1 in the testis of neomales; weak signals in the spermatogonium, primary spermatocytes and Sertoli cells (Fig 4G and 4H). In the negative controls, no specific signals were detected (Fig 4C, 4F and 4I).
Fig 4. Cyto-locations of CS-β-catenin1 mRNA in gonads of C. semilaevis.
(A). Low magnification showing the adult ovaries. (B). High magnification of the framed area in (A). (C). Control of β-catenin1 localization in female ovaries. (D). Low magnification showing the male adult testis of males. (E). High magnification of the framed area in (D). (F). Control of β-catenin1 localization in male testis. (G). Low magnification showing the adult testis of a neomale. (H). High magnification of the framed area in (G). (I). Control of β-catenin1 localization in neomale testis. Three samples were handled. Oocytes at different developmental stages are marked by I, II, III and IV. PSC: primary spermatocytes; SSC: secondary spermatocytes; LE: Leydig cells; SP: spermatid; SE: Sertoli cells. Scale bars: 100 μm.
CS-β-catenin1 and associated genes in ovaries treated with quercetin
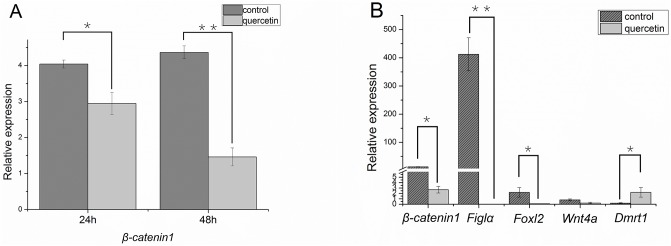
The transcription of the CS-β-catenin1 can be significantly suppressed by quercetin, and the treatment time was also determined as 48 h by comparing the suppression effects (Fig 5A). After 48h, all fish were health and no adverse effects were experienced. After CS-β-catenin1 suppression, the expression of CS-Figla rapidly reduced to almost undetectable levels, CS-foxl2 declined in the ovaries (Fig 5B), while CS-dmrt1 was up-regulated (Fig 5B).
Fig 5. Relative mRNA expression levels of CS-β-catenin1 and other sex-related genes after treatment with quercetin.
(A) Expression of CS-β-catenin1 24 and 48 h after injection with quercetin. (B) Expression of CS-β-catenin1, CS-Figla, CS-Foxl2, CS-Wnt4a and CS-dmrt1 48 h after injection with quercetin. The expression of the β-actin gene was used as an internal control. Three samples were handled. Values with asterisks indicate a significant difference (P<0.05).
Methylation patterns in ovaries, testis and neomale gonads
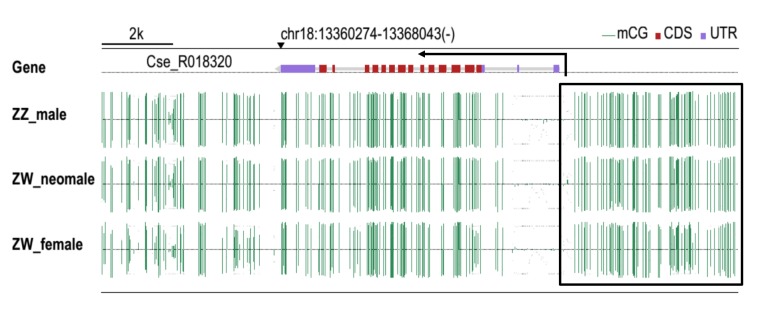
Among females, males and neomales, the location of the 4 kb area upstream of ATG and the CS-β-catenin1 gene body showed no significant methylation in the putative region between females, males and neomales (Fig 6).
Fig 6. Methylation analyses of CS-β-catenin1 in gonads.
The CS-β-catenin1 genomic sequence with gene body, also the 2kb up-and downstream area was analyzed. The methylation level of cytosines is shown with green vertical lines. The boxed area indicated the not different significantly methylation sites distributed in the upstream of 5’UTR (about 2 kb) of CS-β-catenin1 gene. Arrows indicate direction of transcription.
Discussion
Although the β-catenin gene has been reported in a few teleost species, systematic studies of its potential function are limited. In the present study, for the first time, the β-catenin1 gene was cloned and characterized in C. semilaevis, This was named CS-β-catenin1, and its expression was analysed as well as the promoter methylation pattern in the gonads. By using an inhibitor of β-catenin1 gene expression in vivo, we showed that CS-β-catenin1 was a potential regulator of CS-Figla expression in the ovaries.
Two different β-catenin genes, β-catenin1 and β-catenin2, were identified in the genome of teleosts, which may have arisen from whole-genome duplication [29]. Based on the evolutionary conservation of the teleost genome, we speculated that the β-catenin2 gene probably exists in C. semilaevis, and further studies are needed to confirm our speculations.
In this present study, CS-β-catenin1 was shown to contain 12 ARM repeat regions, which are highly conserved among the species. Similarly, the conserved ARM regions were found in Danio rerio, Nile tilapia, Homo sapiens and Chlamys farreri [6, 11, 12, 27]. These regions are thought to be involved in the binding of β-catenin to Axin, glycogen synthase kinase 3β (GSK3β) and adenomatous polyposis coli (APC), which are three key molecules in the canonical Wnt pathway. Thus, these regions play a central regulatory role in this pathway [30–32]. In addition, these regions are thought to be required for β-catenin to interact with cell adhesion molecule E-cadherin, the tumour-suppressor gene adenomatous polyposis coli (APC) and α-catenin, as well as for the mediation of adherens junctions of the plasma membrane to the cytoskeleton [33]. We expect that the ARM regions from CS-β-catenin1 will have similar functions.
In C. semilaevis, histological gonad differentiation was found to begin at 56-62dph, and testicular and ovarian differentiation do not occur simultaneously. Ovarian differentiation begins earlier than testicular differentiation with the appearance of an ovarian cavity. After the cavity has formed, the testes start to differentiate [20]. In our study, we observed clear CS-β-catenin1 mRNA expression at 48 dph, which occurred just before the histological differentiation of the ovaries. Based on these results, we hypothesize that CS-β-catenin1 is involved in ovary growth. Moreover, CS-β-catenin1 probably sustained its expression until 2yph in the adult ovaries.
The cyto-locations of β-catenin in the gonads vary among species. In mice, β-catenin mRNA is mainly expressed in ovarian somatic cells, such as granulosa cells and surface epithelium cells [34]. In C. semilaevis, Nile tilapia and Chlamys farreri, β-catenin is predominately expressed in the oocytes, especially in the early stages of development [12, 27]. However, chicken β-catenin mRNA is transcribed only in the Sertoli cells of the testes from the immature to mature stages [34]. We speculate that this restricted transcription is probably caused by differences in the species. Additionally, it is likely related to the functional diversity of β-catenin among different cell types. In mice, β-catenin is expressed in the testes [7]. However, mutations affecting β-catenin expression did not result in phenotypic changes in the testes [7], which suggested that β-catenin is probably not important for testis development in mammals. In the present study, we also observed weak β-catenin expression in the germ cells and somatic cells of testes in 1 yph C. semilaevis, and there was no significant expression fluctuations during the development of testis. We speculate that, as in mammals, β-catenin is likely not necessary for testicular development in C. semilaevis.
Wnt4 is the key signalling molecule in the canonical Wnt/β-catenin pathway, which has important roles in embryonic development and disease, tissue self-renewal, and adipogenesis of vertebrates [35–38]. Additionally, the Wnt4/β-catenin pathway is associated with gonad development in mammals [39, 40] and teleosts, such as Acanthopagrus schlegelii and zebrafish [41, 42]. For example, the absence of Wnt4 protein results in female to male sex reversal and promotes the development of male reproductive ducts in mice with XX gonads [10]. In our previous studies, we identified a homologous gene of Wnt4 (Wnt4a) and found that it was predominately expressed in oocytes in the ovaries, and in male germ cells, it was mainly expressed in the testes [43]. Furthermore, in the present study, CS-β-catenin1 showed a similar expression pattern as Wnt4a. Our results, together with the reports above, indicate that the canonical Wnt4/β-catenin pathway likely exists in C. semilaevis and plays an important role in gonad development. We speculate the Wnt4/β-catenin1 pathway is likely to be conserved and is an ancient pathway in the determination of gonad development and differentiation in vertebrates.
The regulatory roles of β-catenin in gonad development have been studied extensively in vertebrates [44–46]. In teleosts, however, the regulatory function of β-catenin during gonad development is still poorly understood. In C. semilaevis, Dmrt1 was identified as a testis-related candidate gene which is important in sex determination of males [20], while Figla was suggested to be a female-related gene that was involved in ovary differentiation, i.e., folliculogenesis [47–49]. Additionally, foxl2 had higher expression in female gonads [50]. In addition, Wnt4a was regarded as a potential gene for sex reversal [43]. To investigate the regulatory pathway of β-catenin in C. semilaevis, we measured the expression of dmrt1, foxl2, wnt4a and Figla after in vivo knockdown of β-catenin in the ovaries. Compared to the expression of dmrt1, which significantly increased, the expression of Figla and foxl2 displayed an opposite pattern and markedly decreased. Therefore, we speculated that the normal physiological functions of β-catenin1 in the ovaries were likely related to promoting female-related gene expression and repressing male-related gene expression in C. semilaevis, which was similar to findings in Nile tilapia [12]. Figla was deemed to favour ovarian differentiation by antagonizing spermatogenesis in Nile tilapia [51]. However, differences appeared in the expression of foxl2 after knocking out β-catenin in Nile tilapia. When β-catenin was knocked out, foxl2 expression increased, which suggested that there was a possible compensatory effect for the deficiency. We hypothesized that other pathways would exist to compensate for the lack of β-catenin1 in addition to foxl2 in the development of ovaries in C. semilaevis.
DNA methylation, especially in the promoter region, is a heritable epigenetic modification that plays a crucial role in the modification of phenotypes and the regulation of gene expression [52, 53]. In C. semilaevis, many sex-related genes are regulated by DNA methylation, including the gametogenesis-related genes Altesk1, piwil2, wnt4a and neurl3 [43, 54–56], as well as the gonad differentiation-related genes GATA6 and GATA4 [57, 58]. To explore the reasons why there was differential β-catenin1 mRNA expression among the gonads in different sexes of C. semilaevis, we analysed the degree of methylation of CS-β-catenin1 in males, females and neomales in the promoter region, but no significant results were found. This indicates that β-catenin1 transcription is not primarily regulated by differential methylation between testis and ovaries.
Conclusion
In summary, the β-catenin1 gene was successfully isolated from C. semilaevis. CS-β-catenin1 displayed an expression pattern that was predominantly in the gonads and the germ cells of the ovaries, which suggested that it has a potential function in ovarian germ cells. In addition, we showed that the normal physiological functions of CS-β-catenin1 in the ovaries were to promote female-related gene expression and repress male-related gene expression. However, the molecular mechanism of CS-β-catenin1 function is unclear, and further studies are needed.
Supporting information
(A) Deduced protein sequence of CS-β-catenin1. The predicted ARM domain containing 12 repeats is marked with red and blue text. (B) Schematic illustration of CS-β-catenin1 structure.
(TIF)
(DOC)
(DOC)
Acknowledgments
We would like to thank Yingming Yang and Zhongkai Cui at Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences for sample collection.
Data Availability
The gene sequence files are available from the NCBI database (accession number KX898023)after acceptance.
Funding Statement
This work was supported by grants from the National Nature Science Foundation of China (31130057,31530078,31402293,31472269), Taishan Scholar Climbing Project Fund of Shandong of China. Special Scientific Research Funds for Central Non-profit Institutes, Chinese Academy of Fishery Sciences (2015C03YQ01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kirikoshi H, Sekihara H, Katoh M. Expression profiles of 10 members of frizzled gene family in human gastric cancer. Int J Oncol. 2001. October; 19 (4):767–771. [DOI] [PubMed] [Google Scholar]
- 2.Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, et al. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA. 1999. February; 96(4):1569–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004. July; 20: 781–810. doi: 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- 4.Willert K, Nusse R. β-catenin: a key mediator of wnt signaling. Curr Opin Genet Dev. 1998. February; 8(1): 95–102. [DOI] [PubMed] [Google Scholar]
- 5.Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006. November; 38(11): 1304–1309. doi: 10.1038/ng1907 [DOI] [PubMed] [Google Scholar]
- 6.Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008. October; 17 (9): 2949–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of β-catenin in mouse gonadal development. Hum Mol Genet. 2009. February; 18(3): 405–417. doi: 10.1093/hmg/ddn362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parakh TN, Hernandez JA, Grammer JC, Weck J, Hunzicker-Dunn M, Zeleznik AJ, et al. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires β-catenin. Proc Natl Acad Sci USA.2006. August; 103(33): 12435–12440. doi: 10.1073/pnas.0603006103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard P, Ryan J, Sim H, Czech DP, Sinclair AH, Koopman P, et al. Wnt signaling in ovarian development inhibits sf1 activation of sox9 via the tesco enhancer. Endocrinology. 2012. February; 153(2): 901–912. doi: 10.1210/en.2011-1347 [DOI] [PubMed] [Google Scholar]
- 10.Chassot AA, Gregoire EP, Lavery R, Taketo MM, de Rooij DG, Adams IR, et al. RSPO1/β-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS One. 2011. October; 6 (10): e25641 doi: 10.1371/journal.pone.0025641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Zhang J, Lin SC, Meng A. β-catenin 1 and β-catenin 2 play similar and distinct roles in left-right asymmetric development of zebrafish embryos. Development. 2012. June; 139 (11): 2009–2019. doi: 10.1242/dev.074435 [DOI] [PubMed] [Google Scholar]
- 12.Wu LM, Wu FR, Xie L, Wang DS, Zhou LY. Synergistic role of β-catenin1 and 2 in ovarian differentiation and maintenance of female pathway in Nile tilapia. Mol Cell Endocrino. 2016. May; 427: 33–44. [DOI] [PubMed] [Google Scholar]
- 13.Chen SL, Li J, Deng SP, Tian YS, Wang QY, Zhuang ZM, et al. Isolation of female-specific AFLP markers and molecular identification of genetic sex in half-smooth tongue sole (Cynoglossus semilaevis). Mar Biotechnol. 2007. Mar-Apr; 9(2): 273–280. doi: 10.1007/s10126-006-6081-x [DOI] [PubMed] [Google Scholar]
- 14.Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999. February; 397(6718): 405–409. doi: 10.1038/17068 [DOI] [PubMed] [Google Scholar]
- 15.Liao XL, Xu GB, Chen SL. Molecular method for sex identification of half-smooth tongue sole (Cynoglossus semilaevis) using a novel sex-linked microsatellite marker. Int J Mol Sci. 2014. July; 15(7):12952–12958. doi: 10.3390/ijms150712952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao CW, Li QY, Chen SL, Zhang P, Lian JM, Hu QM, et al. Epigenetic modification and inheritance in sexual reversal of fish. Genome Res. 2014. April; 24(4): 604–615. doi: 10.1101/gr.162172.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao CW, Wu PF, Wang XL, Tian YS, Chen SL. Comparison of chromosome preparation methods for the different developmental stages of the half-smooth tongue sole (Cynoglossus semilaevis). Micron. 2010. January; 41(1):47–50. doi: 10.1016/j.micron.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 18.Hu QM, Chen SL, Gao FT, Liu SS, Liu F, Yang JF, et al. Differences in sex reversion and growth between normal and neomale stock in half-smooth tongue sole, Cynoglossus semilaevis. Aquacult Int. 2014. February; 22(4):1437–1449. [Google Scholar]
- 19.Mitcheson YSD, Liu M. Functional hermaphroditism in teleosts. Fish and Fisheries. 2008. February; 9(1):1–43. [Google Scholar]
- 20.Chen SL, Zhang GJ, Shao CW, Huang QF, Liu G, Zhang P, et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat Genet. 2014. March; 46(3):253–260. doi: 10.1038/ng.2890 [DOI] [PubMed] [Google Scholar]
- 21.Hu QM, Chen SL. Cloning genomic structure and expression analysis of ubc9 in the course of development in the half-smooth tongue sole (Cynoglossus semilaevis). Comp Biochem Physiol B Biochem Mol Biol. 2013. July; 165(3): 181–188. doi: 10.1016/j.cbpb.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 22.Xu WT, Li HL, Dong ZD, Cui ZK, Zhang N, Meng L, et al. Ubiquitin ligase gene neurl3 plays a role in spermatogenesis of half-smooth tongue sole (Cynoglossus semilaevis) by regulating testis protein ubiquitination. Gene. 2016. October; 592 (1): 215–220. doi: 10.1016/j.gene.2016.07.062 [DOI] [PubMed] [Google Scholar]
- 23.Li ZJ, Yang LJ, Wang J, Shi WC, Ravindra AP, Liu Y, et al. β-Actin is a useful internal control for tissue-specific gene expression studies using quantitative real-time PCR in the half-smooth tongue sole Cynoglossus semilaevis challenged with LPS or Vibrio anguillarum. Fish Shellfish Immunol. 2010. July; 29(1): 89–93. doi: 10.1016/j.fsi.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods. 2001. December; 25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Kajiura-Kobayashi H, Nagahama Y. Differential expression of vasa homologue gene in the germ cells during oogenesis and spermatogenesis in a teleost fish, tilapia, Oreochromis niloticus. Mech Dev. 2000. December; 99(1–2):139–142. [DOI] [PubMed] [Google Scholar]
- 26.Park CH, Chang JY, Hahm ER, Park S, Kim HK, Yang CH. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem Biophys Res Commun. 2005. March; 328(1):227–234. doi: 10.1016/j.bbrc.2004.12.151 [DOI] [PubMed] [Google Scholar]
- 27.Li HL, Zhang ZF, Bi Y, Yang DD, Zhang LT, Liu JG. Expression characteristics of β-catenin in scallop Chlamys farreri gonads and its role as a potential upstream gene of Dax1 through canonical wnt signalling pathway regulating the spermatogenesis. PLoS One. 2014. December; 9(12):e115917 doi: 10.1371/journal.pone.0115917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren JF, Li JZ, Liu X, Feng Y, Gui Y, Yang JW, et al. Quercetin Inhibits fibroblast activation and kidney fibrosis involving the suppression of mammalian target of rapamycin and β-catenin Signaling. Sci Rep. 2016. April; 6:23968 doi: 10.1038/srep23968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer A, Van PY. From 2R to 3R: evidence for a fish specific genome duplication (FSGD). Bioessays. 2005. September; 27(9): 937–945. doi: 10.1002/bies.20293 [DOI] [PubMed] [Google Scholar]
- 30.Schneider SQ, Finnerty JR, Martindale MQ. Protein evolution: structure-function relationships of the oncogene beta-catenin in the evolution of multicellular animals. J Exp Zool B Mol Dev Evol. 2003. February; 295(1):25–44. doi: 10.1002/jez.b.6 [DOI] [PubMed] [Google Scholar]
- 31.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012. June; 149 (6): 1192–1205. doi: 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 32.Hülsken J, Hulsken W, Birchmeier, Behrens J. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J Cell Biol. 1994. December; 127 (6 Pt 2):2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, et al. Association of the APC gene product with β-catenin. Science. 1993. December; 262(5140):1731–1734. [DOI] [PubMed] [Google Scholar]
- 34.Seung MB, Whasun L, Wooyoung J, Jin YL, Jinyoung K, Fuller WB, et al. Sex-specific expression of CTNNB1 in the gonadal morphogenesis of the chicken. Reprod Biol Endocrinol. 2013. September; 11: 89 doi: 10.1186/1477-7827-11-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez Gifford JA. The role of WNT signaling in adult ovarian folliculogenesis. Reproduction. 2015. October; 150(4): 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clevers H. Wnt/β-catenin signaling in development and disease. Cell, 2006. November; 127(3): 469–480. doi: 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 37.Pandur P, Maurus D, Kühl M. Increasingly complex: New players enter the wnt signaling network. Bio Essays. 2002. October; 24(10):881–884. [DOI] [PubMed] [Google Scholar]
- 38.Prestwich TC, Macdougald OA. Wnt/β-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol. 2007. December; 19(6):612–617. doi: 10.1016/j.ceb.2007.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vainio S, Heikkila M, Kispert A, Chin N, Mcmahon AP. Female development in mammals is regulated by wnt4 signalling. Nature. 1999. February; 397(6718):405–9. doi: 10.1038/17068 [DOI] [PubMed] [Google Scholar]
- 40.Bernard P, Ryan J, Sim H, Czech DP, Sinclair AH, Koopman P, et al. Wnt signaling in ovarian development inhibits sf1 activation of sox9 via the tesco enhancer. Endocrinology. 2012. February; 153(2):901–912. doi: 10.1210/en.2011-1347 [DOI] [PubMed] [Google Scholar]
- 41.Wu GC. Chang CF. Wnt4 is associated with the development of ovarian tissue in the protandrous black Porgy, Biol Reprod. 2009. December; 81(6):1073–1082. doi: 10.1095/biolreprod.109.077362 [DOI] [PubMed] [Google Scholar]
- 42.Sreenivasan R, Jiang J, Wang XG, Bártfai R, Kwan HY, Christoffels A, et al. Gonad differentiation in zebrafish is regulated by the canonical wnt signaling pathway. Biol Reprod. 2014. February; 90(2):45 doi: 10.1095/biolreprod.113.110874 [DOI] [PubMed] [Google Scholar]
- 43.Hu QM, Zhu Y, Liu Y, Wang N, Chen SL. Cloning and characterization of wnt4a gene and evidence for positive selection in half-smooth tongue sole (Cynoglossus semilaevis). Sci Rep. 2014. November; 4:7167 doi: 10.1038/srep07167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maatouk DM, Napoli LD, Alvers A, Parker KL, Taketo MM, Capel B, et al. Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Human Molecular Genetics, 2008. October; 17(9):2949–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicol B, Yao HH. Gonadal identity in the absence of pro-testis factor sox9 and pro-ovary factor β-catenin in mice. Biol Reprod. 2015. August; 93(2):35 doi: 10.1095/biolreprod.115.131276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mork L, Capel B. Conserved action of β-catenin during female fate determination in the red‐eared slider turtle. Evol Dev. 2013. Mar-Apr; 15(2):96–106. doi: 10.1111/ede.12020 [DOI] [PubMed] [Google Scholar]
- 47.Li HL, Xu WT, Zhang N, Shao CW, Zhu Y, Dong ZD, et al. Two figla homologues have disparate functions during sex differentiation in half-smooth tongue sole (Cynoglossus semilaevis). Sci Rep. 2016. June; 6:28219 doi: 10.1038/srep28219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyake Y, Sakai Y, Kuniyoshi H. Molecular cloning and expression profile of sex-specific genes, figla and dmrt1, in the protogynous hermaphroditic fish, Halichoeres poecilopterus. Zoolog Sci. 2012. October; 29(10):690–701. doi: 10.2108/zsj.29.690 [DOI] [PubMed] [Google Scholar]
- 49.Joshi S, Davies H, Sims LP, Levy SE, Dean J. Ovarian gene expression in the absence of FIGLA, an oocyte-specific transcription factor. BMC Dev Biol. 2007. June; 7:67 doi: 10.1186/1471-213X-7-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong XL, Chen SL, Ji XS, Shao CW. Molecular cloning, characterization and expression analysis of sox9a and foxl2 genes in half-smooth tongue sole (Cynoglossus semilaevis). Acta Oceanol Sin. 2011. May; 30(1):68–77. [Google Scholar]
- 51.Qiu YX, Sun SH, Charkraborty T, Wu LM, Sun L, Wei J, et al. Figla favors ovarian differentiation by antagonizing spermatogenesis in a teleosts, Nile Tilapia (Oreochromis niloticus). PLoS One. 2015. April; 10(4):e0123900 doi: 10.1371/journal.pone.0123900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daugela L, Nüsgen N, Walier M, Oldenburg J, Schwaab R, El-Maarri O. Measurements of DNA methylation at seven loci in various tissues of CD1 mice. PLoS One. 2012. September; 7(9): e44585 doi: 10.1371/journal.pone.0044585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong KY, Huang XJ, Chim CS. DNA methylation of microRNA genes in multiple myeloma. Carcinogenesis. 2012. September; 33(9):1629–1638. doi: 10.1093/carcin/bgs212 [DOI] [PubMed] [Google Scholar]
- 54.Xu WT, Li HL, Zhang N, Dong ZD, Wang N, Shao CW, et al. Expression analysis and characterization of an autosome-localized tesk1 gene in half-smooth tongue sole (Cynoglossus semilaevis). Gene. 2016. May; 582(2):161–167. doi: 10.1016/j.gene.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 55.Zhang LY, Liu WJ, Shao CW, Zhang N, Li HL, Liu K, et al. Cloning expression and methylation analysis of piwil2 in half-smooth tongue sole (Cynoglossus semilaevis). Mar Genomics. 2014. December; 18 Pt A:45–54. [DOI] [PubMed] [Google Scholar]
- 56.Xu WT, Li HL, Dong ZD, Zhang N, Meng L, Zhu Y, et al. Ubiquitin ligase gene neurl3, plays a role in spermatogenesis of half-smooth tongue sole (Cynoglossus semilaevis) by regulating testis protein ubiquitination. Gene, 2016. October; 592(1):215–220. doi: 10.1016/j.gene.2016.07.062 [DOI] [PubMed] [Google Scholar]
- 57.Liu JX, Zhang W, Sun Y, Wang ZJ, Zhang QQ, Wang XB, et al. Molecular characterization and expression profiles of GATA6 in tongue sole (Cynoglossus semilaevis). Comp Biochem Physiol B Biochem Mol Biol. 2016. August; 198:19–26. doi: 10.1016/j.cbpb.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 58.Liu JX, Zhang W, Du XX, Jiang J, Wang C, Wang XB, et al. Molecular characterization and functional analysis of the GATA4 in tongue sole (Cynoglossus semilaevis). Comp Biochem Physiol B Biochem Mol Biol. 2016. March; 193:1–8. doi: 10.1016/j.cbpb.2015.12.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Deduced protein sequence of CS-β-catenin1. The predicted ARM domain containing 12 repeats is marked with red and blue text. (B) Schematic illustration of CS-β-catenin1 structure.
(TIF)
(DOC)
(DOC)
Data Availability Statement
The gene sequence files are available from the NCBI database (accession number KX898023)after acceptance.