Abstract
Starch synthesis in leaves was increased by phosphate starvation or by treatments which decreased cytoplasmic orthophosphate levels (such as mannose feeding). Usually less than 30% of the total carbon fixed during CO2 assimilation was incorporated into starch in spinach (Spinacia oleracea L.), spinach beet (Beta vulgaris), and tobacco (Nicotiana tabacum) leaves.
In isolated spinach chloroplasts, formation of starch from CO2 was usually less than in leaves. In the absence of significant levels of 3-phosphoglycerate, concentrations of phosphate as low as 1 mm (in the medium) or 10 mm (in the stroma) almost completely inhibited starch synthesis. The inhibitory action of phosphate could be overcome by 3-phosphoglycerate. The controlling factor of starch synthesis appeared to be the ratio of phosphoglycerate to orthophosphate rather than the stromal hexose monophosphate concentration, and it is suggested that this control is exerted via the phosphate translocator and the known allosteric regulation of ADP-glucose pyrophosphorylase. Starch synthesis was also favored by the presence of dihydroxyacetone phosphate and by high light and high temperature. Oxygen was inhibitory, probably owing to carbon drain into glycolate. Starch formation by intact chloroplasts could not be promoted by added glucose or glucose 6-phosphate.
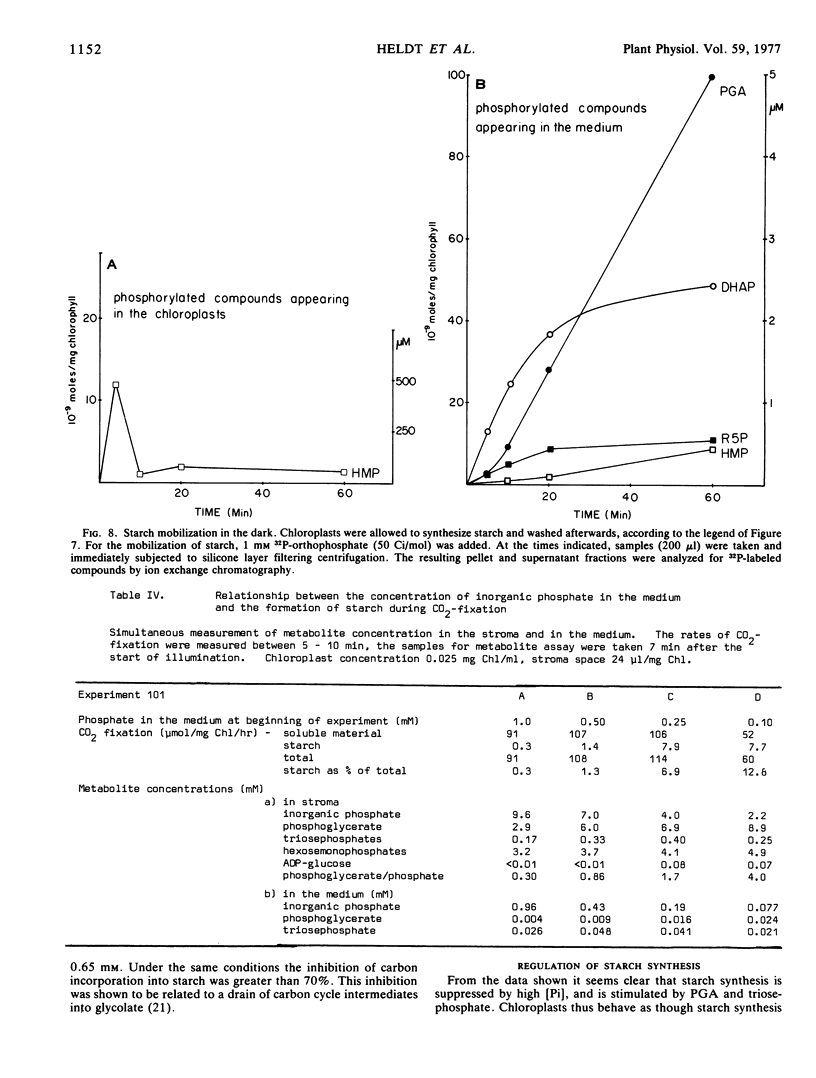
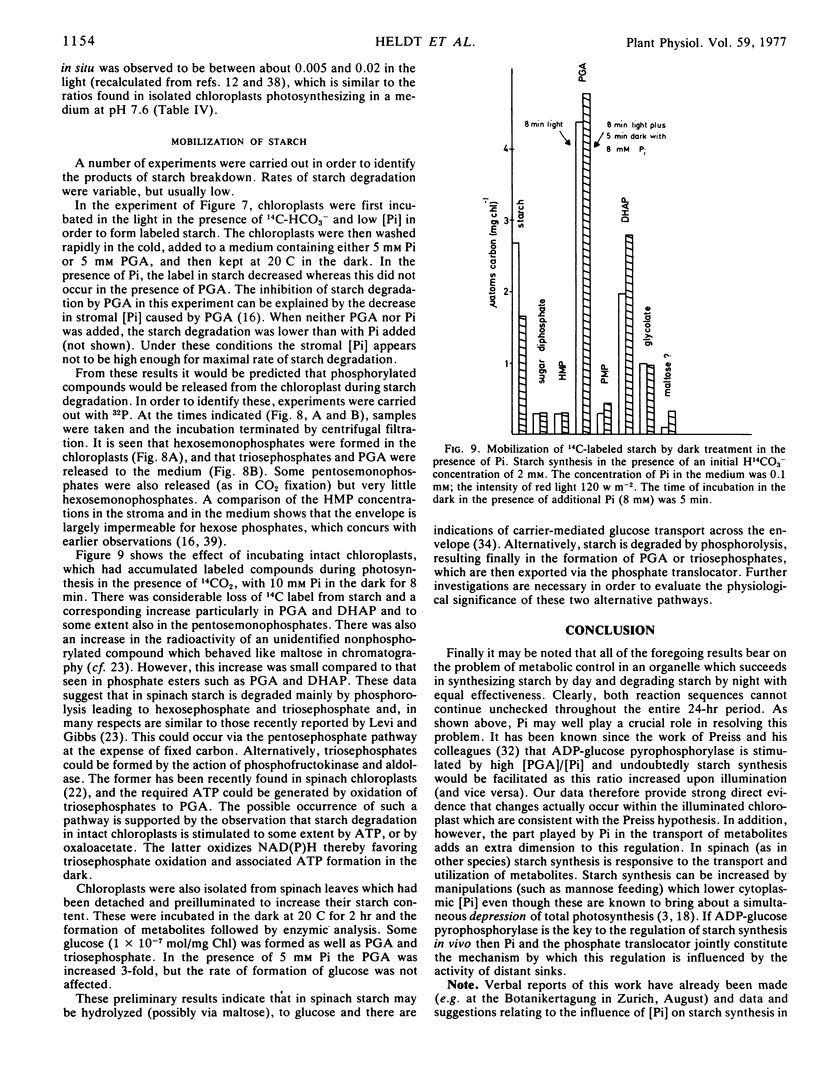
Starch mobilization in the dark was promoted by orthophosphate and phosphate-dependent mobilization was inhibited by phosphoglycerate. The principal products of starch breakdown in the presence of phosphate were the transport metabolites dihydroxyacetone phosphate and 3-phosphoglycerate. Formation of these compounds from starch was stimulated by ATP or oxaloacetate. In a phosphate-independent reaction, starch was also converted to neutral products such as maltose and glucose. The rates of phosphate-dependent starch degradation phosphorolysis were very much higher than those of starch hydrolysis for which there was no phosphate requirement.
Full text
PDF

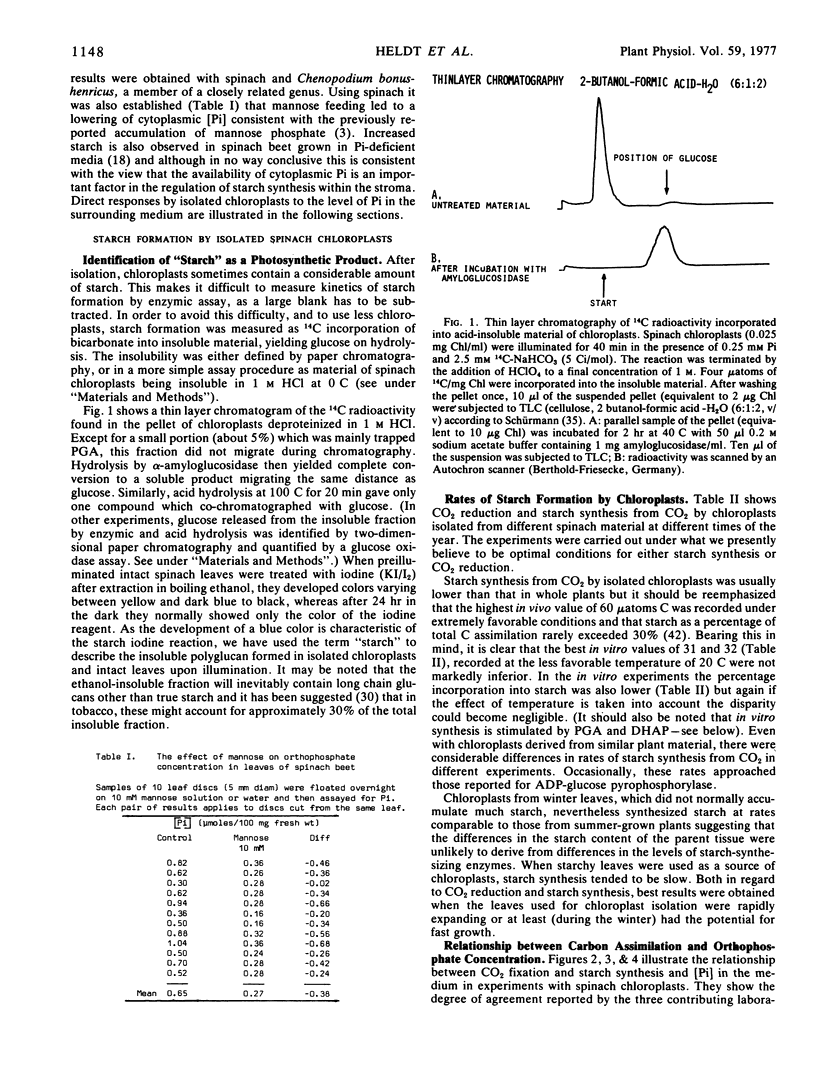
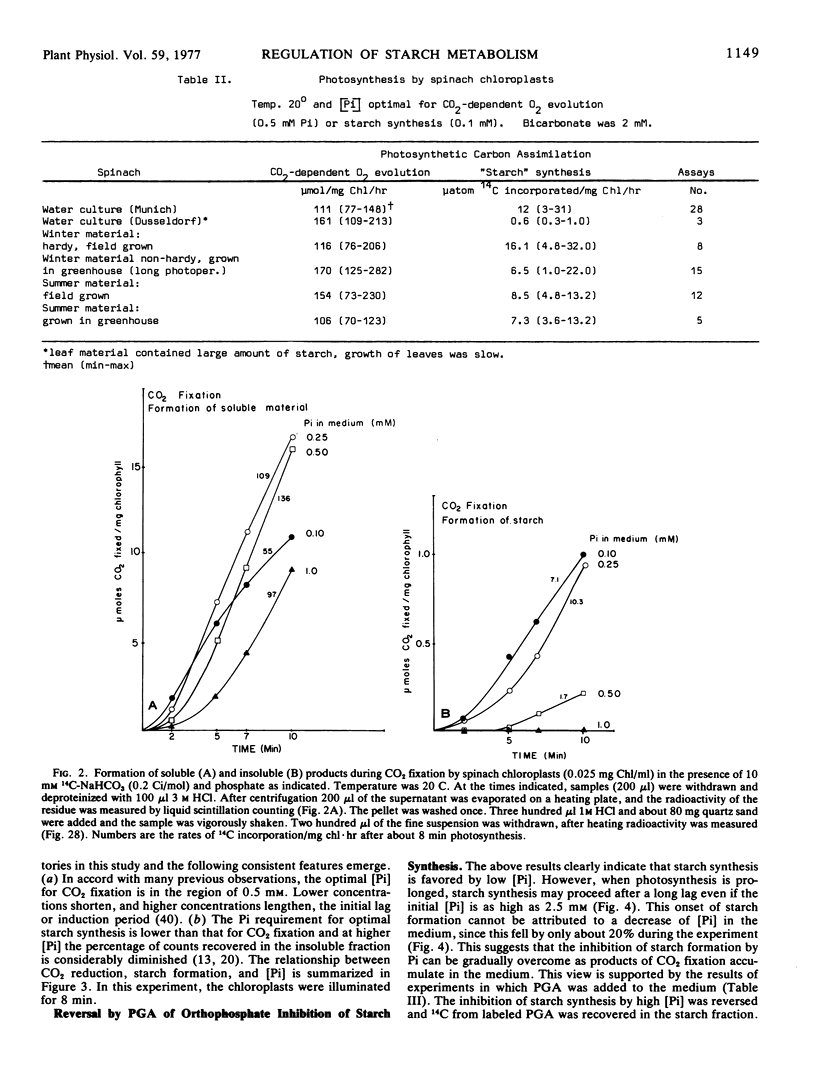
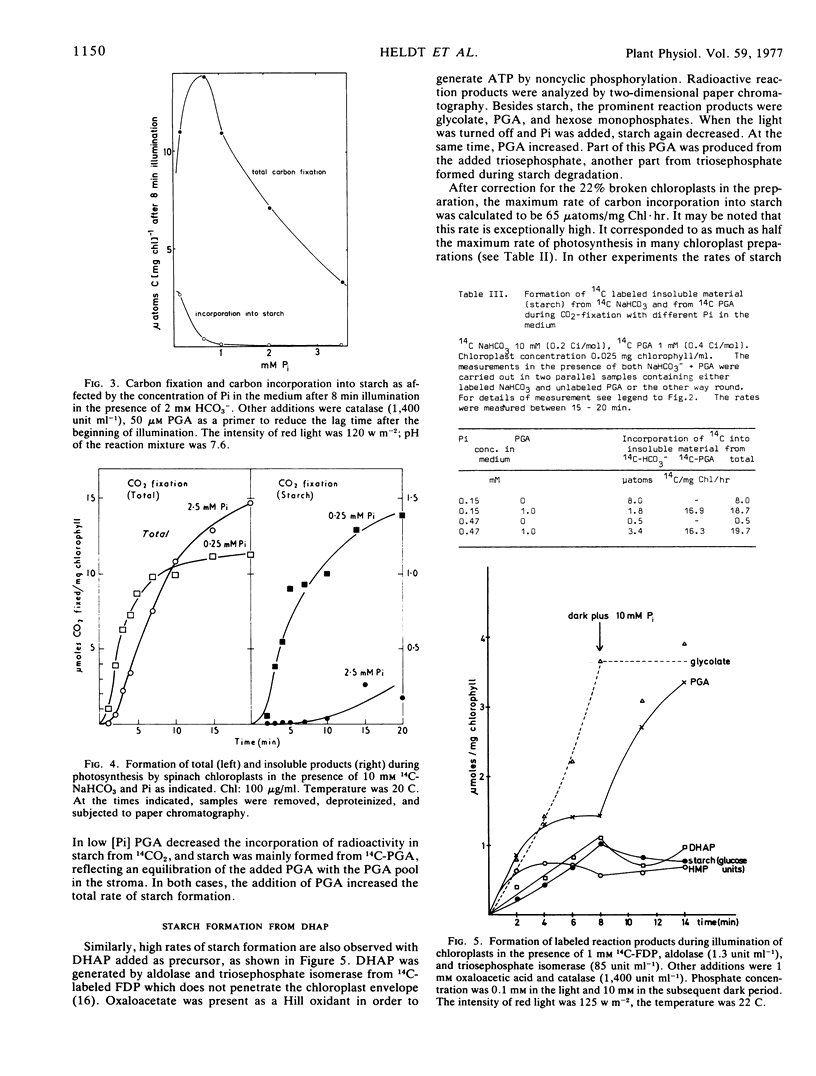
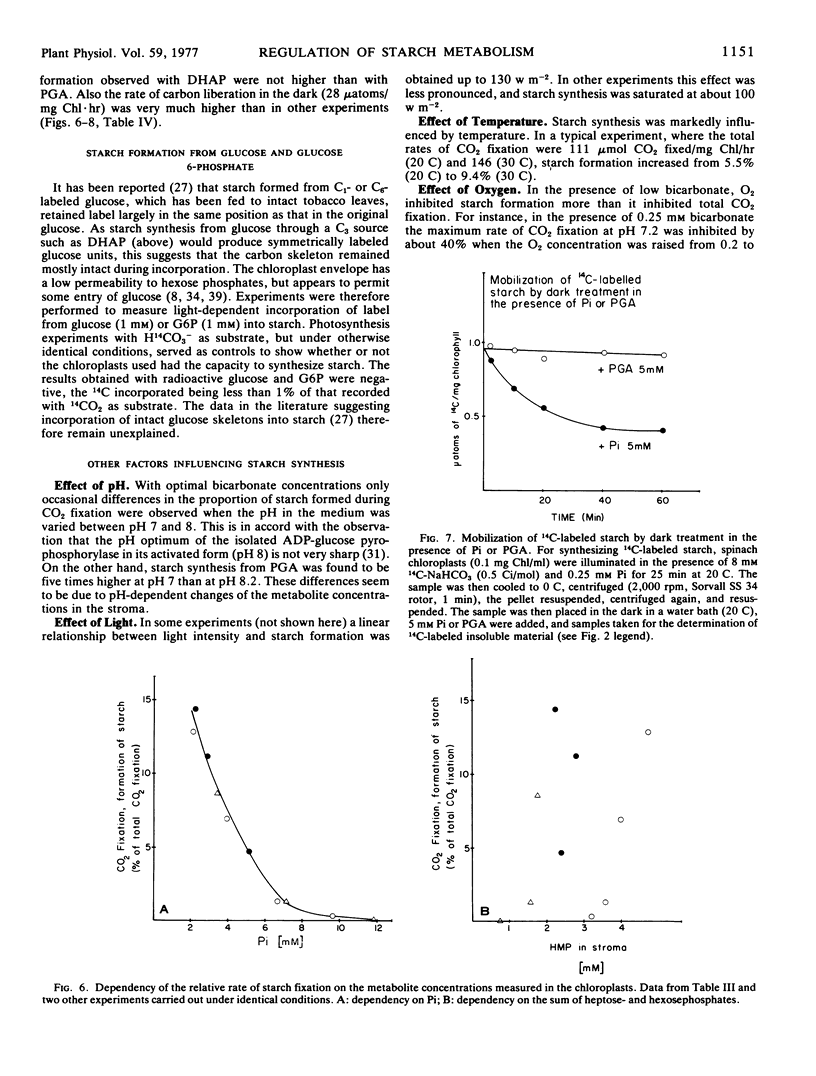


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A., Hudson M. A., Hallier U. W. Untersuchungen zur intrazellulären Verteilung von Enzymen und Substraten in der Blattzelle. I Intrazellulärer Transport von Zwischenprodukten der Photosynthese im Photosynthese-Gleichgewicht und im Dunkel-Licht-Dunkel-Wechsel. Z Naturforsch B. 1967 Nov;22(11):1189–1199. [PubMed] [Google Scholar]
- Heber U. Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta. 1973 Apr 27;305(1):140–152. doi: 10.1016/0005-2728(73)90239-9. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi C., Gibbs M. Starch degradation in isolated spinach chloroplasts. Plant Physiol. 1976 Jun;57(6):933–935. doi: 10.1104/pp.57.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975 Jun;55(6):1087–1092. doi: 10.1104/pp.55.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- MACLACHLAN G. A., PORTER H. K. Replacement of oxidation by light as the energy source for glucose metabolism in tobacco leaf. Proc R Soc Lond B Biol Sci. 1959 Sep 1;150:460–473. doi: 10.1098/rspb.1959.0035. [DOI] [PubMed] [Google Scholar]
- Ozbun J. L., Hawker J. S., Preiss J. Soluble adenosine diphosphate glucose- -1,4-glucan -4-glucosyltransferases from spinach leaves. Biochem J. 1972 Feb;126(4):953–963. doi: 10.1042/bj1260953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T. A., Kirk M., Bassham J. A. Inhibition of photophosphorylation and photosynthetic carbon cycle reactions by fatty acids and esters. Biochim Biophys Acta. 1966 Feb 7;112(2):189–203. doi: 10.1016/0926-6585(66)90320-7. [DOI] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Schürmann P. Separation of phosphate esters and algal extracts by thin-layer electrophoresis and chromatography. J Chromatogr. 1969 Feb 25;39(4):507–509. [PubMed] [Google Scholar]
- Slabas A. R., Walker D. A. Localization of inhibition by adenosine diphosphate of phosphoglycerate-dependent oxygen evolution in a reconstituted chloroplast system. Biochem J. 1976 Jan 15;154(1):185–192. doi: 10.1042/bj1540185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steup M., Peavey D. G., Gibbs M. The regulation of starch metabolism by inorganic phosphate. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1554–1561. doi: 10.1016/s0006-291x(76)80191-x. [DOI] [PubMed] [Google Scholar]
- Urbach W., Hudson M. A., Ullrich W., Santarius K. A., Heber U. Verteilung und Wanderung von Phosphoglycerat zwischen den Chloroplasten und dem Cytoplasma während der Photosynthese. Z Naturforsch B. 1965 Sep;20(9):890–898. [PubMed] [Google Scholar]


