Abstract
Pain is often associated with clinically relevant depression of behavior and mood, and relief of pain-related depression is a common goal of treatment in both human and veterinary medicine. In the development of pharmacological compounds to treat pain and related depression, preclinical studies may be used to evaluate the analgesic potential of new drugs. Such studies require reliable, accurate assays of pain-related behavioral depression in animals. Intracranial self-stimulation (ICSS) is a type of operant conditioning procedure that produces stable baseline behavioral response rates. The author reviews recent research on the use of ICSS to evaluate the expression and pharmacological modulation of pain-related behavioral depression in rats. Results suggest that assays of pain-depressed behavior using ICSS may serve as a useful new tool to improve the translation of preclinical findings to clinical results in analgesic drug development.
The treatment of pain is a clinical priority in both human and veterinary medicine, and as with any disorder, effective treatment begins with accurate diagnosis. Pain has been defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”1, and its presence is inferred from behavior. In verbally competent humans, pain is typically communicated with verbal behavior guided by numerical or visual-analog rating scales2, but in nonverbal humans (e.g., infants) or in nonhuman animals, pain must be inferred from other behaviors3–5. Behaviors used to diagnose pain in nonverbal organisms are identified as those elicited by noxious stimuli that can or do produce tissue damage and that do produce verbal reports of pain in humans. For research purposes, these pain-related behaviors can be categorized into at least two general classes: pain-stimulated behaviors and pain-depressed behaviors6,7 (Table 1).
TABLE 1.
Attributes of pain-stimulated and pain-depressed behaviors
| Pain-stimulated behavior | Pain-depressed behavior | |
|---|---|---|
| Definition | Behaviors that increase in rate, frequency or intensity after presentation of a noxious stimulus | Behaviors that decrease in rate, frequency or intensity after presentation of a noxious stimulus |
| Examples | Reflex withdrawal responses from escapable stimuli (e.g., paw withdrawal response from a noxious thermal stimulus) | Decreases in feeding, grooming, locomotor activity, social interactions or positively reinforced operant behavior after presentation of a noxious stimulus |
| Withdrawal-like behaviors from inescapable stimuli (e.g., paw flinching after intraplantar injection of a noxious chemical stimulus) | ||
| Adaptive functions | Promotes escape from noxious stimuli | Conserves energy for wound protection and healing |
| Recruits aid from conspecifics | Reduces potential of further injury | |
| Manifestation of analgesia | Decreases in pain-stimulated behaviors | Increases in pain-depressed behaviors |
| Potential sources of false positive analgesia | Treatments that produce sedation, paralysis or other forms of motor impairment | Treatments that produce non-selective stimulation of behavior |
PAIN-RELATED BEHAVIORAL DEPRESSION
Preclinical research on the neurobiology and treatment of pain has for decades relied almost exclusively on the assessment of pain-stimulated behaviors6. Recently, several factors have contributed to a growing interest in the use of pain-depressed behaviors as experimental endpoints in preclinical research. First, recent failures in analgesic drug development have eroded confidence in the translational validity of traditional assays of pain-stimulated behavior and promoted consideration of alternative preclinical pain models6,8–12. Second, pain is commonly associated with functional impairment and behavioral depression in both human and veterinary medicine. In humans, this depression of behavior is also often accompanied by comorbid depression of mood13–15. Moreover, signs of behavioral depression are often used clinically both to diagnose the presence of pain and to evaluate the efficacy of analgesic drugs or other treatments. In humans, for example, pain-related depression of behavior can be assessed using instruments such as the Brief Pain Inventory, which scores the impact of pain states on behaviors such as walking, working or social interaction16,17. In animals, pain-related depression of behavior can similarly be assessed by measures of locomotor activity, feeding or social interactions with conspecifics5,18. The clinical utility of assessments of pain-depressed behavior in the diagnosis and treatment of pain suggests that such measures might also be useful for translational research on the expression, neurobiology and treatment of pain. Third, apparent analgesic effects on pain-stimulated behavior may be confounded by drug effects that cause motor impairment19, producing false positive results. In a conventional assay of pain-stimulated behavior, for example, pain is indicated by the stimulation of a target behavior, such as paw withdrawal from a noxious stimulus, and analgesia is indicated by an inhibition of the target behavior, such as slower paw withdrawal. However, such behavioral inhibition can be produced not only by a reduction in sensory sensitivity to the noxious stimulus (i.e., true analgesia) but also by motor effects that impair the ability of the subject to respond (a false positive result). Conversely, in an assay of pain-depressed behavior, pain is indicated by depression of a target behavior, such as locomotor activity, and analgesia is indicated by recovery of the target behavior, such as increased locomotor activity. Hence, motor impairment does not produce a false positive result in assays of pain-depressed behavior and may instead exacerbate pain-related depression of behavior7,19.
OPERANT PROCEDURES IN PAIN RESEARCH
In clinical practice, diagnosis of pain-related depression of behavior typically focuses on unconditioned and overt behaviors such as locomotion, feeding or social interaction and often depends on subjective clinical assessment rooted in familiarity with the normal behavioral routines of a particular subject3,5. The same behavioral endpoints can also be used in research. However, research environments also enable the use of operant conditioning procedures, in which subjects are trained to express a target behavior (e.g., pressing a lever for food), and expression of that behavior is then used to assess pain states or analgesic effects of candidate drugs20–22. The use of operant procedures in research on pain-depressed behavior requires an initial investment in both equipment and animal training but has several advantages. First, operant procedures can generate high behavioral response rates that are stable over time within a given subject, thereby providing a sensitive baseline for detection of within-subject effects produced by experimental manipulations. Second, experimental parameters can be adjusted to generate uniform rates and patterns of behavior across subjects, thereby facilitating comparison of effects produced by experimental manipulations across subjects. Third, the target behavior can be measured objectively, quantitatively and remotely using commercially available equipment, thereby reducing error and variability associated with subjective and qualitative pain metrics. Finally, the target behavior can be reliably elicited and probed at the experimenter’s convenience, thereby permitting precise temporal control over presentation of experimental manipulations and measurement of behavior.
INTRACRANIAL SELF-STIMULATION AS AN OPERANT PROCEDURE IN PAIN RESEARCH
A common example of operant conditioning involves the training of laboratory rats to press a lever in order to earn food pellets. An early study reported that laparotomy produced a morphine-reversible depression of food-maintained operant responding in rats23. Food is thought to function as a reinforcer by activating brain reward pathways. Direct electrical stimulation of these pathways by chronically implanted electrodes can also act as a reinforcer to maintain lever-press responses in rats in a procedure known as intracranial self- stimulation (ICSS; Fig. 1)24,25. In the ICSS procedure, rats are implanted with an electrode that targets a fiber tract called the medial forebrain bundle, which provides excitatory inputs to ‘mesolimbic’ dopamine neurons that originate in the ventral tegmental area of the midbrain and project to rostral limbic areas including the nucleus accumbens. The electrode is connected by a cable to a stimulator. The timing and magnitude of stimulation can be precisely controlled, and contingencies are established such that lever-pressing delivers brief pulses of brain stimulation via the electrode. Rats readily learn to press a lever to receive brain stimulation under these conditions (Fig. 1a), and the rate of lever-pressing varies as a function of the magnitude of the brain stimulation (Fig. 1b). ICSS has a long history of use in research on behavioral effects of drugs26–29 and has recently been appropriated to study the expression and pharmacological modulation of pain7,30.
FIGURE 1.

Intracranial self-stimulation (ICSS) is an operant conditioning procedure that produces stable baseline behavioral response rates for research on pain-related behavioral depression. (a) A rat equipped with an intracranial electrode that targets the brain reward pathway. During experimental sessions, the rat is placed into an operant conditioning chamber, and the electrode is connected by a cable to a stimulator (not shown). Pressing a lever in the chamber illuminates a set of stimulus lights located over the lever and activates the stimulator to deliver pulses of brain stimulation via the electrode. Rats readily learn to press the lever for brain stimulation reinforcement. (b) Illustrative baseline ‘frequency-rate’ curve for ICSS response rates. ICSS response rate (normalized and expressed as a percentage of the maximum control rate, %MCR) increases with increases in the magnitude of brain stimulation reinforcement (expressed on a log scale as the frequency of electrical stimulation in Hz). Data adapted from ref. 31 and represent the mean ± s.e.m. of values from 34 rats.
Specifically, ICSS can be used to assess pain-related behavioral depression in rats31. In one traditional assay of pain-stimulated behavior, a putative pain state is induced by intraperitoneal (i.p.) injection of dilute acid. Tissue acidification is a common component of many inflammatory pain states, and simulation of this state by injection of dilute acid is commonly used in preclinical pain research to stimulate a stretching or writhing behavior, inhibition of which is indicative of analgesia6,32,33. In addition to stimulating stretching or writhing behavior, however, i.p. acid administration also depresses many behaviors including feeding, locomotor activity and ICSS response rate19,30,34. Intraperitoneal administration of 1.8% lactic acid at a dosage of 1 ml per kg body weight produces a depression of lever-press response rates across a range of brain stimulation magnitudes (Fig. 2a) and a decrease in the total response rate summed across all brain stimulation magnitudes (Fig. 2b). Other studies have shown that acid-induced depression of lever-press response rate is concentration-dependent, transient (lasting ~1 h) and replicable at weekly intervals19,30. These features render i.p. acid administration useful as an experimental noxious stimulus in preclinical studies of the expression and pharmacological modulation of pain-depressed behavior using the ICSS procedure.
FIGURE 2.

Pain-related depression of ICSS response rate. (a) Intraperitoneal injection of 1.8% lactic acid at a dosage of 1 ml per kg body weight serves as a visceral noxious stimulus that depresses ICSS response rate across a range of brain stimulation magnitudes. Response rate was significantly lower (#P < 0.05 by two-way analysis of variance followed by Holm-Sidak post hoc test) after administration of acid than after administration of saline vehicle at a dosage of 1 ml per kg body weight (LA vehicle). (b) Administration of lactic acid significantly reduced total ICSS response rate across all stimulation magnitudes (expressed as a percentage of baseline ICSS rate determined before administration of vehicle or lactic acid; #P < 0.05) compared with administration of saline vehicle (LA vehicle). Data adapted from ref. 31 and represent the mean ± s.e.m. of values from 34 rats.
An emerging body of research is assessing the effects of other commonly used noxious stimuli on lever-press response rate in ICSS. ICSS response rate is depressed by noxious stimuli with prominent inflammatory components including intraplantar administration of formalin (M. Leitl and S.S.N., unpublished observations), surgical incision of the hindpaw (E. Ewan and T. Martin, personal communication) and systemic administration of lipopolysaccharide (ref. 35 and M. Leitl and S.S.N., unpublished observations). Conversely, ICSS response rate is not depressed by spinal nerve ligation (ligation of the fifth and sixth lumbar nerves), a procedure commonly used to model neuropathic pain in rats36. Other studies have found weak or negligible effects of common neuropathic manipulations on other measures of functional impairment or pain-related behavioral depression in rodents, such as locomotor activity, feeding, anxiety-related behavior and pain-related facial expression37–39.
EVALUATION OF CLINICALLY EFFECTIVE ANALGESICS USING ICSS
The sensitivity of the depression of ICSS response rate produced by i.p. acid injection to pharmacological modulation can be evaluated as an indicator of analgesic efficacy. For example, the effects of ketoprofen, a nonsteroidal anti-inflammatory drug (NSAID)40, on ICSS response rate can be assessed in the presence and absence of i.p. acid administration (Fig. 3). In the absence of the noxious stimulus, ketoprofen has no effect on the ICSS response rate (Fig. 3a), but in the presence of the noxious stimulus, pretreatment with 1.0 mg ketoprofen per kg body weight blocks the acid-induced depression of ICSS response rate (Fig. 3b,c). These results are in agreement with those of other studies showing that NSAIDs block other forms of pain-related behavioral depression (Table 2) and have clinical efficacy in treating some types of pain. Similar results have been reported for μ-opioid agonists, the second major class of clinically effective analgesic drugs, including morphine, methadone, hydrocodone and buprenorphine30,41. These drugs dose-dependently block acid-induced depression of ICSS response rate while having little or no effect on response rate in the absence of the noxious stimulus30,41.
FIGURE 3.

Ketoprofen blocks acid-induced depression of ICSS response rate. In the absence of lactic acid (LA vehicle), ketoprofen had no effect on ICSS response rate (a), but in the presence of lactic acid, pretreatment with 1.0 mg ketoprofen per kg body weight blocked the acid-induced depression of ICSS response rate (b) across a range of stimulation magnitudes (*P < 0.05). At a relatively high stimulation magnitude of 141 Hz, ketoprofen blocked acid-induced depression of ICSS response rate (thick arrow in b) but had no effect on response rate in the absence of lactic acid (thick arrow in a). However, these data at 141 Hz are not sufficient to show a lack of ketoprofen effect in the absence of acid, because baseline ICSS response rate at this stimulation magnitude was near maximal in the absence of acid and could not be further increased. Consequently, it is important to note that ketoprofen also failed to increase the lower baseline ICSS response rate maintained by a lower stimulation magnitude of 112 Hz in the absence of lactic acid (thin arrow in a). (c) Lactic acid significantly depressed total ICSS response rate across all stimulation magnitudes (#P < 0.05), and ketoprofen significantly blocked the acid-induced depression of ICSS response rate (*P < 0.05). Data adapted from ref. 40 and represent the mean ± s.e.m. of values from 4 rats.
TABLE 2.
Efficacy of NSAIDs in preclinical assays of pain-depressed behavior
| Species | Pain manipulation | Behavioral endpoint | NSAIDs effective? | References |
|---|---|---|---|---|
| Mouse | Vasectomy | Home cage activity | Partial | 54 |
| Mouse | Intraplantar injection of CFA | Wheel running | Yes | 55 |
| Rat | Laparotomy | Food-maintained operant responding | Yes | 23 |
| Rat | Intra-articular injection of CFA | Rearing | Yes | 56 |
| Rat | Intraperitoneal injection of lactic acid | ICSS | Yes | 31,40 |
| Dog | Intra-articular injection of formalin | Locomotion | Yes | 57 |
CFA, complete Freund’s adjuvant.
EVALUATION OF CANDIDATE ANALGESICS USING ICSS
Results of ICSS experiments with ketoprofen and μ-opioid agonists validate the use of acid-induced depression of ICSS response rate to model pain-depressed behavior and its sensitivity to analgesic drugs. Hence, ICSS assays can be used as a research tool in the assessment of candidate analgesic drugs undergoing development, as illustrated by the following three examples. The experimental compound SNC80 is an agonist of the δ-opioid receptor42. Like the NSAID ketoprofen, SNC80 has little effect on ICSS response rate in the absence of i.p. acid but dose-dependently blocks acid-induced depression of ICSS response rate (Fig. 4a). These results support other data suggesting that δ-opioid agonists warrant further consideration as candidate analgesic drugs.
FIGURE 4.
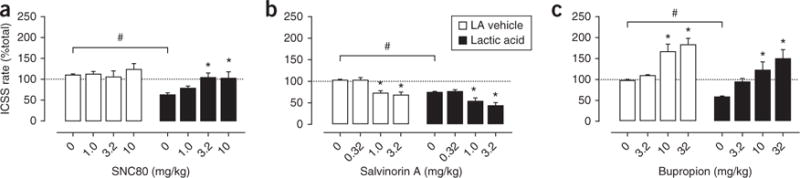
Effects of candidate analgesic compounds on acid-induced depression of ICSS response rate. (a) Like ketoprofen, SNC80 selectively blocked acid-induced depression of ICSS response rate. Thus, SNC80 had little effect on total ICSS response rate in the absence of lactic acid but dose-dependently blocked acid-induced depression of total ICSS response rate. (b) Salvinorin A non-selectively decreased ICSS response rate. Thus, salvinorin A decreased total ICSS response rate in the absence of lactic acid and also exacerbated acid-induced depression of ICSS response rate. (c) Bupropion non-selectively increased ICSS response rate. Thus, bupropion blocked acid-induced depression of total ICSS response rate, but only at doses that also increased total ICSS response rate in the absence of lactic acid. Significant acid-induced depression of ICSS response rate (#P < 0.05) and significant differences in ICSS response rate with a given drug dose compared with no drug in either the presence or the absence of lactic acid (*P < 0.05) are indicated. Data adapted from refs. 31, 42 and 43 and represent the mean ± s.e.m. of values from 5–8 rats.
Salvinorin A is the active constituent of the plant Salvia divinorum and a selective agonist of the κ-opioid receptor31. In contrast to NSAIDs and μ-opioid analgesics, κ-opioid agonists like salvinorin A fail to block acid-induced depression of ICSS response rate (Fig. 4b). These compounds are ineffective at relatively low doses and exacerbate acid-induced depression of ICSS response rate at higher doses, which also depress ICSS response rate in the absence of the noxious stimulus. These findings are illuminating as κ-opioid agonists including salvinorin A have produced robust antinociceptive effects in many preclinical assays of pain-stimulated behavior31 but they have not been effective in clinical studies of analgesia, and they have not been approved for the treatment of pain. Consequently, the case of κ-opioid agonists illustrates both the potential for false positive results in assays of pain-stimulated behavior and the potential for improved translation of preclinical to clinical results using assays of pain-depressed behavior.
Bupropion is a dopamine reuptake inhibitor indicated clinically for the treatment of depression43. Bupropion dose-dependently blocks acid-induced depression of ICSS response rate, but only at doses that also increase ICSS response rate in the absence of the noxious stimulus (Fig. 4c). Cocaine, another compound with prominent activity as a dopamine reuptake inhibitor, has similar effects on ICSS response rate in the absence or presence of acid31. These findings raise the possibility that the apparent antinociceptive effects of dopamine reuptake inhibitors in the ICSS procedure may result from non-selective stimulation of behavior rather than a selective blockade of sensory sensitivity to the noxious stimulus. However, studies in humans have reported analgesic effects of dopamine reuptake inhibitors and related pharmacological and nonpharmacological treatments that increase activity of dopaminergic brain reward systems44–49. This suggests that blockade of dopamine reuptake and enhanced dopaminergic neurotransmission may be associated with clinically relevant analgesia in addition to more general behavioral stimulation. Although there are clearly many circumstances when analgesia coupled with behavioral stimulation is counterproductive (e.g., when increased activity might increase risk for further injury), we have suggested that dopamine reuptake inhibitors may warrant further consideration as treatments for pain when some level of behavioral stimulation is also desirable (e.g., as an adjunct to clinically supervised physical therapy in humans)43.
EVALUATION OF ABUSE LIABILITY USING ICSS
The use of ICSS to study the expression and pharmacological modulation of pain-depressed behavior also resonates with a much larger and older literature on the use of ICSS to study the neurobiology of motivation. For example, the use of opioid analgesics to treat pain is constrained in part by their abuse liability50. Accordingly, one goal of analgesic drug development has been to develop safe analgesics that lack the abuse liability of opioids, and pursuit of this goal requires experimental tools to assess abuse-related drug effects. ICSS is one such tool. Many prominent drugs of abuse, including stimulants (e.g., cocaine and amphetamine) and μ-opioid agonists (e.g., morphine under some conditions), increase or ‘facilitate’ ICSS response rate; such effects are often interpreted as evidence of abuse liability26,27,29,50. By extension, comparison of drug effects on ICSS response rate in the absence and presence of pain can be used to draw inferences not only about the behavioral selectivity of drugs but also about their abuse liability. For example, bupropion facilitates ICSS response rate in the absence of a noxious stimulus (Fig. 4c), whereas SNC80 does not (Fig. 4a). These data could be interpreted to suggest that bupropion has higher abuse liability than SNC80. This conclusion is consistent with other preclinical evidence suggesting a higher abuse liability for bupropion than SNC80; for example, bupropion maintains self-administration in assays of intravenous drug self-administration, whereas SNC80 does not51,52.
ICSS EXPERIMENTAL DESIGN AND DATA INTERPRETATION
The bupropion example emphasizes the importance of experimental design and data interpretation in ICSS assays of pain-depressed behavior in order to evaluate the potential for false positive effects. False positive effects in assays of pain-depressed behavior can result from test drugs that produce non-selective stimulation of behavior rather than true analgesia. This issue can be addressed experimentally by comparing the effects of the test drug on the target behavior in both the presence and the absence of the noxious stimulus, as described in the examples above (Figs. 3 and 4). Analgesia is inferred if pain-related behavioral depression can be blocked by drug doses that do not affect behavior in the absence of pain. A subtle vulnerability to this approach is revealed in cases where the target behavior occurs at a high, near-maximal rate in the absence of pain. In such cases, the target behavior may be difficult to stimulate further, and hence, the lack of a drug-induced increase in behavior in the absence of pain may be misleading. This vulnerability can be mitigated by examining behavior that can be stimulated at both low and high baseline rates in the absence of pain. ICSS is ideal for this purpose. The magnitude of the brain-stimulation reinforcer can be rapidly and efficiently manipulated across a range of values to generate a range of baseline response rates in the absence of pain (Figs. 1–3). High ICSS response rates maintained by high brain-stimulation magnitudes can then be used to examine depressant effects of pain and blockade of these depressant effects by candidate analgesics, whereas low ICSS response rates maintained by low stimulation magnitudes can be used to examine the degree to which candidate analgesics increase ICSS response rate in the absence of pain. The utility of this approach is illustrated by the experiment with ketoprofen (Fig. 3). At a relatively high stimulation magnitude of 141 Hz, ketoprofen completely blocks the acid-induced depression of ICSS response rate (Fig. 3b) but has no effect on response rate in the absence of pain (Fig. 3a); however, the baseline ICSS response rate at this stimulation magnitude is near maximal in the absence of pain and cannot be further increased. Consequently, these data are not sufficient to conclude that ketoprofen has no effect on ICSS response rate in the absence of pain. Stronger evidence for this conclusion is obtained by examining ketoprofen effects on low ICSS response rates maintained by the lower stimulation magnitude of 112 Hz in the absence of pain (Fig. 3a). At this stimulation magnitude, the baseline ICSS response rate in the absence of pain is lower and clearly sub-maximal, but it is still not affected by ketoprofen.
In addition to false positive effects caused by non-selective behavioral stimulation, assays of pain-depressed behavior may also be vulnerable to false negative effects caused by non-selective behavioral depression. The experiment examining the pharmacological modulation of pain-related depression of ICSS response rate by the κ-opioid agonist salvinorin A illustrates the importance of considering this issue (Fig. 4b). Salvinorin A fails to block acid-induced depression of ICSS response rate, a finding that was interpreted to suggest that salvinorin A lacks analgesic effects. However, an alternative possibility is that salvinorin A produces analgesia but only at doses that also produce sedative or other effects that prevent drug-induced increases in pain-depressed behavioral responses. In the case of salvinorin A and other κ-opioid agonists, clinical data support a lack of analgesic efficacy31,53; however, general anesthesia with barbiturates or volatile anesthetics illustrates the potential for other drugs to block pain while also producing sedation that could be expected to prevent increases in pain-depressed behavioral responses. Experimental strategies to address the potential for sedative drug effects to mask analgesia can be customized to particular drugs of interest (e.g., the prototype cannabinoid receptor agonist Δ9-tetrahydrocannabinol40), but assays of pain-depressed behavior will be most useful for identifying drugs or drug doses that produce analgesia without sedation.
CONCLUSIONS
ICSS experiments have been used to evaluate the pharmacological modulation of pain-related behavioral depression by many different drugs (Table 3). Taken together, the results of these experiments suggest three major conclusions. First, acid-depressed ICSS response rate is an example of acute pain-related behavioral depression that is sensitive to two major classes of clinically effective analgesic drugs (NSAIDs and μ-opioid agonists). Second, unlike conventional assays of pain-stimulated behavior, the assay of pain-depressed ICSS response rate does not produce false positive antinociceptive results for several drug classes that are clinically ineffective for the treatment of acute pain in humans (κ-opioid agonists, dopamine receptor antagonists, cannabinoid receptor agonists). Third, ICSS can be used to generate a wide range of baseline behavioral response rates that are sensitive not only to the depressant effects of pain but also to the stimulant and potentially abuse-related effects of candidate analgesics. These three attributes suggest that acid-induced depression of ICSS response rate is a useful experimental tool in the development of safe analgesics to treat acute pain. Future applications of ICSS experiments may include research to characterize the neural mechanisms of acute visceral pain and to examine the expression and pharmacological modulation of depressant effects associated with more chronic forms of pain.
TABLE 3.
Effects of different drug classes on acid-induced depression of ICSS response rate
| Effective in blocking acid-induced depression of ICSS? | Class | Drug | References |
|---|---|---|---|
| Yes | NSAIDs | Ketoprofen | 31,40 |
| μ-opioid agonists | Morphine, methadone, fentanyl, hydrocodone, buprenorphine, nalbuphine | 30,41 | |
| δ-opioid agonists | SNC80 | 42 | |
| Dopamine reuptake inhibitors | Bupropion, cocaine, RTI112, RTI113 | 31,43 | |
| No | κ-opioid agonists | Salvinorin A, U69,593, ICI204448, ffir | 31,53 |
| μ-, δ- and κ-opioid antagonists | Naltrexone, naltrindole, norbinaltorphimine | 41,53,58 | |
| Dopamine receptor antagonists | Flupenthixol | 31 | |
| Serotonin reuptake inhibitors | Citalopram, clomipramine | 43 | |
| Norepinephrine reuptake inhibitors | Nisoxetine, nortriptyline | 43 | |
| Serotonin and norepinephrine reuptake inhibitors | Milnacipran | 43 | |
| Cannabinoid receptor agonists | Δ9-tetrahydrocannabinol, CP55940 | 40 |
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979;6:249–252. [PubMed] [Google Scholar]
- 2.Melzack R, Katz J. In: Textbook of Pain. McMahon LR, Koltzenburg M, editors. Elsevier; London, UK: 2006. pp. 291–304. [Google Scholar]
- 3.Flecknell PA. Refinement of animal use—assessment and alleviation of pain and distress. Lab Anim. 1994;28:222–231. doi: 10.1258/002367794780681660. [DOI] [PubMed] [Google Scholar]
- 4.McGrath P. In: Textbook of Pain. McMahon LR, Koltzenburg M, editors. Elsevier; London, UK: 2006. pp. 305–315. [Google Scholar]
- 5.National Research Council. Recognition and Alleviation of Pain in Laboratory Animals. National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 6.Negus SS, et al. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 7.Negus SS, Bilsky EJ, Pereira Do Carmo G, Stevenson GW. In: Analgesia: Methods and Protocols. Szallasi A, editor. Humana; New York: 2010. pp. 79–91. [Google Scholar]
- 8.Blackburn-Munro G. Pain-like behaviours in animals—how human are they? Trends Pharmacol Sci. 2004;25:299–305. doi: 10.1016/j.tips.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Vierck CJ, Hansson PT, Yezierski RP. Clinical and preclinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Whiteside GT, Adedoyin A, Leventhal L. Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology. 2008;54:767–775. doi: 10.1016/j.neuropharm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 12.Mao J. Current challenges in translational pain research. Trends Pharmacol Sci. 2012;33:568–573. doi: 10.1016/j.tips.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70:890–897. doi: 10.1097/PSY.0b013e318185c510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldenberg DL. Pain/Depression dyad: a key to a better understanding and treatment of functional somatic syndromes. Am J Med. 2010;123:675–682. doi: 10.1016/j.amjmed.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 16.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 17.Dworkin RH, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 18.National Research Council. Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press; Washington, DC: 2011. [Google Scholar]
- 19.Stevenson GW, Bilsky EJ, Negus SS. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain. 2006;7:408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- 20.Ferster CB, Skinner BF. Schedules of Reinforcement. Prentice-Hall; Englewood Cliffs, NJ: 1957. [Google Scholar]
- 21.Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- 22.Skinner BF. The experimental analysis of behavior. American Scientist. 1957;45:343–371. [Google Scholar]
- 23.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 24.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 25.Stellar JR, Stellar E. The Neurobiology of Motivation and Reward. Springer; New York: 1985. [Google Scholar]
- 26.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 27.Reid LD. In: Methods of Assessing the Reinforcing Properties of Abused Drugs. Bozarth MA, editor. Springer; Berlin, Germany: 1987. pp. 391–420. [Google Scholar]
- 28.Stellar JR, Rice MB. In: The Neuropharmacological Basis of Reward. Liebman JM, Cooper SJ, editors. Oxford University Press; New York: 1989. pp. 14–65. [Google Scholar]
- 29.Vlachou S, Markou A. In: Animal Models of Drug Addiction. Olmstead MC, editor. Humana; New York: 2011. pp. 3–56. [Google Scholar]
- 30.Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC. Effects of peripherally restricted κ opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther. 2012;340:501–509. doi: 10.1124/jpet.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendershot LC, Forsaith J. Antagonism of the frequency of phenylquinone-induced writhing in the mouse by weak analgesics and nonanalgesics. J Pharmacol Exp Ther. 1959;125:237–240. [PubMed] [Google Scholar]
- 33.Mogil JS, Wilson SG, Wan Y. In: Methods in Pain Research. Kruger L, editor. CRC Press; Boca Raton, FL: 2001. pp. 11–39. [Google Scholar]
- 34.Stevenson GW, et al. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci. 2009;85:309–315. doi: 10.1016/j.lfs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leitl M, Negus SS. Role of toll-like receptor 4 in pain-depressed behavior. Society for Neuroscience; New Orleans, LA: Oct 13–17, 2012. [Google Scholar]
- 36.Ewan EE, Martin TJ. Rewarding electrical brain stimulation in rats after peripheral nerve injury: decreased facilitation by commonly abused prescription opioids. Anesthesiology. 2011;115:1271–1280. doi: 10.1097/ALN.0b013e3182330448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langford DJ, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 38.Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain. 2011;152:990–1000. doi: 10.1016/j.pain.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seminowicz DA, et al. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009;47:1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther. 2012;343:389–400. doi: 10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altarifi A, Negus SS. Effects of mu opioid receptor agonists on pain-stimulated and pain-depressed behaviors in rats. Society for Neuroscience; New Orleans, LA: Oct 13–17, 2012. [Google Scholar]
- 42.Negus SS, et al. Effects of the δ opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. J Pain. 2012;13:317–327. doi: 10.1016/j.jpain.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg M, Carroll FI, Negus SS. Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain. 2013;14:246–259. doi: 10.1016/j.jpain.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang RI, Johnson RP, Lee JC, Waite EM. The oral analgesic efficacy of bicifadine hydrochloride in postoperative pain. J Clin Pharmacol. 1982;22:160–164. doi: 10.1002/j.1552-4604.1982.tb02157.x. [DOI] [PubMed] [Google Scholar]
- 45.Webb SS, Smith GM, Evans WO, Webb NC. Toward the development of a potent, nonsedating, oral analgesic. Psychopharmacology (Berl) 1978;60:25–28. doi: 10.1007/BF00429174. [DOI] [PubMed] [Google Scholar]
- 46.Villemure C, Laferrière AC, Bushnell MC. The ventral striatum is implicated in the analgesic effect of mood changes. Pain Res Manag. 2012;17:69–74. doi: 10.1155/2012/371362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8:781–797. doi: 10.1586/14737175.8.5.781. [DOI] [PubMed] [Google Scholar]
- 48.Yang JC, Clark WC, Dooley JC, Mignogna FV. Effect of intranasal cocaine on experimental pain in man. Anesth Analg. 1982;61:358–361. [PubMed] [Google Scholar]
- 49.Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: involvement of neural reward systems. PLoS One. 2010;5:e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ling W, Mooney L, Hillhouse M. Prescription opioid abuse, pain and addiction: clinical issues and implications. Drug Alcohol Rev. 2011;30:300–305. doi: 10.1111/j.1465-3362.2010.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:362–375. [PubMed] [Google Scholar]
- 52.Nicholson KL, et al. Preclinical evaluation of the abuse potential of the analgesic bicifadine. J Pharmacol Exp Ther. 2009;330:236–248. doi: 10.1124/jpet.109.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 2010;210:149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roughan JV, Wright-Williams SL, Flecknell PA. Automated analysis of postoperative behaviour: assessment of HomeCageScan as a novel method to rapidly identify pain and analgesic effects in mice. Lab Anim. 2009;43:17–26. doi: 10.1258/la.2008.007156. [DOI] [PubMed] [Google Scholar]
- 55.Cobos EJ, et al. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matson DJ. Inflammation-induced reduction of spontaneous activity by adjuvant: a novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320:194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- 57.Pardo EG, Rodríguez R. Reversal by acetylsalicylic acid of pain induced functional impairment. Life Sciences. 1966;5:775–781. [Google Scholar]
- 58.Negus SS, Leitl M, Banks ML. Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous kappa opioids. American College of Neuropsychopharmacology 51st Annual Meeting; Hollywood, FL. 2–6 December 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]


