Abstract
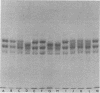
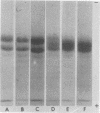
Discontinuous sodium dodecyl sulfate slab gel electrophoresis of G1 globulin from several strains of Phaseolus vulgaris L. seed permitted clear resolution of the constituent polypeptides. Three strains (Tendergreen, Canadian Wonder, and BBL 240) had subunits of molecular weight 53,000, 47,000 and 43,000 while two strains (Seafarer and PI 229,815) had 50,500, 47,000 and 43,000 molecular weight subunits. F1 seed from the cross BBL 240 × PI 229,815 showed four polypeptides on dissociation of the G1 protein; however, the amount of each of the 53,000 and 50,500 subunits was half that of the 47,000 subunit. This is interpreted as evidence that both the maternal and paternal loci for these polypeptides are transcribed and translated with similar efficiency. All of the polypeptides were found to have associated sugar residues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Knowland J. Protein synthesis directed by the RNA from a plant virus in a normal animal cell. Genetics. 1974 Sep;78(1):383–394. doi: 10.1093/genetics/78.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Romero J., Sun S. M., McLeester R. C., Bliss F. A., Hall T. C. Heritable Variation in a Polypeptide Subunit of the Major Storage Protein of the Bean, Phaseolus vulgaris L. Plant Physiol. 1975 Dec;56(6):776–779. doi: 10.1104/pp.56.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman D. R., Hall T. C., Ryan D. S. Affinity Chromatography of the Major Seed Protein of the Bean (Phaseolus vulgaris L.). Plant Physiol. 1976 Sep;58(3):272–275. doi: 10.1104/pp.58.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sun S. M., Buchbinder B. U., Hall T. C. Cell-free Synthesis of the Major Storage Protein of the Bean, Phaseolus vulgaris L. Plant Physiol. 1975 Dec;56(6):780–785. doi: 10.1104/pp.56.6.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. M., Hall T. C. Solubility characteristics of globulins from Phaseolus seeds in regard to their isolation and characterization. J Agric Food Chem. 1975 Mar-Apr;23(2):184–189. doi: 10.1021/jf60198a004. [DOI] [PubMed] [Google Scholar]
- Sun S. M., McLeester R. C., Bliss F. A., Hall T. C. Reversible and irreversible dissociation of globulins from Phaseolus vulgaris seed. J Biol Chem. 1974 Apr 10;249(7):2118–2121. [PubMed] [Google Scholar]