Abstract
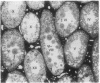
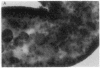
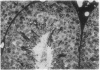
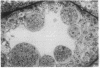
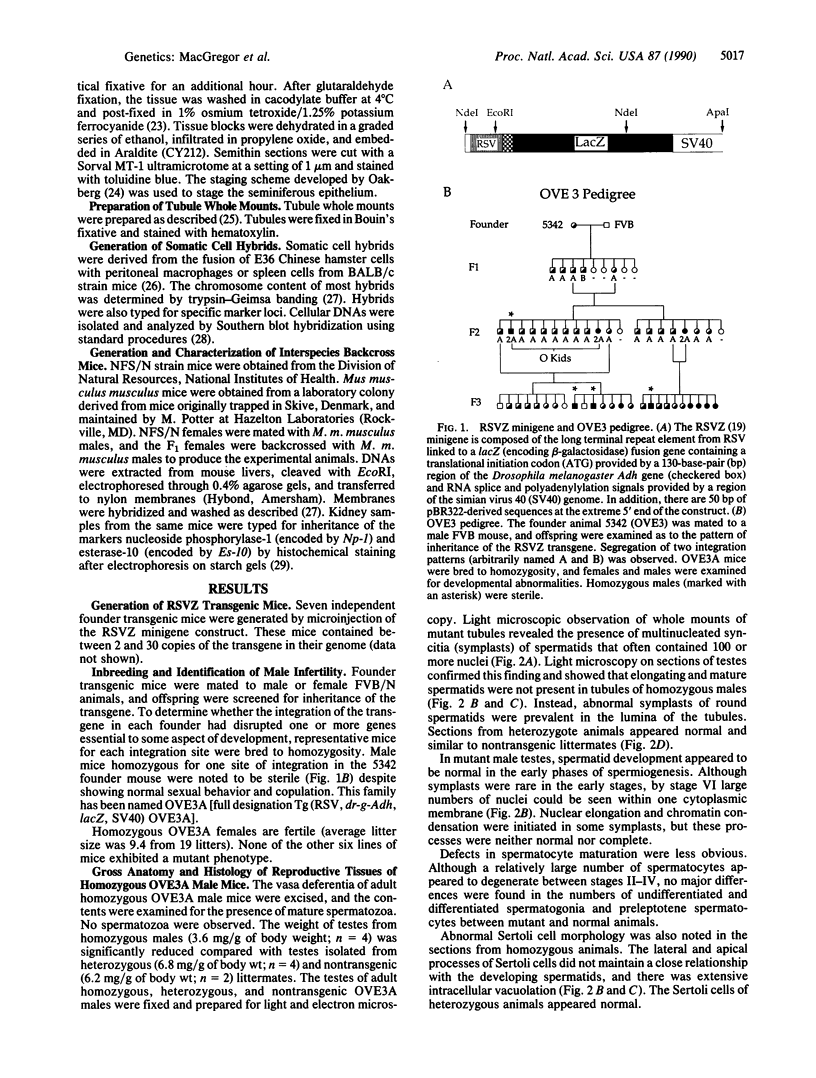
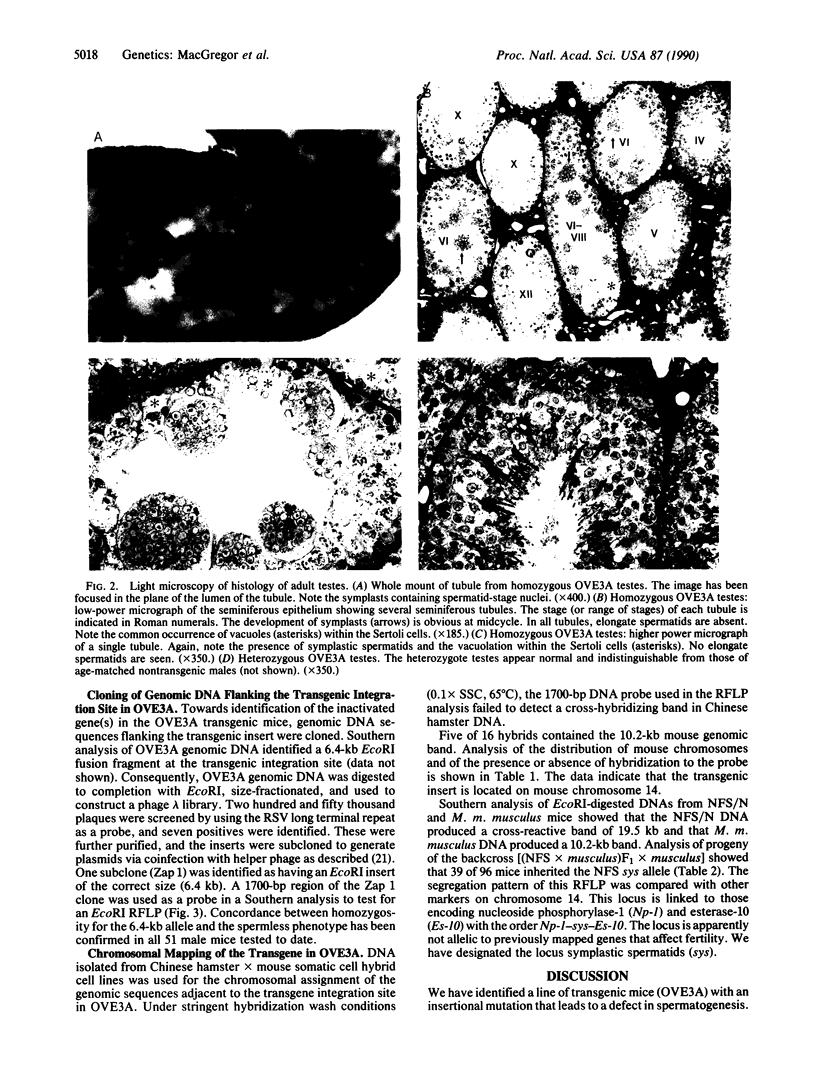
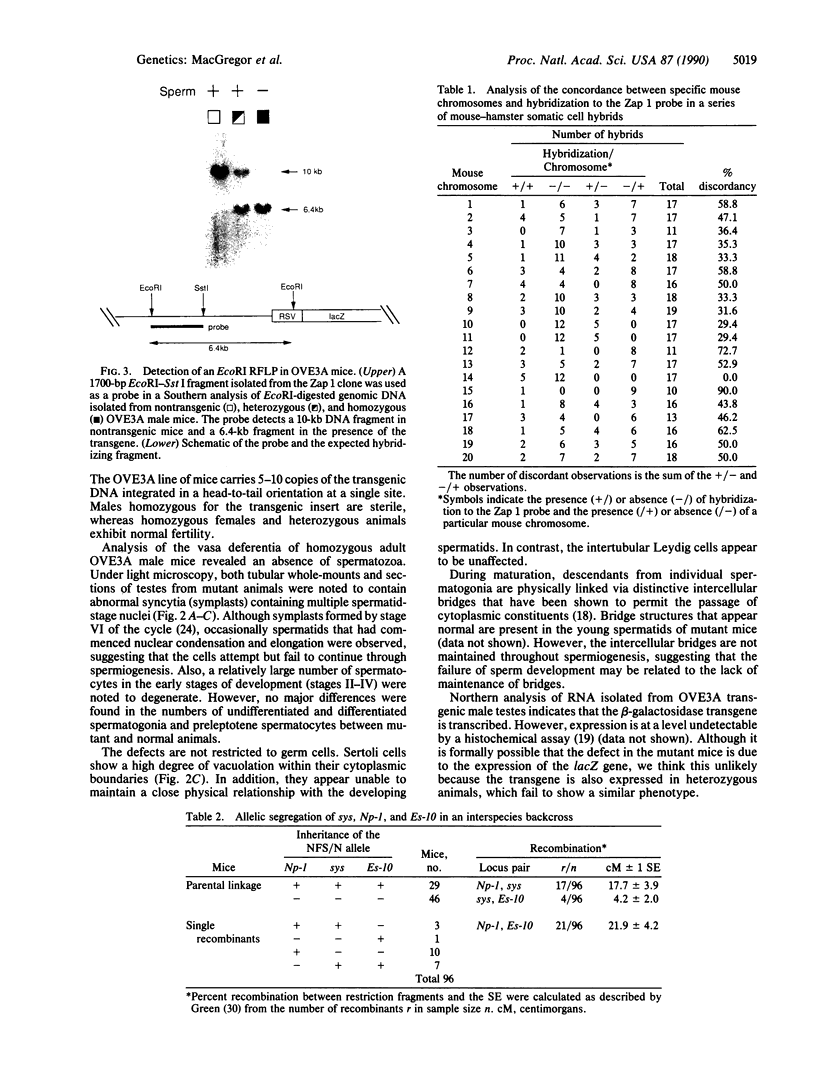
A line of transgenic mice that carries an insertional mutation in a gene essential for spermatogenesis is described. Males homozygous for the transgenic insert are sterile, while female homozygotes and both male and female heterozygotes exhibit normal fertility. Developing spermatids in homozygous males form prominent abnormal multinucleated syncytia (symplasts) and do not complete maturation. In addition, abnormal cytoplasmic vacuolation is commonly seen in Sertoli cells. One flank of the transgenic integration site within the genome has been cloned and used to show linkage between homozygosity for the transgene and the mutant phenotype. The flank maps to mouse chromosome 14 approximately 4 centimorgans proximal to the gene encoding esterase-10 (Es-10). As no other gene that is known to be essential for spermatogenesis has been mapped to this region of the genome and as the mutant phenotype is unique, the transgenic insert appears to affect a previously unidentified gene. We have named the mutation "symplastic spermatids" (sys).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Shawi R., Burke J., Jones C. T., Simons J. P., Bishop J. O. A Mup promoter-thymidine kinase reporter gene shows relaxed tissue-specific expression and confers male sterility upon transgenic mice. Mol Cell Biol. 1988 Nov;8(11):4821–4828. doi: 10.1128/mcb.8.11.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. E., Behringer R. R., Peschon J. J., Brinster R. L., Palmiter R. D. Genetically haploid spermatids are phenotypically diploid. Nature. 1989 Jan 26;337(6205):373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- Clermont Y., Bustos-Obregon E. Re-examination of spermatogonial renewal in the rat by means of seminiferous tubules mounted "in toto". Am J Anat. 1968 Mar;122(2):237–247. doi: 10.1002/aja.1001220205. [DOI] [PubMed] [Google Scholar]
- Costantini F., Radice G., Lee J. L., Chada K. K., Perry W., Son H. J. Insertional mutations in transgenic mice. Prog Nucleic Acid Res Mol Biol. 1989;36:159–169. doi: 10.1016/s0079-6603(08)60169-5. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Nishida Y., Terao M., D'Eustachio P., Mintz B. Cellular DNA rearrangements and early developmental arrest caused by DNA insertion in transgenic mouse embryos. Mol Cell Biol. 1987 Jun;7(6):2243–2247. doi: 10.1128/mcb.7.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W., Pravtcheva D., Poorman P. A., Moses M. J., Brock W. A., Ruddle F. H. Association of foreign DNA sequence with male sterility and translocation in a line of transgenic mice. Somat Cell Mol Genet. 1989 Nov;15(6):569–578. doi: 10.1007/BF01534918. [DOI] [PubMed] [Google Scholar]
- Handel M. A. Genetic control of spermatogenesis in mice. Results Probl Cell Differ. 1987;15:1–62. doi: 10.1007/978-3-540-47184-4_1. [DOI] [PubMed] [Google Scholar]
- Hekman A. C., Trapman J., Mulder A. H., van Gaalen J. L., Zwarthoff E. C. Interferon expression in the testes of transgenic mice leads to sterility. J Biol Chem. 1988 Aug 25;263(24):12151–12155. [PubMed] [Google Scholar]
- Hoggan M. D., Halden N. F., Buckler C. E., Kozak C. A. Genetic mapping of the mouse c-fms proto-oncogene to chromosome 18. J Virol. 1988 Mar;62(3):1055–1056. doi: 10.1128/jvi.62.3.1055-1056.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary R., Clapoff S., Brown A., Campbell R., Peterson A., Rossant J. A transgene containing lacZ inserted into the dystonia locus is expressed in neural tube. Nature. 1988 Sep 29;335(6189):435–437. doi: 10.1038/335435a0. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Lawrence J. B., Ruddle F. H. A sequential staining technique for the chromosomal analysis of the interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp Cell Res. 1977 Mar 1;105(1):109–117. doi: 10.1016/0014-4827(77)90156-2. [DOI] [PubMed] [Google Scholar]
- Krulewski T. F., Neumann P. E., Gordon J. W. Insertional mutation in a transgenic mouse allelic with Purkinje cell degeneration. Proc Natl Acad Sci U S A. 1989 May;86(10):3709–3712. doi: 10.1073/pnas.86.10.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhler J., Timpl R., Jaenisch R. Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell. 1984 Sep;38(2):597–607. doi: 10.1016/0092-8674(84)90514-2. [DOI] [PubMed] [Google Scholar]
- MacGregor G. R., Mogg A. E., Burke J. F., Caskey C. T. Histochemical staining of clonal mammalian cell lines expressing E. coli beta galactosidase indicates heterogeneous expression of the bacterial gene. Somat Cell Mol Genet. 1987 May;13(3):253–265. doi: 10.1007/BF01535207. [DOI] [PubMed] [Google Scholar]
- Mark W. H., Signorelli K., Lacy E. An insertional mutation in a transgenic mouse line results in developmental arrest at day 5 of gestation. Cold Spring Harb Symp Quant Biol. 1985;50:453–463. doi: 10.1101/sqb.1985.050.01.057. [DOI] [PubMed] [Google Scholar]
- McNeish J. D., Scott W. J., Jr, Potter S. S. Legless, a novel mutation found in PHT1-1 transgenic mice. Science. 1988 Aug 12;241(4867):837–839. doi: 10.1126/science.3406741. [DOI] [PubMed] [Google Scholar]
- OAKBERG E. F. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat. 1956 Nov;99(3):391–413. doi: 10.1002/aja.1000990303. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Lai S. P., Van Quill K. R., Westphal H. Tissue-specific expression in transgenic mice of a fused gene containing RSV terminal sequences. Science. 1986 Mar 28;231(4745):1574–1577. doi: 10.1126/science.3006249. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkert C. A., Widera G., Cowing C., Heber-Katz E., Palmiter R. D., Flavell R. A., Brinster R. L. Tissue-specific, inducible and functional expression of the E alpha d MHC class II gene in transgenic mice. EMBO J. 1985 Sep;4(9):2225–2230. doi: 10.1002/j.1460-2075.1985.tb03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel L., Burguet S. Ultrastructure of leydig cells as revealed by secondary tissue treatment with a ferrocyanide-osmium mixture. Tissue Cell. 1977;9(4):751–766. doi: 10.1016/0040-8166(77)90040-4. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Gridley T., Jaenisch R. Retroviruses and insertional mutagenesis in mice: proviral integration at the Mov 34 locus leads to early embryonic death. Genes Dev. 1987 Jun;1(4):366–375. doi: 10.1101/gad.1.4.366. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Covarrubias L., Stewart T. A., Mintz B. Prenatal lethalities in mice homozygous for human growth hormone gene sequences integrated in the germ line. Cell. 1983 Dec;35(3 Pt 2):647–655. doi: 10.1016/0092-8674(83)90097-1. [DOI] [PubMed] [Google Scholar]
- Zeller R., Jackson-Grusby L., Leder P. The limb deformity gene is required for apical ectodermal ridge differentiation and anteroposterior limb pattern formation. Genes Dev. 1989 Oct;3(10):1481–1492. doi: 10.1101/gad.3.10.1481. [DOI] [PubMed] [Google Scholar]